Abstract
Background
Metallic taste (MT) is a taste abnormality often reported by cancer patients. The aim of this systematic review was to exhaustively report MT incidences in cancer patients and to evaluate the risk of bias in the pertinent studies in accordance with a meta-analysis approach.
Methods
The research objective was to determine the prevalence of MT in patients treated for cancer. A literature search was conducted using PubMed, Web of Science, and Embase. The authors each screened articles and evaluated the eligibility and individual risk of bias for each article. Then, all of the results were compared. A meta-analysis was conducted on studies that specifically focused on MT evaluation.
Results
Very few articles have been published on the incidence of MT among taste and smell abnormalities in cancerology (22 of 1674, 1.3%), and the quality of the reports on MT was often low. The most common bias was the methodology used for MT evaluation. Pooling the results of the 22 studies led to an estimated MT incidence in the cancer patient population of 29% (95% CI [0.21; 0.39]) with high and significant heterogeneity observed among the studies. A heterogeneity analysis was performed to identify the causal factors of this heterogeneity. The specific impact of MT on nutritional status (two) and quality of life (five) studies were reported, respectively, and without a specific evaluation of MT. There was no mention of oral health in any of the studies.
Conclusion
Although in clinical practice cancer patients often report MT, its incidence has only been reported in 22 studies, most of which have a moderate to severe risk of bias. Considering the rather high prevalence of MT, more research should be conducted in this field to better identify its causes and mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taste and smell abnormalities (TSAs) are frequent in oncology regardless of the type of cancer (any solid or hematological type [1, 2, 3, 4, 5]) and are often considered caused by the treatments administered. For instance, dysgeusia affects 50 to 75% of patients depending on the administered treatment(s) (chemotherapy, radiotherapy, or radiochemotherapy) [2, 6]. Perception thresholds can be modified (increased or decreased), perceptions can be distorted (parageusia or parosmia), and/or patients may have perceptions of flavors that do not exist (phantogeusia or phantosmia). TSAs can also impact nutritional status by limiting oral intake [7, 8] and subsequently decreasing quality of life [2, 9, 10].
Metallic taste (MT) is an unusual form of parageusia. Patients describe MT as a spontaneous, unpleasant, persistent metallic sensation in the mouth. Very little is known about the trigger mechanisms for this sensation, possibly due to oncologists’ inconsistent reporting of this symptom, one illustration of which being the large difference in MT incidence reported by patients and their oncologists [11] in which oncologists believed that only 9.9% of their patients suffered from MT, whereas 32.2% of patients reported MT. A few conditions associated with MT are well known, such as oral candidiasis and/or dry mouth [12, 13]. However, the specific mechanisms underlying MT sensation are poorly understood, although several hypotheses have been suggested. The origin of MT may be (i) biochemical, through oxidation between Fe2+ and oral lipids in the mouth [14]; (ii) electrical, induced by local hydrolysis and a spontaneous electrical current between dental amalgams [15]; (iii) neurological, due to the removal of inhibition of the facial nerve on the glossopharyngeal nerve [16, 17, 18]; and/or (iv) proteomic in origin linked to the overexpression of bitter receptors on the tongue [19, 20].
Little is known about the impact of TSAs on cancer patients’ food behavior and nutritional status. However, several studies have reported correlations between chemical sensory alterations and the food behavior of patients while receiving chemotherapy. A recent review [21] noted that the occurrence of chemosensory disorders was associated with a decrease in the oral intake of patients diagnosed with cancer and the evolution of patients’ food preferences and food behavior while receiving chemotherapy. Moreover, among TSAs, the sensation of MT is likely one of the most negatively influential factors on food behavior [22].
To gain further insights into the impact of MT, we performed a systematic review and meta-analysis of the literature to delineate the prevalence of MT in cancer patients.
Methods
The research objective was to determine the prevalence of metallic taste in patients treated for cancer.
PICO question
-
Population: patients treated for cancer.
