Abstract
Purpose
The purpose of this study is to evaluate compliance with and safety of a novel independent home exercise program for patients with high-grade brain tumors. We designed this program around the preferences and individual capabilities of this population as well as the potential barriers to exercise in cancer patients. Demographics were collected to better understand those that persisted with exercise.
Methods
Subjects with high-grade brain tumor received one-time training that included watching an exercise video and live demonstration of resistance band exercises, a balance exercise, and recommendations for walking. Subjects were instructed to do the exercises every day for 1 month. Main outcome measures were percentage of subjects who exercised throughout the month, frequency of exercising, demographic factors, quality of life scores (assessed by FACT-BR), and self report of adverse events.
Results
Fourteen of the 15 (93%) subjects started the exercises during the course of the month. Nine of the fifteen (60%) continued the exercises throughout the month. Three additional subjects would have continued to exercise if formal or supervised rehabilitation had been offered. Among the subjects who continued the exercises regularly, higher frequency of exercising was significantly associated with living as married (p = 0.033), annual income >$50,000 (p = 0.047), scores of physical well-being (p = 0.047), and brain cancer specific well-being (p = 0.054) subscales. Among those who exercised frequently, there was also a trend towards increase in total FACT-BR scores (p = 0.059). The subjects who scored higher on the social well-being subscale of the FACT-BR at baseline self-reported a higher likelihood to continue the exercises after 1 month of participation in the study (p = 0.018). No adverse events were reported.
Conclusions
Our small group of subjects with high-grade brain tumors demonstrated compliance with and safety of a novel independent strength and balance exercise program in the home setting. Higher frequency of exercising was associated with life quality parameters as well as marriage and income.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compliance is defined as “the extent to which a patient’s behavior coincides with medical advice.” [1] Oncology providers are now advising patients to exercise due to an increasing body of evidence on the beneficial effects of exercise in cancer patients [2]. A national survey demonstrated that Canadian oncologists agreed that exercise was beneficial, important, and safe for patients, even during cancer treatment [3]. Forty-three percent of these survey participants tried to recommend exercise to their patients when appropriate. Burke’s group described the importance of designing exercises around individual capabilities in cancer patients and expressed concern that less than 25% of European cancer survivors meet the current physical activity guidelines [4]. Jones and Courneya explained that most cancer patients will not exercise without a structured intervention [5]. Their survey of prostate, breast, colorectal, and lung cancer subjects found that 84% preferred to receive exercise counseling at some point during their cancer experience. Since the majority (40%) of their survey participants preferred to exercise at home, they concluded that programs should be offered with a minimal amount of equipment and supervision.
In considering exercise preferences and individual capabilities, barriers must also play a role. Little evidence is available on barriers to exercise in the brain tumor population. In breast and head and neck cancer, the vast majority of barriers cited are treatment-related side effects [6, 7]. However, breast and prostate cancer patients have cited “not sure what to do, no access to exercise, and nowhere to do it” as barriers to exercise [8]. Head and neck cancer patients have expressed concern over “lack of equipment, lack of facilities, and lack of knowledgeable exercise” as barriers. A study that included one brain tumor patient (of 401 subjects with cancer) found most frequent barriers were “illness” and “lack of motivation,” but “weather extremes, lack of facilities, weakness, and fear of falling” were also cited [9].
We hoped to address exercise compliance in brain tumor patients at our institution by addressing their preferences and individual capabilities. Weakness and balance impairments affect individual capabilities, and motor weakness is typically the focus of rehabilitation, especially in the outpatient setting [2]. One reason for this may be that severe muscle wasting and weakness can be seen in brain tumor patients after glucocorticoid use. Administration of glucocorticoids to brain tumor patients for symptom management has been part of treatment to reduce cerebral edema for over 50 years [10]. Resistance and endurance exercises can reverse muscle atrophy and weakness in non-cancer patients treated with glucocorticoids [11]. Although deficits related to steroid myopathy may be more salient in this subset of patients [12], weakness in brain tumor patients is also related to location and size of the tumor. Not only does exercise help with weakness but also, in malignant recurrent glioma patients, exercise behavior is a strong predictor of survival [13].
Jones and his group have shown that brain cancer patient preference for exercise increases in the post-treatment period as compared to during chemotherapy and radiation and that most patients prefer to exercise at home or at an outpatient supervised setting rather than at a hospital-based setting [14]. In their study at a large academic medical center, subjects preferred to receive exercise information with technologically based approaches. They acknowledge that patient preference for technology may vary related to the demographics of the participating subjects.
