Abstract
Purpose
This study explores the prevalence of self-reported taste and smell changes (TSCs) during chemotherapy and relationships between TSCs and demographic and clinical factors.
Materials and methods
Consecutive patients who had received chemotherapy for ≥6 weeks at 11 outpatient chemotherapy units completed a questionnaire developed for this survey.
Results
Seventy-five percent of the 518 participants reported TSCs, with TSCs more prevalent among women and younger patients. After adjustment for age and sex, we found that patients reporting TSCs more often reported: previous smell changes, less responsibility for cooking, concurrent medication, higher educational levels, and being on sick leave. Participants reporting oral problems, nausea, appetite loss, and depressed mood more frequently reported TSCs. Diagnosis and type of chemotherapy regimen did not predict TSCs.
Conclusion
TSCs were found to be common during cancer chemotherapy and were related to sociodemographic rather than clinical factors. TSCs were also found to be closely related to many other side effects of chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients undergoing cancer chemotherapy typically experience multiple side effects and/or treatment-related problems. Griffin et al. [13] found that chemotherapy patients reported experiencing an average of 20 symptoms from a list of 73, while Youngblood et al. [33] found 12 symptoms of 37 studied. Common chemotherapy-related problems include nausea, tiredness, hair loss, concerns about effects on other family members, sleeping difficulties, taste changes, and loss of appetite [6, 11, 13, 22, 29, 33]. Research in this area has most often focused on chemotherapy-induced nausea and vomiting [13], while taste and/or smell changes (TSCs) have received less attention in general, with only a few recent studies published. Hence, there is scant evidence underlying care provision for patients with TSCs.
Although TSCs have been associated with a variety of diseases, conditions, and pharmaceutical agents, they are typically frequent during cancer chemotherapy. The few studies that have investigated TSCs among patients receiving cancer chemotherapy have focused on physiological tests of taste and smell sensitivity [3, 19, 21] or prevalence of disturbance [10, 25] rather then on how these chemosensory changes affect patients’ daily lives. While such physiological knowledge is important in developing approaches to prevent or treat disturbances, knowledge about patients’ experiences of TSCs is also needed to enable support during chemotherapy. There is otherwise a risk that healthcare providers may overlook these problems [28].
Studies that focus on patients’ perceptions indicate that TSCs have an impact on patients’ daily life [2, 5, 32]. In a qualitative interview study [2], patients reported both social and emotional consequences of TSCs. In the same study, all 21 interviewed patients reported receiving information about TSCs, but this information appeared to be poorly understood.
This poor understanding, combined with a lack of adequate language for communication about TSCs [2], may indicate that it is particularly challenging for healthcare providers to discuss TSCs with patients. There is a need for further investigation to complement physiological data and expands the evidence base for constructive information and clinical action. The aim of this study was therefore to explore the prevalence of self-reported TSCs during cancer chemotherapy and to study the relationships between TSCs and demographic and clinical factors.
Materials and methods
As we found no Swedish questionnaire that addressed our research questions, we designed a survey questionnaire to investigate TSCs among patients receiving cancer chemotherapy. The questionnaire was based on findings from a previous qualitative interview study [2], clinical experience, and existing literature [23, 32]. It contained 33 questions, using both open-ended and closed response alternatives. One section concerned taste changes and appetite (seven questions), and another section focused on smell changes (eight questions). We also collected data on socio-demographic factors postulated to impact on meal-related situations, clinical background data, other potential chemotherapy-related symptoms (nausea, vomiting, oral problems, depressed mood, weight, and an open question about other problems the participants believed to be related to chemotherapy) and communication about TSCs between patients and health care providers. The last two questions were open-ended, encouraging patients to provide additional relevant information and to comment on the questionnaire itself. Information about cancer diagnosis chemotherapy regimen, number of cycles, previous chemotherapy, and medication given in conjunction with the chemotherapy was obtained from medical records.
An initial draft of the questionnaire was reviewed by clinical chemotherapy nurses and by a group of experienced cancer nurse researchers. The draft was then pilot-tested in a group of selected patients receiving chemotherapy that included adults of both sexes with a wide range of ages and degrees of TSCs. A think-aloud method [7, 15] was used to ensure that items were understandable and that the respondent interpreted the questions as intended. After three think-aloud interviews, the questionnaire was revised. Seven additional think-aloud interviews were held without necessitating revision. As a further control, three additional patients filled the questionnaire alone with the researcher checking that analysis and interpretation were in line with the patients’ intended responses.