-
Intervention: chemotherapy, radiotherapy, surgery, other (target and hormonal therapies)
-
Comparison: N/A
-
Outcomes: the prevalence of MT
Data sources and search strategy
An initial search was performed in September 2019 in PubMed, Web of Science (WoS), and Embase. Two updates were performed in January 2020 and November 2020. The search terms used are given below.
-
EMBASE: ‘neoplasm’/exp AND (‘taste disorder’ OR ‘metallic taste’)
-
PubMed: “neoplasms”[MeSH] AND (“taste disorders”[MeSH] OR (“metallic”[All Fields] AND “taste”[All Fields]) OR “metallic taste”[All Fields])
-
Web of Science: ALL = (neoplasm* OR tumor* OR cancer*) AND (ALL = (taste* OR ageusia OR hypogeusia* OR gustat*)) AND (ALL = (disorder* OR alteration* OR metal*))
This systematic review was conducted following the PRISMA 2020 guidelines [23], which are described in the supplementary material (Tables S1 and S2). The study protocol was registered in November 2019 on PROSPERO (an international database of prospectively registered systematic reviews) under the number CRD42020157450. Several changes to the initial protocol were made: (i) nutritional and quality of life aspects could not be assessed because very few articles have been published on MT and nutritional and/or quality-of-life issues and (ii) a meta-analysis on MT prevalence was performed that was not part of the initial plan.
Study selection
Screening step
The screening was conducted by the three investigators (GB, GF, and TTD). GB and GF screened all of the identified titles and abstracts, and discrepancies were resolved by the third investigator (TTD). The inclusion criteria for the screening steps were (a) title or abstract describing any taste disorder in cancer patients, not necessarily MT, or (b) full papers mentioning MT and its prevalence. When only the title (without an abstract) was accessible in the first step and there was doubt concerning eligibility, the corresponding record was considered for the second step. No restrictions were placed on language or publication dates. Abstracts and full papers were included. Case reports, reviews, meta-analyses, commentaries, and editorials were excluded.
Determination of eligibility
Eligibility was assessed by all three investigators. GB and GF screened all the retrieved articles. Discrepancies were resolved by the third investigator (TTD). The eligibility criteria consisted of a specific description of metallic taste (MT), including data on the prevalence (number and/or % of patients).
The screening and eligibility steps were performed using the Rayyan web app for systematic reviews [24].
Data extraction
The three investigators extracted the following data for each study: author names, year, title, journal, study design, sample size, sex, age (mean, SD, and range), country, type of cancer, type of treatment, prevalence of MT (n/N and/or %), date of measurement (in months), and methodology for MT evaluation. When detailed data on MT prevalence were not provided in a paper, the respective authors were contacted.
Individual risk of bias
A bias analysis was performed for each study using the ROBIN-I tool following the Cochrane guidelines for nonrandomized studies and the RoB2 tool for randomized studies [25, 26].
The main confounding domain was used to evaluate MT based on three criteria, namely, the date of MT measurement after treatment, MT awareness of participant, and specificity of MT evaluation. Other identified confounding variables were age, sex, toxicity factors (smoking and alcohol consumption), and oral health.
Outcome
The outcome was the prevalence of MT in cancer patients.
Data analysis
Prevalence was expressed as the number of patients reporting MT divided by the total number of patients. MT prevalence was estimated using a random-effects meta-analysis model. The random-effects model was chosen assuming that clinical and methodological heterogeneity were likely to exist and affect the results. The DerSimonian and Laird method was used to pool the studies [27]. The Clopper-Pearson interval, also called the “exact” binomial interval, was used to calculate confidence intervals [28, 29].
Heterogeneity was assessed by the I2 statistic [30]. Contour-enhanced funnel plots were used to investigate small study effects [31]. Funnel plot asymmetry was tested using the weighted linear regression method [32]. Leave-one-out analysis was performed on the included studies. The analyses were conducted with R software (v3.5.3) using the package Meta and the function Metaprop for meta-analysis of single proportions (the R code is provided in Doc S1).
Registration of the review
This review was not previously registered.
