Summary
Background
Aim of this study was to describe longitudinal assessments of handgrip strength, strength of thigh muscles, and self-reported physical performance in patients with glioblastoma after neurosurgical intervention undergoing chemoradiation.
Methods
Strength testing was performed in 24 Austrian glioblastoma patients (m:f = 19:5, 52 ± 14a, BMI = 26 ± 3 kg/m²) at baseline and follow up after chemoradiation (interval between baseline and follow up = 14 ± 9 weeks). Isokinetic testing of knee extension/flexion was performed by using a Biodex 3 dynamometer. Handgrip strength was measured by using a Jamar hand-dynamometer. Physical performance was assessed by using the subscales “physical functioning” and “role physical” of the SF-36 Health Survey.
Results
Peak torque of knee extensors (peak torque) were clearly lower than expected for age- and sex-related values (p < 0.0001). In comparison with age- and sex-related reference values, deficits of “role physical” (p < 0.0001) and “physical functioning” (p = 0.010) were found.
Effects of measurements of muscle strength on “physical functioning” were significant (peak torque:p < 0.001; handgrip strength:p < 0.001).
No significant change could be detected after follow up for peak torque (p = 0.337), handgrip strength (p = 0.995), “physical functioning” (p = 0.824), and “role physical” (0.594).
Conclusions
In this study, notable deficits especially in muscular strength of thigh muscles and general physical performance of patients with glioblastoma have been found before and after chemoradiation. Reduced muscle strength and impaired self-reported physical performance seem to be clinically relevant functional deficits in (Austrian) glioblastoma patients. Therefore, rehabilitation and supportive care should also include options to increase muscle strength.
Zusammenfassung
Grundlagen
Die vorliegende Untersuchung hatte die Beschreibung der motorischen Grundeigenschaft „Kraft“ (vor und nach Chemoradiatio) und deren Auswirkung auf die Leistungs- und Funktionsfähigkeit von Glioblastom (GBM)-Patienten zum Ziel.
Methodik
24 Patienten (m:f = 19:5, 52 ± 14a, BMI = 26 ± 3 kg/m²) wurden in die vorliegende Pilot-Untersuchung eingeschlossen. Es wurden bei jedem Patienten eine „Handdynamometrie“ mit Ermittlung der Faustschlusskraft (Jamar®) sowie eine isokinetische Dynamometrie der Extensoren und Flexoren der Kniegelenke (Biodex®-3) vor und nach Chemoradiatio (Abstand zwischen den Messungen = 14 ± 9 Wochen) durchgeführt. Die körperliche Leistungs- und Funktionsfähigkeit der Studienteilnehmer wurde mittels der Subskalen „Körperliche Funktionsfähigkeit“ und „Körperliche Rollenfunktion“ des SF-36 Health Survey erfragt.
Ergebnisse
Das maximale Drehmoment der Knieextensoren (maximales Drehmoment) lag deutlich unter den alters- und geschlechtsspezifischen Erwartungswerten (p < 0,0001). Verglichen mit alters- und geschlechtsspezifischen Referenzwerten konnten signifikante Defizite der selbsterfassten Leistungs- und Funktionsfähigkeit der Patienten („Körperliche Rollenfunktion“:p < 0,0001; „Körperliche Funktionsfähigkeit“:p = 0,010) gefunden werden. Sowohl Faustschlusskraft als auch maximales Drehmoment zeigten signifikante (negative) Korrelationen mit der Subskala „Körperliche Funktionsfähigkeit“ (maximales Drehmoment:p < 0,001; Faustschlusskraft:p < 0,001). Maximales Drehmoment (p = 0,337), Faustschlusskraft (p = 0,995), „Körperliche Funktionsfähigkeit“ (p = 0,824), und „Körperliche Rollenfunktion“ (0,594) änderten sich im Zeitverlauf (vor und nach Chemoradiatio) nicht signifikant.