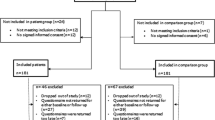
The finalized questionnaire was then used to survey a consecutive sample of adult cancer patients receiving cancer chemotherapy at 11 oncology or gynecology outpatient units in three of the six health care regions in Sweden independent of the diagnosis and regimen. Patients eligible for inclusion had received chemotherapy for ≥6 weeks, were able to communicate in Swedish, had no disabilities or problems that contra-indicated participation, and gave informed consent. Participants completed the questionnaire once, usually in hospital.
The study protocol was approved by the Regional Research Ethics Review Board in Stockholm (2005/77–31/3).
Statistical considerations
A power calculation indicated that to reliably assess differences of 20% between sub-groups based on chemotherapy regimen, groups of 50–100 patients were required if 40–60% of the patients reported TSCs (α = 0.05, 1 − β = 0.80). Statistics from one of the participating units indicated that the most common chemotherapy regimens were each administered to about 10% of the total number of chemotherapy patients. The target sample size was therefore set at 500–600 patients to obtain adequate groups of patients treated with the four–five most common regimens. A multi-center study was considered to be needed to recruit adequate numbers of patients during a feasible time period of 3 weeks.
Differences in proportions were analyzed with a chi-squared test. The relationships between self-reported occurrence of TSCs and putative predictors (6 demographic variables and 14 clinical variables) were analyzed using univariate and multivariate logistic regression models (SPSS, Version 15). Multiple response alternatives to questions about symptoms were dichotomized as follows for analysis: “never” or “seldom” were defined as “without symptoms,” while “sometimes," “often,” or “always” were defined as “experiencing symptoms.” Open-ended questions were analyzed inductively [30, 31].
Results
Patients and background data
A total of 675 patients were eligible for inclusion. Ninety-two patients declined to participate, and 61 did not participate because of administrative errors. Four questionnaires lacked sufficient data for analysis. The results are thus based on 518 questionnaires (77% response rate), of which 77% were from women.
Table 1 shows that women were significantly younger than men (p = 0.04), more likely to live alone (p < 0.01), and more likely to be responsible for cooking (p < 0.01). More women than men reported experiencing smell changes (SC) in the past (p < 0.01), typically in relation to pregnancy, surgery, or viral infections. More women than men reported cancer chemotherapy-induced TSCs (p < 0.01). More men than women were able to continue working full time or part time (p < 0.01). Table 2 shows details of diagnosis and treatment. Participants had received 2–100 cycles of the current chemotherapy regimen, with 50% of the participants interviewed after 4–8 cycles.
The prevalence of symptoms commonly occurring in conjunction with chemotherapy were TSCs 75%, oral problems 56%, depressed mood 49%, nausea 39%, appetite loss 22%, and vomiting 10%. Other symptoms reported in response to an open-ended question were fatigue (22%), neurological disturbances (13%), bowel problems (9%), muscular pain (7%), eye problems (7%), alopecia (7%), concentration problems (3%), sleep disturbances (3%), and mood swings (3%). Twenty-six other symptoms of psychosocial and somatic character were reported to a less extent. Seven participants reported positive changes, such as less pain and time to reflect on life.
Taste and smell changes
A total of 387 (75%) patients reported some form of chemosensory change; 40 of these participants reported SCs alone and 134 reported taste changes (TCs) alone. Most participants reported that the TSCs began directly after the first treatment, while some patients experienced the onset of TSCs up to 10 weeks after the first treatment. Taste changes occurred intermittently for 59% of those reporting TCs, while the TCs were constant for 35%. Smell changes were intermittent for 39% of those reporting SCs, while they were constant for 32%, although 29% of those with SCs did not respond to this question. Intermittent TSCs were reported to last from 1 to approximately 14 days, with some participants commenting that the TSCs developed and ceased gradually.
As shown in Table 3, all types of TCs (salt, sweet, sour, bitter, or other taste changes) were reported, with “other taste changes,” “salt,” and “sweet” most frequently reported. Descriptions of “other taste changes” included metallic tastes (n = 50), decreased taste sensations (n = 36), or other sensations said to be indescribable (n = 30). Over half of the participants who reported TCs reported only one type of TC. Eight identified odors were assessed: “perfume” and “cooking” were those most frequently affected, although typically more than one odor was affected in those reporting SCs (Table 3).