Results
Search results
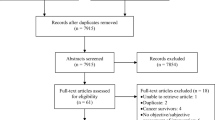
The PRISMA flowchart for article selection is presented in Fig. 1. The search of the three databases identified 2050 records in the cancer area mentioning dysgeusia; 1678 articles remained after removing duplicates. A further 1451 articles were excluded through title and abstract screening because the selection criteria were not met. Specifically, 507 articles dealt with an irrelevant population, 358 articles dealt with an unrelated outcome, and 396 articles were reviewed. Of the 224 remaining articles, 206 were excluded after full-text review principally because the text did not mention MT (n = 154) or MT prevalence (n = 30). Despite our efforts to access all the references, including contacting the authors directly, 10 articles remained inaccessible. Of these 10 articles, seven were published before 2000, only titles were available for six, and six were in a language other than English and published in local journals. Finally, 22 articles were included in the meta-analysis [8, 11, 33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
Characteristics of included studies
The characteristics of the included studies are presented in Table 1. Among the 22 included articles, there were 11 cross-sectional studies, three randomized controlled trials (RCTs), four cohort studies, one observational retrospective study, one study in which variables were controlled before and after the study was performed, one case–control study, and one quasi-randomized control study. The studies were categorized in terms of the type of cancer as follows: 13 dealt with any solid or hematological cancer, three with head and neck cancer, one with breast cancer, two with lung cancer, one with colorectal cancer, one with testicular cancer, and in one study the cancer location was not specified. In this last study, the treatments were chemotherapy (platinum based, taxan based, and others), hormonal therapies, target therapies (tyrosine-kinase inhibitors, others), and concomitant radiochemotherapy [42].
Assessment of MT
Most of the studies used a questionnaire to evaluate MT in cancer patients. However, there were significant variations regarding questionnaire implementation. Only 6 studies reported the time at which MT was evaluated after treatment; 8 studies explicitly included training patients for MT recognition; and 4 studies used an evaluation methodology specific to MT. These variations led to a significant risk of bias for MT evaluation, as presented below and in Figures S1A and S1B of the supplementary material.
Risk of bias
The risk of bias analyses for the 3 RCTs and the 19 non-RCTs are presented in the supplementary material (Table S3). The distribution of the risk of bias for the 3 RCTs and 19 non-RCTs is presented in Fig. 2A and B, respectively.
None of the included non-RCTs had a low risk of bias regarding MT evaluation. The risk of bias in the non-RCT studies was moderate for four (21%), serious for 13 (68%), and critical for 2 (11%) of the studies. The 3 available RCTs were judged to have a high risk of bias (100%) for MT evaluation.
The main issue encountered was a risk of bias for the MT evaluation confounding domain, especially the absence of precision in assessing oral health status (mucositis, candidiasis, and dry mouth) or tobacco consumption. Twelve studies showed a high risk of bias; we had concerns regarding 6 studies; and only two studies were evaluated as having a low risk of bias (see Figs. S1A and S1B in the supplementary material).
MT incidence
A forest plot is presented in Fig. 3. The incidence of MT (with random effects) was estimated at 29% in the general cancer population (95% CI [0.21; 0.39]).
The heterogeneity among studies was high and significant (I2 = 97%, tau = 0.85, p < 0.01) which prompted us to perform subgroup analyses in an attempt to explain this heterogeneity, and the test results are presented in the supplementary material. Many factors were considered to account for the heterogeneity: the cancer type (Fig. S2), treatment type (Figure S3), overall risk of bias (Figure S4), and risk of bias of the sensory evaluation method (Figure S5). The heterogeneity remained close to 1 and significant for every factor considered, except lung cancer. However, only two studies [43, 46] on lung cancer were included, making it difficult to draw sound conclusions. Finally, leave-one-out analysis (Figure S6) confirmed that no single study substantially influenced the MT prevalence estimation. The funnel plot analysis (Figure S7) showed that several of the studies (n = 16) lay outside the 95% confidence interval, which confirmed the heterogeneity among the studies. However, the funnel plot did not provide any evidence indicating that the results of studies with few patients were systematically different from those with a larger number of patients (test of funnel asymmetry: t = 0.87, p value = 0.40).