Schlussfolgerungen
Im Rahmen der vorliegenden Studie an GBM-Patienten (vor und nach Chemoradiatio) konnten deutliche Defizite der Kraft der Knieextensoren und der selbsterfassten körperlichen Leistungs- und Funktionsfähigkeit gefunden werden. Da diese Defizite funktionell relevant für den Alltag der Patienten zu sein scheinen, sollte die Rehabilitation dieser Patienten Optionen zur Verbesserung der motorischen Grundeigenschaft Kraft inkludieren.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma, the most common primary brain tumor in adults, is an aggressive form of brain tumor the treatment of which usually entails neurosurgical intervention where possible followed by chemoradiation, allowing more patients with malignant glioma than ever to survive longer [1,2].
Glioblastoma leads to a decrease in muscular strength due to neuromuscular dysfunction caused by glioblastoma itself, and due to corticoid treatment which is needed to decrease intracranial pressure [1–8].
Reduced muscle strength, sensorimotor deficits, and corticoid-related loss of bone mineral density lead to higher risk of falls and consecutively fractures [9–11]. Decreases of muscular strength and endurance capacity have negative effects on functional outcomes [2]. The consequences are deconditioning, muscle wasting, decrease of quality of life (QOL), mood disorders (depression), limitations of activities of daily living (ADL), and decrease of (social) participation [1–3]. Muscle wasting and decreased muscle strength were shown to be independent predictors of mortality in other severe diseases such as chronic heart failure [12,13].
To our knowledge, muscular strength of different muscle groups and its impact on self-reported physical performance of glioblastoma patients have not been described in prior publications.
Aim of this study was to describe longitudinal assessments of handgrip strength, strength of thigh muscles, and of their impact on self-reported physical performance in a sample of Austrian patients with glioblastoma undergoing chemoradiation.
Methods and materials
The present study was approved by the ethics committee of the Medical University of Vienna (EK Nr: 122/2008). The study was performed in accordance with the Declaration of Helsinki (1964). All participants gave their informed consent prior to their inclusion in the study. A sample of 31 consecutive Austrian patients with histologically confirmed, clinically stable, postsurgical, and previously untreated glioblastoma were included in the present study. Due to logistic reasons, the interval between baseline and follow up was 14 ± 9 weeks.
Additional inclusion criteria included: treatment with radiotherapy plus concomitant and adjuvant temozolomide-therapy, and no contraindications to strength testing [1,2]. In our glioblastoma center, the corticoid treatment is tapered off as soon as possible after neurosurgical intervention. Postoperatively, patients are free from corticosteroids within 2 weeks unless they develop signs of central edema. During and after radiation therapy, patients are prescribed dexamethasone only after complaining of symptoms related to cerebral edema. By minimizing the use of dexamethasone, we intend to spare the patients’ side effects from corticosteroids.
Demographic and clinical data were documented. Karnofsky performance status (KPS) measured ³ 70 % for 23 patients and 50 % for 1 patient. Two (8 %) patients suffered from hemiparesis and 2 (8 %) from upper extremity weakness because of glioblastoma. Furthermore, 8 (33 %) patients had sufficiently treated epilepsy.
Assessment of muscular strength
Isokinetic testing of dominant thighs (isokinetic knee extension and flexion strength) was performed by using a Biodex 3 dynamometer (Biodex Medical Systems, Shirley, New York, USA) [14]. Isokinetic testing is considered the “gold standard” for the objective assessment of muscle strength in vivo, and is therefore usually used for assessment of muscular strength of thighs in previous clinical studies [14]. Furthermore, quadriceps strength is known to relate to different basic ADL (ability of walking, stair climbing), and to falling [14]. Isokinetic testing was performed at an angular velocity of 60°/s. Isokinetic testing at 60°/s is suggested by the manufacturer for evaluation of maximal voluntary strength, and was proved to be useful and valid for assessing maximal muscular strength of cancer patients and other patients with severe diseases such as heart failure [13,15–17].