Putative predictors of TSCs
Univariate logistic regression (Table 4) indicated that the following factors were statistically significant predicators of self-reported TSCs: young age (p < 0.01), female (p < 0.01), high education level (p < 0.01), on sick leave (p = 0.01), living with children (p < 0.03), taking concurrent medicationFootnote 1 (p < 0.01), history of smell changes (p < 0.01), and breast cancer diagnosis (p < 0.02). Treatment with gemcitabine (p < 0.01) predicted fewer reports of TSCs (Table 5). Sex and age can be regarded as basic explanatory variables, as both remained statistically significant when adjusted for the other. After adjustment for age and sex in multivariate logistic regression analyses, the following factors remained statistically significant: higher educational level (p = 0.02), on sick leave (p < 0.05), taking concurrent medication (p < 0.01), and history of SCs (p < 0.01). One additional factor was found to be significant: not responsible for cooking (p = 0.02). Treatment with gemcitabine (p < 0.01) continued to predict fewer reports of TSCs.
Other symptoms and their association to TSCs
Univariate logistic regression indicated that participants with TSCs reported significantly more oral problems (p < 0.01), nausea (p < 0.01), appetite loss (p < 0.01), and depressed mood (p = 0.01) than participants without TSCs. When adjusted for age and sex in multivariate regression analyses, oral problems (p < 0.01), nausea (p < 0.01), appetite loss (p < 0.01), and depressed mood (p < 0.01) remained predictors for TSCs (Table 6). Participants’ comments on oral problems, nausea, and depressed mood were analyzed inductively. This analysis indicated that of the 292 participants who reported oral problems, 196 reported dry mouth, 100 reported blisters, and 38 reported mucositis. Comments on nausea generally concerned duration and frequency, with 55 participants reporting nausea lasting 1 to 7 days after treatment. The most frequent comments on depressed mood were descriptions of depression that arose in response to the cancer experience in its entirety or in response to chemotherapy-induced problems, rather than descriptions of depressed mood as a side-effect in it self.
Discussion
This survey includes a systematic sample of patients receiving chemotherapy for ≥6 weeks on oncology or gynecology outpatient units, independent of the diagnosis and regimen. The study design excluded the ‘sickest’ and most vulnerable patients, such as those receiving treatments with long infusion times and those requiring more surveillance than is possible in outpatient care, and has resulted in a sample dominated by women. However, it is reasonable to assume that this sample reflects the outpatient chemotherapy population in Swedish oncology and gynecology departments, whereas it excludes patients receiving chemotherapy at other units, such as surgical and lung medicine departments. This survey approach enabled us to reach a broad sample of patients who were undergoing many different treatment regimens to detect whether particular treatment modalities should be the subject for focus in further studies.
In this sample, 75% reported experiencing TSCs in relation to cancer chemotherapy. This is in line with other studies in which 46–77% of patients undergoing chemotherapy reported TCs and 35–87% reported SCs [18, 25]. Chemosensory changes are thus extremely common side effects in all diagnoses and chemotherapy regimens investigated in this study. This is in contrast to clinical assumptions, which seem to be common despite the lack of research on TSCs’ association with different chemotherapeutic agents [1, 25]. Another assumption without support in this data is that smoking habits are related to TSCs. As in the study of Brämerson et al. [4] of olfactory disturbances in a normal population, we found no association between TSCs and smoking. It can also be noted that TSCs showed no association with self-reported weight changes. Instead, we found that a number of socio-demographic factors, e.g., age and sex, predict TSCs. As it is known that sense of smell and taste are affected by age [26, 27], it is possible that a change in an already decreased chemosensory ability is not as apparent as such changes in younger persons.
In contrast to Wickham et al. [32] who found no difference in chemotherapy-induced TSCs by gender, women in our sample reported TSCs more often than men. Gender differences in olfaction are recognized [24], although there is some discrepancy as to whether men [4] or women [12] experience most olfactory dysfunction. Less data can be found on gender and taste dysfunction.
Our results show that a previous history of SCs was associated with TSCs. This is similar to findings on chemotherapy-induced nausea, which link past experiences of nausea to higher risk of nausea during chemotherapy [16]. Possible explanations that have been discussed in relation to chemotherapy-induced nausea [14, 20] may also be relevant in this study: past experiences of SCs may facilitate recognition of present changes or it may be the case that certain individuals have a predisposition to chemosensory changes.