Potential barriers to exercise such as lack of knowledge of appropriate exercises, cost of exercise, and lack of space were considered in our study design. Weakness was addressed as both affecting individual capability and as a barrier to exercise. Although we could not address all treatment-related side effects, weakness is certainly one common side effect that is amenable to exercise. Balance impairment may also affect individual capability and be an exercise barrier. We hoped to better understand compliance with our exercise program by recording the demographics of our brain tumor population in relation to their exercise frequency and quality of life.
The effect of teaching a specific independent home exercise program following treatment for brain tumor has not been systematically described in the past. We designed a program to address exercise preferences and individual capabilities. The program was also designed with consideration of barriers to exercise in cancer patients. We monitored compliance with and safety of this program and recorded demographics and quality of life. We chose exercises with a focus on improving muscle weakness and balance in the post-treatment period. We made a video to potentially enhance our ability to encourage compliance in subjects who might prefer a technologically based approach.
Our home exercise program had three components: strengthening, balance, and conditioning. The strengthening portion targeted the upper and lower body muscles used during walking: deltoids, hip abductors, and quadriceps. The balance component targeted standing lower extremity stability. The conditioning component consisted of recommendations for walking. While exercises targeting these muscle groups are employed in inpatient and outpatient physical therapy settings, to our knowledge, there have been no studies evaluating the compliance and safety of this particular set of exercises performed independently at home after face-to-face teaching in a population of patients with brain tumors.
The purpose of this phase 1 trial was to evaluate compliance with and safety of our exercise program designed with consideration of the preference for a home-based, post-treatment program and individual capability, such as likelihood of weakness and balance impairment, in the brain tumor patient. Secondary aims included its efficacy in improving quality of life and any correlation between compliance with the home exercise program and demographics.
Methods
Inclusion criteria for our single arm, prospective trial were as follows: above 18 years of age, diagnosis of high-grade astrocytoma (grade 3 or 4), or any primary tumor with metastasis to the brain, at least 28 days out from surgery, currently undergoing follow-up for standard oncology care at our center, and able to perform exercises independently at home. We included subjects with high-grade primary central nervous system tumors as well as those with tumors metastatic to the brain since they demonstrate similar impairments in weakness and balance. We defined the ability for home exercise as oriented to person, place, time; can follow two-step commands; no functional joint range of motion limitations with the exception of those related to hemiplegia as a result of their condition, as determined by clinical musculoskeletal exam; no current untreated thromboembolic disease; and not on home oxygen. This was approved by our Internal Review Board, #H00006774.
Potential confounders included relying on oncology providers to decline approach of a subject for any reason before offering for the subject to decline and our location at a multi-site academic cancer center in an economically depressed region of the state. Of note, providers were not discouraged from ordering rehabilitation during the study period and subjects who had participated in rehabilitation were not excluded. By nature of the design of the study, subjects were not doing formal rehabilitation concurrently with the home exercise program, but were asked about participation in inpatient and outpatient rehabilitation services received between diagnosis and study participation and desire to participate in rehabilitation services after or during the study period.
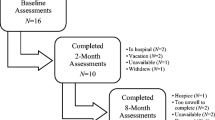
Forty-seven subjects were screened from the UMass cancer center tumor registry, Medical Oncology provider schedules, and direct physician referral at three medical centers affiliated with UMass Memorial Healthcare (University Ambulatory Care Center, Health-Alliance Fitchburg, and Marlborough Hospital). This number was arrived at as this was the total number of available subjects during the 8 months that we had research staff available. Thirteen subjects declined to participate and eight subjects were declined for approach by their treating physician. Seven potential subjects were not screened as they were in hospice care or deceased at the time of the study and one was ineligible at screening. Three potential subjects had no further follow-up scheduled at the cancer center and thus were not eligible. As such, 32 potential subjects were either declined or ineligible.
Fifteen subjects met the eligibility criteria, consented to participate, and completed most study procedures. Thirteen subjects completed all study procedures. Eight subjects had astrocytoma, and seven had brain metastases from other primary cancers. They were an average of 55 years of age and 15 months post-diagnosis. Despite significant time since diagnosis, seven subjects were currently undergoing chemotherapy. Three subjects were on steroids during the home exercise program, but only one was currently undergoing radiation. Sixty percent (nine subjects) had brain surgery after diagnosis. Two subjects had inpatient rehabilitation, and one had outpatient rehabilitation after diagnosis. Clinical and demographic characteristics are shown in Table 1.