Discussion
We carried out a systematic literature review and meta-analysis of the prevalence of MT in cancer patients. Five main results can be highlighted. First, very few articles reported MT incidence among TSAs in oncology (22 out of 1678 (1.3%)). Then, the pooled results led to an estimated MT incidence of 29% in the general cancer population (95% CI [0.21; 0.39]). The specific impact of MT on nutritional status and quality of life has rarely been evaluated. Only five studies reported MT and nutritional status, and only two studies mentioned MT and the quality of life of patients, but without specific evaluation. Furthermore, the quality of the reports concerning MT was often low (Figs. 2, 3, and 4) and we did not identify any studies with a low risk of bias; only 18% of the studies had a moderate risk of bias. Finally, there was no mention of oral health in any study.
Most of the included studies were observational cross-sectional studies. Three RCTs and three longitudinal cohort studies were included. The primary outcome of the latter was the incidence of TSAs, but not MT specifically.
Exploration of heterogeneity
A heterogeneity test assesses whether the results of studies can be considered to be similar (homogeneity hypothesis); if all the results are similar, the studies can be considered together. If the heterogeneity is significant, then there is at least one study for which the results cannot be considered identical to those of the others, making it inappropriate to pool the studies. Significant heterogeneity can usually be resolved by subgroup analyses [53]. Considering the significant and high heterogeneity (0.97, p < 0.01) encountered in our meta-analysis, several subgroup analyses were carried out (see the supplementary material). However, the heterogeneity remained high and significant according to the cancer type (except for the two studies on lung cancer [43, 46]), the treatment type, the global risk of bias, and the risk of bias for sensory evaluation. Several hypotheses can be proposed as a possible explanation of this heterogeneity. First, the treatments in the 22 included articles were mainly chemotherapies using many different drugs and administration schemes (depending on the cancer type). A subgroup analysis by drug type could not be performed because individual data was not available. Second, a large variability in the time of MT measurement was observed (e.g., immediately after chemotherapy or many months after the end of the treatment), which might account for a large part of the observed heterogeneity. Third, several confounding variables, such as tobacco use, alcohol consumption, or oral health status were not controlled. Indeed, it has been shown that chemotherapy can cause oral mucosal lesions, oral candidiasis, and an increased abundance of acidophilic oral microflora, which may induce the measured taste disturbances [13, 54].
Two studies [37, 49] reported a high MT incidence but were based on the same cohort; only the population size varied between these two studies. None of the included studies reported MT incidence in the noncancer population, and we found no corresponding data in the literature. However, one of the coauthors of Logan’s paper [44] (Prof. L. Bartoshuk) shared data for the control group with us, in which the MT incidence was 8%. Many factors were considered in the analysis of MT sensation for the general population [15, 42, 44, 55]: high intense sweetener consumption, anodic stimulation of the tongue, chorda tympani damage or direct stimulation, pregnancy, burning mouth syndrome, the presence of metals (iron, copper, zinc, platin) or salts (calcium, magnesium) in the mouth, and medical treatments. However, no clear mechanism explaining MT has been reported.
Impact of MT on nutritional status and quality of life of cancer patients
There was a presumed impact of MT on weight and/or quality of life in several of the included studies; however, only five studies reported weight changes in the investigated population [8, 22, 41, 43, 49]. Although every included study reported TSAs in cancer patients, none of the studies was dedicated to MT. Therefore, we were not able to evaluate the specific impact of MT on nutritional status and weight in patients treated for cancer. Similarly, neither of the two studies in which TSAs were mentioned as affecting the quality of life [38, 39] reported a specific impact of MT on quality of life.