In this study, we found that concurrent medications predicted TSCs. Many drugs induce TSCs [8], but this study lacks the specificity necessary to examine the potential effects of particular substances. It is possible that this finding is not related to pharmacological effects but that those patients on concurrent medication may represent a particularly vulnerable subset.
Another vulnerable group may be those who experience multiple symptoms. There is limited knowledge at present about the interactions between symptoms. We found a relationship between reported TCSs and oral problems, nausea, depressed mood, and appetite loss, suggesting that these symptoms may be a part of a symptom cluster [17].
It should be recognized that patients completed the survey in hospital during treatment, which means that some patients have had to recall experiences of TSCs during the past month. This limitation was compensated for by the high response rate obtained in this manner. This response rate, in conjunction with comments to an open question, also suggests that the questionnaire was easy to complete. However, there is more missing data for questions toward the end of the questionnaire, which may be due to a lack of uninterrupted time in a clinical setting.
Some participants commented that questions were repetitive. This may be due to our questionnaire design, which distinguished between taste and smell, but posed similar questions about both. One difficulty for participants in responding in such detail may have been that these senses are not usually as clearly differentiated in daily life as in this questionnaire. Another indication of difficulties in distinguishing tastes was the high use of the response alternative “other” rather than salt, sweet, sour, or bitter in regard to what tastes were affected. While physiological tests of taste are able to distinguish four–five tastes, these results are not always in line with participants’ experience. Mattson et al. [19] found that although some patients were able to describe an increased sensitivity to sweet, this could not always be discerned by physiological testing. Duffy et al. [9] argue that there are discrepancies between results from experimental studies and patients’ anecdotes. It is possible that this may reflect the influence of olfactory sensations on taste. One might question if the sensation of smell is influenced by taste to the same degree. Data from the present study and results of a previous qualitative study [2] suggest that SCs without TCs may be a discrete experience.
In general, there is little evidence at present with which to guide health care providers in dealing with different aspects of TSCs. Duffy et al. [9] recommend distinguishing sensory changes from hedonic complaints to provide information about the source of the dysfunction. They also emphasize the need for individualized care. The results of the present study suggest that support for TSCs during cancer chemotherapy may be improved by assessing nausea, oral problems, depressed mood and appetite loss, as well as assessing patients’ concurrent medications. In addition, questions about previous SCs should be included when preparing the patient for chemotherapy.
Notes
Most frequent medications (excluding antiemetics) were painkillers (69), antihypertensives (64), proton-pump inhibitors (59), cardiac medications (58), levotyroxin (39), antidepressants (34), diuretics (27), and anticoagulants (24).
References
Bartoshuk LM (1990) Chemosensory alterations and cancer therapies. NCI Monogr 9:179–184
Bernhardson BM, Tishelman C, Rutqvist LE (2007) Chemosensory changes experienced by patients undergoing cancer chemotherapy: a qualitative interview study. J Pain Symptom Manage (in press).
Berteretche MV, Dalix AM, d’Ornano AM, Bellisle F, Khayat D, Faurion A (2004) Decreased taste sensitivity in cancer patients under chemotherapy. Support Care Cancer 12(8):571–576
Bramerson A, Johansson L, Ek L, Nordin S, Bende M (2004) Prevalence of olfactory dysfunction: the skovde population-based study. Laryngoscope 114(4):733–737
Cameron B, Quested Evans (2003) A matter of taste: the experience of chemotherapy related taste changes. Aust J Cancer Nursing 4(1):3–9
Carelle NPE, Bellanger A, Germanaud J, Thuillier A, Khayat D (2002) Changing patient perceptions of the side effects of cancer chemotherapy. Cancer 95(1):155–163
Collins D (2003) Pretesting survey instruments: an overview of cognitive methods. Qual Life Res 12(3):229–238
Doty RL, Bromley SM (2004) Effects of drugs on olfaction and taste. Otolaryngol Clin North Am 37(6):1229–1254