The subjects received one-time training with the research staff that included live demonstration of the exercises and watching an exercise video. [15] Research assistants were trained by a board-certified physiatrist. The home exercise program was developed by this physiatrist to help with potential weakness and balance impairments. The program included a set of resistance band exercises for strengthening muscles used in walking (deltoids, quadriceps, and hip abductors), a balance exercise, and recommendations for walking. The balance exercise was standing on a single leg while holding onto a chair as needed for balance. The walking recommendations were to walk at one’s own pace for 20 min indoors or outdoors, to “not exert yourself,” and to separate walking time into two sets of 10 min if 20 min in a row was perceived as difficult.
The subjects received the resistance band and an exercise instruction pamphlet with a link to the exercise video on “youtube” [9]. The resistance band and pamphlet were provided free of charge, but internet access was not provided. The resistance band was a “medium red TheraBand resistance band,” purchased by the roll from an exercise supply company [16]. They were instructed to do the exercises every day for 1 month. We aimed to address the barriers of knowledge, cost, and space by educating our subjects on the program, providing the equipment free of charge, and designing a program that required very little space. They were contacted after the month in-person or by phone to obtain feedback on their experience with the home exercise program and quality of life. Fifteen subjects reported their frequency of exercising, but only 13 reported adverse events or lack thereof.
Compliance was determined by the percentage of subjects who started and continued the exercises throughout the 1-month study period and frequency of exercising. Safety was determined by the absence of significant adverse events recorded during the study period to include experiencing chest pain, shortness of breath, falls, and dizziness (without falls) during exercising. Minor adverse events, including muscle pain and use of OTC pain medication after exercising, were also noted. Quality of life scores (assessed by FACT-BR) [17] and demographic factors were collected. Statistical analysis was performed on all results.
Results
Fourteen of the 15 (93%) subjects started the exercises during the course of the month. Five subjects did the exercises four or more times per week for the first month. Nine of the 14 (64%) who started, continued the exercises throughout the month on a regular basis. Of the subjects who stopped the exercises, none would have continued if a group setting had been offered. One subject would have continued if offered formal outpatient rehabilitation, and two would have continued if any form of supervised exercise had been offered. Two subjects had previously been prescribed inpatient rehabilitation, and one had been prescribed outpatient rehabilitation. Sixty percent of subjects (nine subjects) were likely or very likely to continue with the home exercises.
During the study period, no falls or cardiovascular adverse events were reported. Eight percent of subjects reported dizziness without fall, and 17% of subjects had muscle soreness lasting less than 24 h after the exercise. Eight subjects never had muscle soreness after exercising. Two subjects required over-the-counter pain medications for muscle soreness after exercise. When asked if they watched the video on their own after the initial teaching, 10/14 answered never, two subjects left it blank, and two said always while performing the exercises.
Among the subjects who continued the exercises regularly, higher frequency of exercising was significantly associated with living as married (p = 0.033), an annual income >$50,000 (p = 0.047), and less change in scores of physical well-being (p = 0.047). In this subgroup, there was also a trend towards increase in brain cancer specific well-being (p = 0.054) and total FACT-BR scores (p = 0.059) as compared to subjects who chose not to exercise as frequently. This data is illustrated in Fig. 1. The subjects who scored higher on the social well-being subscale of the FACT-BR at baseline self-reported a higher likelihood to continue the exercises after 1 month of participation in the study (p = 0.018).
Discussion
To our knowledge, this is the first study to propose a sample home exercise program for brain tumor patients in the treatment or post-treatment period and monitor their compliance and adverse events for at least 1 month. Jones and his group reported that 45% of their survey participants would have liked to receive information on exercise during adjuvant treatment for brain tumor [8]. This is consistent with prostate cancer patients who expressed that access to professional expertise alleviated exercise concerns [18] and breast cancer patients who desired increased knowledge of exercise treatments [19]. We hoped that providing a program that required one simple tool, a resistance band, with exercise instruction developed by a professional would remove the potential knowledge, cost, and space barriers to exercise in our population and provide a framework for continued home exercise.