Quality of evidence
The quality of evidence for MT prevalence in the cancer patient population was considered to be low for different reasons. First, the substantial heterogeneity observed among studies could reflect the influence of the different types of cancer and/or of the different types of treatments considered in the included studies. Moreover, for several studies, the absence of a description of the location and dose used for the treatment might also contribute to the discrepancies among studies. Second, most of the included studies were unclear regarding MT evaluation or had a high risk of bias in the MT evaluation. These findings highlight the necessity of using a more rigorous and/or standardized methodology for MT evaluation.
The perceptual status of MT
This systematic review addresses the question of MT as a symptom of cancer patient treatments and highlight the difficulty to evaluate this symptom in an adequate manner. Indeed, it is likely that MT is not a basic taste since its perception vanished when retro-olfaction was suppressed in many studies [14, 15, 28, 29, 56, 57, 58, 5960]; MT is thus more in line with the concept of flavor that could involve the activation of several chemosensory systems [61]. Therefore, we suggest the need to introduce an appropriate testing methodology of MT as a flavor. We decided to explore, in healthy volunteers, if there was a significant difference of intensity in the perception of MT when a ferrous sulfate solution is specifically applied by swabs to the anterior portion (facial nerve) or to the posterior portion (glossopharyngeal nerve) of the tongue. Another prospective line of study is currently led by our team, the TORCAD program (NCT03558789), and tries to better objectify the MT in head and neck cancers by a study of saliva composition, basic tastes, and MT testing [42] as well as employing serial quality of life [62, 63] questionnaires at specific times of treatments. This prospective study shall enable to clarify potential causes of MT, namely oxidation between Fe2+ and oral lipids in the mouth [14], removal of inhibition of the facial nerve on the glossopharyngeal nerve [16, 17, 18]. In addition, such a study would further explore implications for patient care and potential management strategies identified by IJpma et al. [42], such as the use of plastic cutlery, the addition of herbs, spices, sweetener, or acid to foods; to eat cold or frozen foods; to use “miracle fruit” supplements; and to rinse the mouth with chelating agents (e.g., bovine lactoferrin).
Limitations
Bias in the selection phase cannot be excluded. First, all relevant studies may not have been indexed in the three searched databases (WoS, PubMed, Embase). Second, the search was based on a specific list of terms related to the subject of this review. The possibility that additional articles may have been identified using other terms cannot be excluded, although the search was intended to be as extensive as possible.
Another limitation is the systematic absence of evaluations of oral health status and Candida in the selected articles. These health factors may not affect the incidence of MT, although they could be critically related to the etiology of MT. To clarify this, they should be considered in future MT studies.
Conclusion
This systematic review and meta-analysis revealed that approximately one-third of cancer patients suffer from MT disorder. However, these results should be interpreted with caution due to the low quality of the included studies which evaluated MT. The findings of this review highlight that a standardized procedure needs to be employed to evaluate MT in order to obtain high-quality results. Moreover, sensory and/or survey protocols should be followed to maximally reduce confounding MT with other tastes, particularly the bitter taste. The timing of MT evaluation should also be systematically reported to reduce the heterogeneity among studies. One of the initial objectives of this review was to evaluate the relationship between MT prevalence and the nutritional and quality-of-life status of cancer patients. However, insufficient information was available in the literature to accomplish this objective, revealing a gaping lack of knowledge in this field. This analysis clearly demonstrated that taste disorders, particularly MT, are considered independently of the quality of life and nutritional issues in prospective clinical studies. Therefore, it appears to be necessary to (i) promote rigorous evaluation of both nutritional status and quality of life in prospective clinical and biological studies, (ii) consider oral health and treatment in cases of mucositis or candidiasis, and (iii) conduct high-quality controlled trials to account for MT as well as other taste and smell disorders to improve the nutritional status and quality of life of patients treated for cancer. To this end, we are currently conducting a prospective study implementing these recommendations in patients with head and neck neoplasms. We expect that the results will provide better insights into the causes and possible treatments of MT.
Data availability
Data are provided in Table 1 and the supplementary files.
Code availability
Provided in the supplementary files.