The dynamometer was calibrated for torque and range of motion and measurements were conducted according to the manufacturer’s specification [18]. Based on the Biodex System 3 Pro manual and recommendations from a previous study on cancer patients by our group, the test protocols were established [15,18].
Patients were stabilized in the chair with abdominal and shoulder straps [18]. The anatomical axis of rotation was aligned to the dynamometer axis using manual palpation and visual inspection [18]. For the purpose of acclimatization, testing was explained to the subjects and isokinetic contractions were demonstrated until the patients felt confident about the manoeuvres [18]. During the acclimatization, only contractions with less effort were allowed. The warming up consisted of three sets of three submaximal isokinetic repetitions [18]. After a relaxation period of a few minutes, test series started [18]. Two isokinetic sets of 5 reciprocal knee extension and flexion maximal contractions with an angular speed of 60°/s were performed [15,18]. Highest achieved value was used. Muscle strength of thighs was measured as a moment of maximal force (torque) during knee movement at a constant (isokinetic) velocity of 60°/s (= peak torque/normalized to participant’s body weight in Nm/kg) [18,19]. Peak torque/normalized to participant’s body weight (peak torque) was measured for dominant knee extensors/flexors at baseline and at follow up.
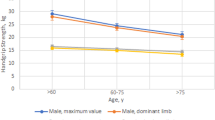
In order to reduce interrater variability, all tests were performed by the same physiatrists, who gave a moderate standardized verbal encouragement during the test [15,18]. Age- and sex-predicated values for isokinetic muscle strength of knee extensors were obtained from the Biodex System 3 Pro manual, and from prior reports [18,20].
Handgrip strength of dominant hand was measured by using a Jamar hand-dynamometer [21,22]. Handgrip strength measurement by dynamometry is well standardized, and handgrip correlates with a number of important life functions (e.g. self-care, carrying bags, homework, etc.) [21]. Participants were examined in a standardized position [23]. During the test, the patients were seated, their elbow by their side and flexed to right angles, and a neutral wrist position, the dynamometer handle position was chosen according to individual hand size. Each participant was tested at the same handle position at baseline and follow up. In alternating order, three maximum voluntary grip strength contractions were taken for each hand [22,23]. The mean value of dominant hand was used for analysis [24]. Maximal handgrip strength was measured in pounds (lbs) [22–24]. Revised normative age- and sex-related values for grip strength with the Jamar dynamometer were obtained from a prior report [22].
Assessment of self-reported physical performance
Physical performance was assessed by using the SF-36 Health Survey [25]. The SF-36 Health Survey is a generally accepted generic instrument for assessing eight different domains of health related quality of life (HRQOL) [25]. In this study, patients filled in the subscales “physical functioning” and “role physical” of the German version of the SF-36 Health Survey [25]. Among the strengths of the SF-36 Health Survey is its applicability to many disease groups as well as the general population, so that it can be used to compare cancer patients with healthy populations [15,25]. Since most studies of HRQOL of cancer survivors lack either a noncancer control group or broad normative data on noncancer patients for comparisons, this seems to be a main advantage in attempting to sort out the effects of cancer on differences in HRQOL versus the influence of concomitant diseases and natural aging [15,25]. The SF-36 Health Survey has been used in several studies to investigate HRQOL of cancer patients and has shown not only different deficits in HRQOL, depending on the cancer entity, tumor stage, treatment-related adverse effects, and progression of cancer, but also differences which are related to concomitant diseases, sex, age, sociodemographic status, and other conditions [15,25]. It has been shown that administration of generic HRQOL questionnaires may help physicians to look beyond “what’s wrong” with their patients [15,25]. The SF-36 Health Survey seems to be a very useful instrument to describe overall HRQOL of patients, and to detect more specific symptoms and QOL deficits related to different cancer entities, disease-specific questionnaires should be preferred [15,25]. In the present study, only the subscales “physical functioning” and “role physical” were used for self-reported assessment of physical performance.