Duffy V, Fast K, Lucchina L, Bartoshuk L (2002) Oral sensation and cancer. In: Berger A, Portenoy R, Weissman D (eds) Principles and practice of palliative care and supportive oncology. Lippincott Williams and Wilkins, Philadelphia, pp 178–193
Epstein JB, Phillips N, Parry J, Epstein MS, Nevill T, Stevenson-Moore P (2002) Quality of life, taste, olfactory and oral function following high-dose chemotherapy and allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 30(11):785–792
Foltz AT, Gaines G, Gullatte M (1996) Recalled side effects and self-care actions of patients receiving inpatient chemotherapy. Oncol Nurs Forum 23(4):679–683
Frasnelli JTH (2004) Olfactory dysfunction and daily life. Eur Arch Oto-Rhino-Laryngol Hand Neck 265(3):231–235
Griffin AM, Butow PN, Coates AS, Childs AM, Ellis PM, Dunn SM, Tattersall MH (1996) On the receiving end. V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 7(2):189–195
Higgins SC, Montgomery GH, Bovbjerg DH (2007) Distress before chemotherapy predicts delayed but not acute nausea. Support Care Cancer 15:171–177
Jobe JB, Mingay DJ (1989) Cognitive research improves questionnaires. Am J Public Health 79(8):1053–1055
Jordan K, Schmoll HJ, Aapro MS (2007) Comparative activity of antiemetic drugs. Crit Rev Oncol Hematol 61(2):162–175
Kim HJ, McGuire DB, Tulman L, Barsevick AM (2005) Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs 28(4):270–282 (quiz 283–4)
Lindley C, McCune JS, Thomason TE, Lauder D, Sauls A, Adkins S, Sawyer WT (1999) Perception of chemotherapy side effects cancer versus noncancer patients. Cancer Pract 7(2):59–65
Mattsson T, Arvidson K, Heimdahl A, Ljungman P, Dahllof G, Ringden O (1992) Alterations in taste acuity associated with allogeneic bone marrow transplantation. J Oral Pathol Med 21(1):33–37
Miller M, Kearney N (2004) Chemotherapy-related nausea and vomiting-past reflections, present practice and future management. Eur J Cancer Care (Engl) 13(1):71–81
Mulder NH, Smit JM, Kreumer WM, Bouman J, Sleijfer DT, Veeger W, Schraffordt Koops H (1983) Effect of chemotherapy on taste sensation in patients with disseminated malignant melanoma. Oncology 40(1):36–38
Nail LM, Jones LS, Greene D, Schipper DL, Jensen R (1991) Use and perceived efficacy of self-care activities in patients receiving chemotherapy. Oncol Nurs Forum 18(5):883–887
Nordin S, Bramerson A, Murphy C, Bende M (2003) A Scandinavian adaptation of the Multi-Clinic Smell and Taste Questionnaire: evaluation of questions about olfaction. Acta Otolaryngol 123(4):536–542
Olofsson JK, Nordin S (2004) Gender differences in chemosensory perception and event-related potentials. Chem Senses 29(7):629–637
Rhodes VA, McDaniel RW, Hanson B, Markway E, Johnson M (1994) Sensory perception of patients on selected antineoplastic chemotherapy protocols. Cancer Nurs 17(1):45–51
Schiffman SS (1997) Taste and smell losses in normal aging and disease. Jama 278(16):1357–1362
Schiffman SS, Gatlin CA (1993) Clinical physiology of taste and smell. Annu Rev Nutr 13:405–436
Sherry VW (2002) Taste alterations among patients with cancer. Clin J Oncol Nurs 6(2):73–77
Sitzia J, North C, Stanley J, Winterberg N (1997) Side effects of CHOP in the treatment of non-Hodgkin’s lymphoma. Cancer Nurs 20(6):430–439
Thorne S, Kirkham S, MacDonald-Emes J (1997) Interpretive description: a noncategorical qualitative alternative for developing nursing knowledge. Res Nurs Health 20:169–177
Thorne S, O’Flynn-Magee K (2004) The analytic challenge in interpretive description. Int J Qual Methods 3(1):1–21
Wickham RS, Rehwaldt M, Kefer C, Shott S, Abbas K, Glynn-Tucker E, Potter C, Blendowski C (1999) Taste changes experienced by patients receiving chemotherapy. Oncol Nurs Forum 26(4):697–706
Youngblood M, Williams PD, Eyles H, Waring J, Runyon S (1994) A comparison of two methods of assessing cancer therapy-related symptoms. Cancer Nurs 17(1):37–44
Acknowledgements
We thank the following agencies for economic support: the Swedish Health Care Sciences Postgraduate School, Karolinska Institutet (BMB), the Swedish Research Council (CT), and the Cancer & Traffic Injury Fund (project support). The authors also thank statisticians Hemming Johansson and Sara Runesdotter for their advice and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bernhardson, BM., Tishelman, C. & Rutqvist, L.E. Self-reported taste and smell changes during cancer chemotherapy. Support Care Cancer 16, 275–283 (2008). https://doi.org/10.1007/s00520-007-0319-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-007-0319-7