References
De Conno F, Ripamonti C, Sbanotto A, Ventafridda V (1989) Oral complications in patients with advanced cancer. J Palliat Care 5:7–15
Hovan AJ, Williams PM, Stevenson-Moore P, Wahlin YB, Ohrn KE, Elting LS et al (2010) A systematic review of dysgeusia induced by cancer therapies. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 18:1081–1087
Heckel M, Stiel S, Ostgathe C (2015) Smell and taste in palliative care: a systematic analysis of literature. Eur Arch Otorhinolaryngol 272:279–288
Spotten LE, Corish CA, Lorton CM, Ui Dhuibhir PM, O’Donoghue NC, O’Connor B et al (2017) Subjective and objective taste and smell changes in cancer. Ann Oncol 28:969–984
van Oort S, Kramer E, de Groot JW, Visser O (2018) Taste alterations and cancer treatment. Curr Opin Support Palliat Care 12:162–167
Spotten L, Corish C, Lorton C, Dhuibhir PU, O’Donoghue N, O’Connor B et al (2016) Subjective taste and smell changes in treatment-naive people with solid tumours. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 24:3201–3208
Boltong A, Keast R (2012) The influence of chemotherapy on taste perception and food hedonics: a systematic review. Cancer Treat Rev 38:152–163
Coa KI, Epstein JB, Ettinger D, Jatoi A, McManus K, Platek ME et al (2015) The impact of cancer treatment on the diets and food preferences of patients receiving outpatient treatment. Nutr Cancer 67:339–353
Belqaid K, Orrevall Y, McGreevy J, Mansson-Brahme E, Wismer W, Tishelman C et al (2014) Self-reported taste and smell alterations in patients under investigation for lung cancer. Acta Oncol 53:1405–1412
Brisbois TD, de Kock IH, Watanabe SM, Baracos VE, Wismer WV (2011) Characterization of chemosensory alterations in advanced cancer reveals specific chemosensory phenotypes impacting dietary intake and quality of life. J Pain Symptom Manage 41:673–683
Newell S, Sanson-Fisher RW, Girgis A, Bonaventura A (1998) How well do medical oncologists’ perceptions reflect their patients’ reported physical and psychosocial problems? Data from a survey of five oncologists. Cancer 83:1640–1651
Epstein JB, Thariat J, Bensadoun RJ, Barasch A, Murphy BA, Kolnick L et al (2012) Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA: A Cancer Journal for Clinicians. 62:400–422
Jensen SB, Mouridsen HT, Bergmann OJ, Reibel J, Brunner N, Nauntofte B (2008) Oral mucosal lesions, microbial changes, and taste disturbances induced by adjuvant chemotherapy in breast cancer patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:217–226
Epke EM, McClure ST, Lawless HT (2009) Effects of nasal occlusion and oral contact on perception of metallic taste from metal salts. Food Qual Prefer 20:133–137
Lawless HT, Stevens DA, Chapman KW, Kurtz A (2005) Metallic taste from electrical and chemical stimulation. Chem Senses 30:185–194
Kveton JF, Bartoshuk LM (1994) The effect of unilateral chorda tympani damage on taste. Laryngoscope 104:25–29
Lehman CD, Bartoshuk LM, Catalanotto FC, Kveton JF, Lowlicht RA (1995) Effect of anesthesia of the chorda tympani nerve on taste perception in humans. Physiol Behav 57:943–951
Yanagisawa K, Bartoshuk LM, Catalanotto FA, Karrer TA, Kveton JF (1998) Anesthesia of the chorda tympani nerve and taste phantoms. Physiol Behav 63:329–335
Ijpma I, Renken RJ, Gietema JA, Slart R, Mensink MGJ, Lefrandt JD et al (2017) Changes in taste and smell function, dietary intake, food preference, and body composition in testicular cancer patients treated with cisplatin-based chemotherapy. Clin Nutr. 36:1642–1648
Hirai R, Takao K, Onoda K, Kokubun S, Ikeda M (2012) Patients with phantogeusia show increased expression of T2R taste receptor genes in their tongues. Ann Otol Rhinol Laryngol 121:113–118
Drareni K, Dougkas A, Giboreau A, Laville M, Souquet PJ, Bensafi M (2019) Relationship between food behavior and taste and smell alterations in cancer patients undergoing chemotherapy: a structured review. Semin Oncol 46:160–172