Statistical analysis
The initial analysis provided descriptive information on the demographic and treatment characteristics of participants. To assess strength of skeletal muscle in patients with glioblastoma, their maximum peak torque of thigh muscles (knee extensor and flexor muscles) and handgrip strength after neurosurgical intervention were compared to age- and sex-predicated expected values by a t-test (peak torque) or a Wilcoxon signed rank test (handgrip strength), depending on normality of the data as assessed by qq-plots.
Peak torque of knee extensor and flexor muscles was highly correlated for both sides at both measurements (p < 0.0001). Therefore, the analysis was restricted to peak torque of the knee extensor muscles.
For self-assessment of physical performance values for the SF-36 subscales, the “physical functioning” and “role physical” were used. SF-36 values at baseline were compared with mean values from a normal population depending on age, class, and sex using Wilcoxon signed rank tests.
Changes of peak torque of knee extensor muscles, handgrip strength, “physical functioning” and “role physical” over time were analyzed by linear models.
To determine correlations between skeletal muscle strength and self-reported physical performance of patients on these two SF-36 subscales, mixed models were analyzed, where measurement of muscle strength was treated as fixed effect and patients were treated as random effects for the SF-36 subscales “physical functioning” and “role physical”.
Bonferroni Holm correction for simultaneous testing of hypotheses with respect to peak torque of knee extensor muscles, handgrip strength, the subscales “physical functioning”, and “role physical” and dependence of “physical functioning” and “role physical” on peak torque of knee extensor muscles and handgrip strength was performed by the stepwise Bonferroni Holm procedure with levels α/16, α/15 … α.
In the tables II–VII, uncorrectedp-values are given; in the text significance after correction for multiplicity is reported.
All statistical analyses were done using R 2.10.2.
Results
Demographic and clinical data are presented (Table 1).
Out of 31 participants, 1 patient dropped out due to death before follow up, 5 patients worsened so much that they were no longer able to come to the hospital to perform the follow up testing. Assessment was performed in 24 patients (m:f = 19:5, 52 ± 14a, BMI = 26 ± 3 kg/m²) at baseline and follow up after concomitant treatment with temozolomide and radiation.
Muscle strength of thigh muscle and handgrip strength
Table 2 gives summary statistics of peak torque of dominant thigh muscles (knee extensor and flexor muscles) and of grip strength of the dominant hand.
Table 3 gives summary statistics of the difference of peak torque of knee extensor muscles and handgrip strength of glioblastoma patients from expected and normative values, respectively.
The unadaptedp-value for peak torque of knee extensor muscles indicated significant reduction compared to normative values (p < 0.001), reduction of handgrip strength was not significant (p = 0.275). Bonferroni Holm correction did not change significances.
A linear regression of the difference of peak torque of knee extensor muscles and handgrip strength on the time between baseline measurement and follow up was performed. Neither intercepts (peak torque:p = 0.924; handgrip strength:p = 0.763) nor slopes (peak torque:p = 0.337; handgrip strength:p = 0.995) were significant. Table 4 gives summary of this regression.
Self-reported physical performance
Table 5 shows the results of a mixed model modelling regression of “physical functioning” and “role physical”, with peak torque of knee extensor muscles and on handgrip strength, respectively, as fixed effects and patients as random effects.
Effects of both measurements of skeletal muscle strength on “physical functioning” were significant (peak torque:p < 0.001; handgrip strength:p < 0.001) and remained significant after Bonferroni Holm correction of thep-values.
Effects of both measurements of skeletal muscle strength on “role physical” were significant before (peak torque:p = 0.014; handgrip strength:p = 0.047) but not after Bonferroni Holm correction of thep-values.
Table 6 shows the differences between SF-36 subscales “physical functioning”/“role physical” and reference values.
Reduction of “physical functioning” of glioblastoma patients compared to normative values was significant before (p = 0.010) Bonferroni Holm correction, but not after.
Reduction of “role physical” of glioblastoma patients compared to normative values was significant before (p < 0.001) and remained significant after the Bonferroni Holm correction.