Subjective comments from exit interviews included that the subjects who continued the exercises found them “easy to do while brushing teeth” (balance) and gave them “something to do.” They could do them “in the winter” and hoped for “long-term benefit.” Most said they “liked the exercises,” but chose not to view the video. This is consistent with the feedback from a prior study on home exercise in the breast cancer population at the same center that video teaching did not improve compliance as compared to in-person teaching [20]. However, it is in contrast to the study by Jones that suggested a preference for the use of technology rather than in-person teaching [8]. We suspect this is related to the differences in our patient populations. Our study included subjects from the local area who generally prefer not to travel for their cancer care, and the Jones study included primarily subjects that had specifically traveled to get expert care.
Study limitations included small sample size and large heterogeneity of tumor biology as both subjects with primary brain tumor and metastatic disease were included. As such, multivariate analysis could not be performed to determine the quality of life benefits. Additionally, presence or absence of adverse effects was only reported by 13 subjects of the 15. Those subjects who exercised in the most frequent category had a trend towards brain cancer specific and overall quality of life; however, they also improved the least throughout the study. Their overall higher quality of life or higher socioeconomic status at the beginning of the study may have made exercise easier to perform more frequently. Of note, leisure time physical activity is far less available to those with lower socioeconomic status [21].
Only three subjects in our study were offered formal rehabilitation prior to the home exercise program. We were unable to determine if this was due to the lack of significant impairments or barriers to therapy services, such as distance from the cancer center or cost. Outpatient physical and occupational therapy are offered within our system, but outpatient speech and language therapy is not. Outpatient therapy is not offered at the same location as the main cancer care site, and there is typically a co-pay and a marginal cost for parking with each therapy visit. Three subjects that stopped exercising throughout the month would have continued if formal or supervised rehabilitation had been offered.
Although the video link was shown during the initial education session and made available afterwards to those with internet access, this did not influence subject participation in exercise by subjective feedback at exit interview and the low numbers of subjects who chose to view it independently. A simple explanation sheet, a resistance band, and face-to-face counseling were most frequently used by our subjects and may be all that is necessary to get started.
We were unable to measure specific improvements in muscle strength or balance. Objective measures such as isokinetic muscle strength by dynamometry, skeletal muscle cross-sectional area, body composition, and cardiopulmonary exercise testing have been proposed as feasible longitudinal assessment tools in this population [22]. Since our program was targeted to weakness and balance, dynamometry measured before and after the program could have shown if strength was improved with 1 month of independent home exercise. The Brunel Balance Assessment may have been helpful to show any change in balance [23]. However, it is reassuring that no patient reported a fall while exercising, which is a clinically important subjective measure of balance. The majority of subjects continued to exercise throughout the month, suggesting that this program is feasible to implement in the post-treatment and home setting.
Conclusion
In this study, the subjects that did a novel independent exercise program designed with careful consideration of the exercise preferences, individual capabilities, and potential barriers to exercise of the brain tumor population demonstrated compliance and safety in the home setting. No major adverse events were reported. Dizziness in the post-treatment period could be related to the primary tumor or adverse medication side effect. Muscle soreness is not uncommon even in healthy subjects after exercise, and this responded to over-the-counter medications in our small study. A larger study with dynamometry and the Brunel Balance Assessment may provide insight into the frequency, intensity, and duration of exercise needed to achieve objective improvements in strength and balance in outpatients with brain tumor.
Higher frequency of exercising was associated with life quality parameters as well as being married and higher income. Nurse navigators or other Oncology staff may be an importance resource to offer accountability to an exercise program for patients without family or financial support. The lack of knowledge of an appropriate home exercise program for their condition that requires little equipment and space may be a barrier to exercise in this population that can be overcome with face-to-face instruction or an exercise pamphlet and one resistance band. Offering formal or supervised rehabilitation could be considered to improve exercise compliance in this population.