Speck RM, DeMichele A, Farrar JT, Hennessy S, Mao JJ, Stineman MG et al (2013) Taste alteration in breast cancer patients treated with taxane chemotherapy: experience, effect, and coping strategies. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 21:549–555
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 134:103–112
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5:210
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 355:i4919
Schunemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K et al (2019) GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of clinical epidemiology. 111:105–114
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Agresti A, Coull BA (1996) Order-restricted tests for stratified comparisons of binomial proportions. Biometrics 52:1103–1111
Newcombe RG (1998) Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med 17:873–890
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J et al (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 343:d4002
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ. 315:629–634
Al-Taie A, Köseoğlu A (2019) Determination of radiotherapy-related acute side effects; a starting point for the possible implementation of a clinical pharmacy services in the radiological unit in Turkey. J Young Pharm 11:434–438
Alvarez-Camacho M, Martinez-Michel L, Gonella S, Scrimger RA, Chu KP, Wismer WV (2016) Physical symptom burden of post-treatment head and neck cancer patients influences their characterization of food: Findings of a repertory grid study. Eur J Oncol Nurs 22:54–62
Amezaga J, Alfaro B, Rios Y, Larraioz A, Ugartemendia G, Urruticoechea A et al (2018) Assessing taste and smell alterations in cancer patients undergoing chemotherapy according to treatment. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 26:4077–4086
Bernhardson BM, Tishelman C, Rutqvist LE (2008) Self-reported taste and smell changes during cancer chemotherapy. Supportive care in cancer 16:275–283
Cusnir M, Soares H, Schwartz M, Pizzolato J, Ludsky J, Campbell R, et al. (2012) Treatment of taste alterations in chemotherapy patients using “Miracle fruit”. Psycho-oncology. p. 58.
Derksen JWG, Koopman M, Ten Bokkel Huinink D, Sommeijer DW, Dorresteijn B, Jourdan M et al (2019) Taste and smell alterations (TSAs) in patients (pts) with stage II-III colon cancer (CC): A pilot within the PROTECT study. Annals of Oncology 30:v735
Genvresse I, Lange C, Schanz J, Schweigert M, Harder H, Possinger K et al (2001) Tolerability of the cytoprotective agent amifostine in elderly patients receiving chemotherapy: a comparative study. Anticancer Drugs 12:345–349
Halyard MY, Jatoi A, Sloan JA, Bearden JD 3rd, Vora SA, Atherton PJ et al (2007) Does zinc sulfate prevent therapy-induced taste alterations in head and neck cancer patients? Results of phase III double-blind, placebo-controlled trial from the North Central Cancer Treatment Group (N01C4). Int J Radiat Oncol Biol Phys 67:1318–1322
Ijpma I, Renken RJ, Ter Horst GJ, Reyners AK (2016) The palatability of oral nutritional supplements: before, during, and after chemotherapy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 24:4301–4308
Ijpma I, Timmermans ER, Renken RJ, Ter Horst GJ, Reyners AK (2017) Metallic taste in cancer patients treated with systemic therapy: a questionnaire-based study. Nutrition and cancer. 69:140–145
Lindsey AM, Piper BF (1986) Anorexia, serum zinc, and immunologic response in small cell lung cancer patients receiving chemotherapy and prophylactic cranial radiotherapy. Nutr Cancer 8:231–238
Logan HL, Bartoshuk LM, Fillingim RB, Tomar SL, Mendenhall WM (2008) Metallic taste phantom predicts oral pain among 5-year survivors of head and neck cancer. Pain 140:323–331
McDaniel RW, Rhodes VA (1998) Development of a preparatory sensory information videotape for women receiving chemotherapy for breast cancer. Cancer Nurs 21:143–148