Table 7 gives the differences between SF-36 subscales at baseline and after follow up.
A linear regression of the difference of “physical functioning” and “role physical” on the time between baseline measurement and follow up was performed. Table 7 shows the estimates and thep-values. Only the intercept for “role physical” was significant before (p = 0.047), but not after Bonferroni Holm correction.
One patient had a Karnofsky index less than 70 when entering the study due to a hemiparesis and showed a drastic improvement of all functions from baseline to follow up (peak torque of right extensor muscles from 22.5 to 169.4 Nm/kg and right handgrip strength from 1.3 to 16.3 lbs).
However, excluding this patient from the analysis did not change the significance for any test.
Discussion
Therapies consisting of surgery and chemoradiation allow more patients with malignant glioma than ever to survive longer [1,2,26–29]. Nevertheless, many of these patients are suffering from muscle weakness, fatigue, and rapid exhaustion in the early course of disease and probably related to the prescription of dexamethasone against increased cranial pressure [4–8,30]. These problems result in limitations of activity and participation and decrease in QOL [31,32].
In summary, the results of this study showed notable deficits in muscular strength of thigh muscles but not for handgrip strength of Austrian patients with glioblastoma. Effects of measurements of muscle strength on “physical functioning” were significant. At follow up, strength of thigh muscles did not improve, which seems to be due to the impact of the clinical course of glioblastoma on muscular strength of thigh muscles. Furthermore, deficits of the baseline values of SF-36 subscales “physical functioning” and of “role physical” could be shown in patients with glioblastoma. No significant changes of these two subscales could be detected over time.
Jones et al. investigated different parameters of physical performance of patients with glioma in two studies [1,2]. In the cross-sectional study a notable reduction of endurance capacity, cross-sectional area (CSA) of the quadriceps, hamstrings, and total mid-thigh muscle could be shown [1]. Isokinetic muscle strength of the right quadriceps muscle at an angular velocity of 90°/s was notably (50 %) below age- and sex-matched expected values [1,33]. The cross-sectional study showed that muscle strength was predictive for VO2peak in exercise testing [1]. Therefore, exercise testing and dynamometry seem to be important assessment options which allow objective and feasible assessment of physical functioning in patients with malignant glioma [1]. Longitudinal measurements indicated improvements of isokinetic strength of thigh muscles at follow up after 6 weeks that were no longer significant at 24 weeks. In both studies of Jones et al., the isokinetic testing was performed with an angular velocity of 90°/s with three reciprocal repetitions [1,2].
In comparison with the cross-sectional study of Jones et al., our results showed notable deficits of muscle strength of thigh muscles, too [1].
In comparison to prior reports, the present study in an Austrian population sample was the first to assess both handgrip and isokinetic strength (maximal voluntary strength at 60°/s) of thighs, and to correlate these measurements with self-reported physical performance by using a generic QOL-questionnaire, respectively [1,2]. This seems to be one of the strengths of the present study because for performing different ADL (e.g. walking, homework, etc.) different muscle groups as knee extensor/knee flexor muscles and forearm and hand muscles are necessary.
We assessed isokinetic strength and related it to weight of the participants (Nm/kg) because strength in most muscle groups was proven to be related not only to age and height but also to body mass [18,19]. Furthermore, some of our patients changed their weight during the follow up period, and during daily activities like walking and stair climbing—from the patients point of view—the whole body mass has to be moved forward.
In the present study, deficits in strength of thigh muscles as well as impairment in physical performance of glioblastoma patients have been observed (Tables 3 and6). Weakness of thigh muscles and of handgrip strength was shown to be associated with decrease in ability to perform daily activities (e.g. climbing stairs, walking without help, carrying shopping bags) as measured on the SF-36 subscale “physical functioning” (Table 5). Therefore, usefulness of strength testing can be seen due to correlations of dynamometric results self-reported HRQOL.