References
Thomas CL (1997) Taber’s cyclopedic medical dictionary, 18th edn. FA Davis Company, Philadelphia, p 425
Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC and Snyder C (2012) Exercise interventions on health-related quality of life for cancer survivors. The Cochrane Library
Jones LW, Courneya KS, Peddle C, Mackey JR (2005) Oncologists’ opinions towards recommending exercise to patients with cancer: a Canadian national survey. Support Care Cancer 13(11):929–937
Bourke L, Homer KE, Thaha MA, Steed L, Rosario DJ, Robb KA, Saxton JM, Taylor SJC (2014) Interventions to improve exercise behaviour in sedentary people living with and beyond cancer: a systematic review. Br J Cancer 110(4):831–841
Jones LW, Courneya KS (2002) Exercise counseling and programming preferences of cancer survivors. Cancer Pract 10(4):208–215
Courneya KS, McKenzie DC, Reid RD, Mackey JR, Gelmon K, Friedenreich CM, Ladha AB, Proulx C, Lane K, Vallance JK, Segal RJ (2008) Barriers to supervised exercise training in a randomized controlled trial of breast cancer patients receiving chemotherapy. Ann Behav Med 35(1):116–122
Rogers LQ, Courneya KS, Robbins KT, Malone J, Seiz A, Koch L, Rao K (2008) Physical activity correlates and barriers in head and neck cancer patients. Support Care Cancer 16(1):19–27
Ottenbacher AJ, Day RS, Taylor WC, Sharma SV, Sloane R, Snyder DC, Kraus WE, Demark-Wahnefried W (2011) Exercise among breast and prostate cancer survivors—what are their barriers? J Cancer Surviv 5(4):413–419
Blaney JM, Lowe-Strong A, Rankin-Watt J, Campbell A, Gracey JH (2013) Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: a questionnaire–survey. Psycho-Oncology 22(1):186–194
Ruderman NB, Hall TC (1965) Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer 18(3):298–306
Horber FF, Scheidegger JR, Grunig BE, Frey FJ (1985) Evidence that prednisone-induced myopathy is reversed by physical training*. The Journal of Clinical Endocrinology & Metabolism 61(1):83–88
Keilani M, Krall C, Marosi C, Flechl B, Dieckmann K, Widhalm G, Marhold M, Crevenna R (2012) Strength of skeletal muscle and self-reported physical performance in Austrian glioblastoma-patients. Wien Klin Wochenschr 124(11–12):377–383
Ruden E, Reardon DA, Coan AD, Herndon JE, Hornsby WE, West M, Fels DR, Desjardins A, Vredenburgh JJ, Waner E, Friedman AH (2011) Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol 29(21):2918–2923
Jones LW, Guill B, Keir ST, Carter K, Friedman HS, Bigner DD, Reardon DA (2007) Exercise interest and preferences among patients diagnosed with primary brain cancer. Support Care Cancer 15(1):47–55
https://www.youtube.com/watch?v=_VbPqb8Nr9I. Accessed November 15, 2016
http://www.ballsnbands.com. Accessed November 30,2016
Weitzner MA, Meyers CA, Gelke CK et al (1995) The Functional Assessment of Cancer Therapy (FACT) scale: development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 75(5):1151–1161
Bruun DM, Krustrup P, Hornstrup T, Uth J, Brasso K, Rørth M, Christensen JF, Midtgaard J (2014) “All boys and men can play football”: a qualitative investigation of recreational football in prostate cancer patients. Scand J Med Sci Sports 24(S1):113–121
Rogers LQ, Matevey C, Hopkins-Price P, Shah P, Dunnington G, Courneya KS (2004) Exploring social cognitive theory constructs for promoting exercise among breast cancer patients. Cancer Nurs 27(6):462–473
Baima J, Reynolds SG, Edmiston K, Larkin A, Ward BM and O’Connor A (2015) Teaching of independent exercises for prehabilitation in breast cancer. Journal of Cancer Education :1–5
Ford ES, Merritt RK, Heath GW, Powell KE, Washburn RA, Kriska A, Haile G (1991) Physical activity behaviors in lower and higher socioeconomic status populations. Am J Epidemiol 133(12):1246–1256
Jones LW, Friedman AH, West MJ, Mabe SK, Fraser J, Kraus WE, Friedman HS, Tresch MI, Major N, Reardon DA (2010) Quantitative assessment of cardiorespiratory fitness, skeletal muscle function, and body composition in adults with primary malignant glioma. Cancer 116(3):695–704
Tyson SF and Connell LA (2009) How to measure balance in clinical practice. A systematic review of the psychometrics and clinical utility of measures of balance activity for neurological conditions. Clinical Rehabilitation
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Baima, J., Omer, Z.B., Varlotto, J. et al. Compliance and safety of a novel home exercise program for patients with high-grade brain tumors, a prospective observational study. Support Care Cancer 25, 2809–2814 (2017). https://doi.org/10.1007/s00520-017-3695-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3695-7