McGreevy J, Orrevall Y, Belqaid K, Wismer W, Tishelman C, Bernhardson BM (2014) Characteristics of taste and smell alterations reported by patients after starting treatment for lung cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 22:2635–2644
Ponticelli E, Clari M, Frigerio S, De Clemente A, Bergese I, Scavino E, et al. (2017) Dysgeusia and health-related quality of life of cancer patients receiving chemotherapy: a cross-sectional study. European Journal of Cancer Care 26
Rehwaldt M, Wickham R, Purl S, Tariman J, Blendowski C, Shott S et al (2009) Self-care strategies to cope with taste changes after chemotherapy. Oncol Nurs Forum 36:E47-56
Soares HP, Cusnir M, Schwartz MA, Pizzolato JF, Lutzky J, Campbell RJ et al (2010) Treatment of taste alterations in chemotherapy patients using the “miracle fruit”: Preliminary analysis of a pilot study. J Clin Oncol. 28:e19523
Ui Dhuibhir P, Barrett M, O’Donoghue N, Gillham C, El Beltagi N, Walsh D (2020) Self-reported and objective taste and smell evaluation in treatment-naive solid tumour patients. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 28:2389–2396
Wickham RS, Rehwaldt M, Kefer C, Shott S, Abbas K, Glynn-Tucker E et al (1999) Taste changes experienced by patients receiving chemotherapy. Oncol Nurs Forum 26:697–706
Wilken MK, Satiroff BA (2012) Pilot study of “miracle fruit” to improve food palatability for patients receiving chemotherapy. Clin J Oncol Nurs 16:E173–E177
Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI (2009) Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract 63:1426–1434
Sakashita S, Takayama K, Nishioka K, Katoh T (2004) Taste disorders in healthy “carriers” and “non-carriers” of Candida albicans and in patients with Candidosis of the tongue. J Dermatol 31:890–897
Reith AJM, Spence C (2020) The mystery of “metal mouth” in chemotherapy. Chem Senses 45:73–84
Epke EM, Lawless HT (2007) Retronasal smell and detection thresholds of iron and copper salts. Physiol Behav 92:487–491
Hettinger TP, Myers WE, Frank ME (1990) Role of olfaction in perception of non-traditional ‘taste’ stimuli. Chem senses 15:755–760
Hong JH, Kim KO (2011) Operationally defined solubilization of copper and iron in human saliva and implications for metallic flavor perception. Eur Food Res Technol 233:973–983
Lim J, Lawless HT (2005) Oral sensations from iron and copper sulfate. Physiol Behav 85:308–313
Stevens DA, Baker D, Cutroni E, Frey A, Pugh D, Lawless HT (2008) A direct comparison of the taste of electrical and chemical stimuli. Chem Senses 33:405–413
Thomas-Danguin T (2009) Flavor. In: M. D. Binder, N. Hirokawa, & U. Windhorst (Éds.), Encyclopedia of Neuroscience. Springer-Verlag and Heidelberg GmbH: Berlin, pp 1580–1582
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF et al (1999) Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol 17:1008–1019
Acknowledgements
The authors acknowledge L. Umubyeyi-Mutel, F. Pithon and C. Molta, MD, for revising the language of the manuscript.
Author information
Authors and Affiliations
Contributions
All three authors contributed to the study conception and design. G. Buiret and G. Féron conceptualized the writing of the article, and all three authors performed the literature search and data analysis (as described in the Methods section). The first draft of the manuscript was written by G. Buiret, and all authors commented on subsequent versions of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
N/A.
Competing interests
The authors declare no competing interests.
Consent for publication
All authors read and approved the final manuscript.
Consent to participate
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Buiret, G., Thomas-Danguin, T. & Feron, G. Metallic taste prevalence in patients treated for cancer: a systematic literature review and meta-analysis. Support Care Cancer 30, 5691–5702 (2022). https://doi.org/10.1007/s00520-022-06904-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-06904-y