These deficits of muscular strength with consecutive notable limitations of physical health related QOL lead to need for rehabilitation concepts for patients with glioblastoma in order to improve muscular strength especially of thigh muscles. Therefore, adequate active exercise options should be initiated as soon as possible. The individual physical load during exercise should be considered individually, according to general condition of the patients and to the results of dynamometry.
Nevertheless, there is now a need for randomized clinical trials recruiting larger samples which evaluate rehabilitation programs in order to produce more generalizable data.
On the other side, we did not perform tests to investigate possible impairments of endurance capacity and sensimotor deficits as well as fatigue and mental health which also can lead to notable limitations in physical performance of patients with glioblastoma [1]. In clinical practice, deficits of glioblastoma patients should be evaluated individually, in order to perform targeted rehabilitation interventions.
In the present study, no deficits of handgrip strength in comparison with age- and sex-related normative values could be shown (Table 3) [22]. One reason might be that corticosteroids usually lead first to decrease of proximal muscle groups like thigh muscles [6].
Longitudinal strength assessment of thigh muscles and handgrip showed no significant difference between values at baseline and at follow-up after 14 ± 9 weeks (Table 4). These results are underlined by the fact that self-reported physical performance showed no significant changes after follow-up (Table 7).
One notable limitation of this study is the selection bias due to inclusion of patients with Karnofsky-indices ³ 70 %, who were able to perform assessments. Therefore, conclusions for patients with poorer performance status (KPS) and poor general condition cannot be drawn from this study. Furthermore, the relatively small sample size and the lack of further functional performance measures (such as skeletal muscle cross-sectional area, cardiopulmonary exercise testing) could be seen as limitation of the present study. Nevertheless, to our knowledge, the present study represents the first follow up observation in a Central European (Austrian) glioblastoma population which describes muscular strength and its impact on general physical performance.
Conclusions
The results of this study showed notable deficits in muscular strength of thigh muscles and general physical performance of patients with glioblastoma. At follow-up, none of the variables under observation showed significant changes which show the impact of clinical course of glioblastoma on muscle strength of thigh muscles and general physical performance.
Further studies with larger sample sizes are urgently needed to assess muscle strength, endurance capacity, sensimotor features, and physical performance of patients with glioblastoma in order to define objective and clinical goals in rehabilitation of patients with glioblastoma.
Nevertheless, a major clinical implication of the present study is that rehabilitation and supportive care of patients with glioblastoma should also include options to increase muscle strength in order to improve ability to ADL.
References
Jones LW, Friedman AH, West MJ, et al. Quantitative assessment of cardiorespiratory fitness, skeletal muscle function, and body composition in adults with primary malignant glioma. Cancer. 2010;116:695–704.
Jones LW, Mourtzakis M, Peters KB, et al. Changes in functional performance measures in adults undergoing chemoradiation for primary malignant glioma: a feasibility study. Oncologist. 2010;15:636–47.
Jones LW, Cohen RR, Mabe SK, et al. Assessment of physical functioning in recurrent glioma: preliminary comparison of performance status to functional capacity testing. J Neurooncol. 2009;94:79–85.
Sturdza A, Millar BA, Bana N, et al. The use and toxicity of steroids in the management of patients with brain metastases. Support Care Cancer. 2008;16:1041–8.
Stieber VW. Low-grade gliomas. Curr Treat Options Oncol. 2001;2:495–506.
Batchelor TT, Taylor LP, Thaler HT, Posner JB, DeAngelis LM. Steroid myopathy in cancer patients. Neurology. 1997;48:1234–8.
Koehler PJ. Use of corticosteroids in neuro-oncology. Anticancer Drugs. 1995;6:19–33.
Dropcho EJ, Soong SJ. Steroid-induced weakness in patients with primary brain tumors. Neurology. 1991;41:1235–9.
Mitra R. Adverse effects of corticosteroids on bone metabolism: a review. PM&R. 2011;3:466–71.
Maruotti N, Corrado A, Cantatore FP. Glucocorticoid induced risk of fractures. Panminerva Med. 2010;52:339–43.
Cohen SB. Comorbidities: glucocorticoids and osteoporosis: predicting fracture risk. Nat Rev Rheumatol. 2010;6:681–2.
Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–3.
Hülsmann M, Quittan M, Berger R, et al. Muscle strength as a predictor of long-term survival in severe congestive heart failure. Eur J Heart Fail. 2004;6:101–7.
Drouin JM, Valovich-mcLeod TC, Shultz SJ, Gansneder BM, Perrin DH. Reliability and validity of the Biodex system 3 pro isokinetic dynamometer velocity, torque, and position measurements. Eur J Appl Physiol. 2004;91:22–9.
Crevenna R, Mähr B, Fialka-Moser V, Keilani M. Strength of skeletal muscle and quality of life in patients suffering from “typical male” carcinomas. Support Care Cancer. 2009;17:1225–8.
Feiereisen P, Vaillant M, Eischen D, Delagardelle C. Isokinetic versus one-repetition maximum strength assessment in chronic heart failure. Med Sci Sports Exerc. 2010;42:2156–63.
Quittan M, Wiesinger GF, Crevenna R, et al. Isokinetic strength testing in patients with chronic heart failure—a reliability study. Int J Sports Med. 2001;22:40–4.
Biodex Multi joint System 3 PRO. Application/operations manual. Biodex Medical Systems Shirley. New York; 2000.
Harbo T, Brincks J, Andersen H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur J Appl Physiol. 2001 May 3. Epub ahead of print.
Perrin DH. Isokinetic exercise and assessment. Champaign: Human Kinetics; 1993.
Harkonen Harju R, Alaranta H. Accuracy of the Jamar dynamometer. J Hand Ther. 1993;6:259–62.
Peters MJ, van Nes SI, Vanhoutte EK, on behalf of the PeriNomS Study group, et al. Revised normative values for grip strength with Jamar Dynamometer. J Peripher Nerv Syst. 2011;16:47–50.
American Society of Hand Therapists. Clinical Assessment Recommendations. 2nd edn. Chicago: The Society; 1992. p. 41–5.
Mathiowetz V. Grip and pinch strength measurements. In: Amundsen LR, editor. Muscle strength testing instrumented and noninstrumented systems. New York: Churchill Livingstone; 1990. p. 163–77.
Bullinger M. German translation and psychometric testing of the SF-36 Health Survey: preliminary results from the IQOLA Project. International Quality of Life Assessment. Soc Sci Med. 1995;41:1359–66.
Marosi C. Chemotherapy for malignant gliomas. Wien Med Wochenschr. 2006;156:346–50.
Gorlia T, Van Den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9:29–38.
Stupp R, Hegi ME, Mason WP, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups, National Cancer Institute of Canada Clinical Trials Group, et al. Effects of radiotherapy with concomitant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66.
Weiler M, Hartmann C, Wiewrodt D, et al. Chemoradiotherapy of newly diagnosed glioblastoma with intensified temozolomide. Int J Radiat Oncol Biol Phys. 2010;77:670–6.
Pereira RM, Freire de C. Glucocorticoid-induced myopathy. Joint Bone Spine. 2011;78:41–4.
Kvale EA, Murthy R, Taylor R, Lee JY, Nabors LB. Distress and quality of life in primary high-grade brain tumor patients. Support Care Cancer. 2009;17:793–9.
Lamperti E, Pantaleo G, Finocchiaro CY, et al. Recurrent brain tumour: the impact of illness on patient’s life. Support Care Cancer. 2011 Jul 3. Epub ahead of print.
Wijndaele K, Duvigneaud N, Matton L, et al. Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc. 2007;39:233–40.
Conflict of interest
The authors declare that there is no actual or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keilani, M., Krall, C., Marosi, C. et al. Strength of skeletal muscle and self-reported physical performance in Austrian glioblastoma-patients. Wien Klin Wochenschr 124, 377–383 (2012). https://doi.org/10.1007/s00508-012-0186-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-012-0186-1