Abstract
Purpose
Taste and smell changes (TSCs) are common in head and neck (H&N) cancer and during and after chemotherapy (CT) and radiotherapy (RT). It is an area that has been under-investigated, particularly in the treatment-naive, but can negatively impact nutritional status. This study examined the prevalence, severity and characteristics of TSCs in people with non-H&N solid tumours, before CT and RT, and their relationship with co-occurring symptoms.
Methods
A prospective, observational study was conducted. Forty consecutive pre-treatment cancer patients, referred to radiation oncology outpatients over 6 weeks, were recruited. Data on TSCs, symptoms and nutritional status were obtained using the ‘Taste and Smell Survey’ and the ‘abridged Patient-Generated Subjective Global Assessment’ (abPG-SGA). BMI was measured. SPSS® was used for statistical analysis. Two-sided P values <0.05 were considered statistically significant.
Results
Most patients were newly diagnosed (n = 28; 70 %). Nineteen (48 %) reported TSCs; nine noted a stronger sweet and seven a stronger salt taste. Of these, four reported a stronger and four a weaker smell sensation. Those at nutritional risk reported more TSCs (n = 13/20). TSCs were significantly associated with dry mouth (P < 0.01), early satiety (P < 0.05) and fatigue (P < 0.05).
Conclusions
TSCs preceded CT or RT in almost half of treatment-naive patients with solid tumours, notably stronger sweet and salt tastes. Half of the study group were at nutritional risk; the majority of these reported TSCs. TSCs were significantly associated with other symptoms. Future research and clinical guidelines, with a common terminology for assessment, diagnosis and management of cancer TSCs, are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chemical senses of taste and smell are fundamental to life. They warn of danger (e.g. gas, fire), deter ingestion of toxins and encourage dietary intake [1]. Disturbance of these senses is termed chemosensory dysfunction [2]. One estimate of the prevalence of taste and smell changes (TSCs) in the USA general population is 0.49 % [3]. This increases 11-fold with age from 0.19 % for those aged 18–24 years to 2.06 % for those ≥85. Chronic illnesses like allergic rhinitis, chronic inflammatory middle ear disease and head injury can adversely affect taste and smell [4, 5].
Taste and smell changes have frequently been reported in cancer. Most of the literature has focused on TSCs related to chemotherapy (CT) or head and neck (H&N) radiotherapy (RT). Prevalence estimates range from 16 to 70 % in the former [6, 7] and 50–100 % in the latter [8, 9]. To our knowledge, there are no dedicated studies characterising subjective TSCs prior to oncological treatment in people with non-H&N solid tumours.
Possible pathophysiological mechanisms are poorly understood [2] and understudied [10, 11]. Both subjective and objective methods can assess TSCs; there is a considerable variation in the methodology. Self-reporting more accurately represents the psychosocial consequences not identified by objective tests [12].
Changes in taste can affect sensitivity (threshold) and/or perception (distortion) of the five basic sensations, i.e. sweet, sour, salty, bitter and umami [13]. Of the five, bitter is most distorted by cancer treatment, in terms of both prevalence and severity [14]. With regard to smell, both higher [15] and lower [1] subjective and objective smell thresholds are found. Distorted smell perception is often described as rancid [16]. TSCs can significantly impair food intake and nutritional status and cause weight loss [2]. Energy intake is reduced [2] and food diversity decreases [17]. Adequate dietary intake is essential at all stages of cancer, since 20 % of patients die from malnutrition, rather than the malignancy [18].
Clinical experience and research suggest that many cancer symptoms, e.g. anorexia, dry mouth, taste changes and weight loss occur in groups or clusters [19]. Categorisation of symptom clusters may be therapeutically important because treatment of one symptom may be influenced by another in the same cluster [20], e.g. taste changes and anorexia. Possible relationships between TSCs, symptom clusters and nutritional status are not widely studied, despite the direct relationship between TSCs and cancer malnutrition [2].
The aim of the present study was to examine the prevalence, severity and characteristics of TSCs in people with non-H&N solid tumours prior to CT or RT. Secondary objectives were to investigate if tumour primary site is associated with TSCs and examine the relationship between patient demographics, clinical characteristics and TSCs and determine any association between TSCs and malnutrition risk and other cancer symptoms.
Methods
Participants and procedure
A prospective, observational study was conducted. A sample of forty consecutive, treatment-naive, non-H&N cancer patients with solid tumours were recruited over a 6-week period at radiation oncology outpatient clinics in a large tertiary care teaching hospital. Patients were screened by consultant radiation oncologists during their outpatient consultation. The inclusion and exclusion criteria are described in Table 1. Eligible patients were asked if they were interested in further information about the study. A brief information leaflet was provided; those who expressed interest were approached by the researcher (L.S.) immediately after their outpatient consultation or at their subsequent RT planning session. Written informed consent was sought after a full verbal explanation of the study and any queries were addressed.
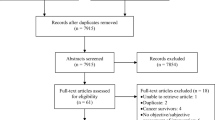
A 15-min interview was conducted with each participant during which two structured, interviewer-assisted questionnaires, the ‘Taste and Smell Survey’ [21] and the ‘abridged Patient-Generated Subjective Global Assessment’ [22], were completed. Demographic and clinical data were obtained from the hospital ARIA® Oncology Information System (Varian Medical Systems, CA, USA). If intravenous contrast was given on the interview day, this was noted as a potential confounder. Current smoking status was also documented. An overview of the recruitment and selection process is in Fig. 1. Ethical approval was obtained from the Research Ethics Committee at St. Luke’s Radiation Oncology Network and the joint St. James’s Hospital/Adelaide and Meath Hospital, Dublin Research Ethics Committee.
Interviewer-assisted questionnaires
Taste and smell changes
Data on TSCs were obtained using the ‘Taste and Smell Survey’ [21], an instrument initially developed for the human immunodeficiency virus (HIV) population to identify self-reported TSCs and their impact on food choices. Although not yet validated, it has been used to investigate cancer TSCs [2, 10, 11]. It can generate a chemosensory complaint score based on frequency and severity of reported TSCs [10, 11]. TSC severity was stratified based on the following frequencies: 0–1 = insignificant; 2–4 = mild; 5–9 = moderate; 10–14 = severe. The original survey contained 16 items, which included 4 questions about the chemosensory influence of HIV medications. Other studies in cancer excluded these questions [10, 11]; they were also excluded from this study and a 12-item questionnaire was used. The questionnaire included un-scored, open-ended questions for qualitative descriptions of TSCs and their impact on quality of life. Responses to these questions were analysed inductively by content analysis.
Nutritional status and Co-occurring symptoms
Nutritional status and symptom data were assessed with an abridged version of the scored Patient-Generated Subjective Global Assessment (abPG-SGA), a validated cancer nutrition screening tool, which foregoes the PG-SGA physical examination [24]. The abPG-SGA includes questions about (1) reported weight and height and weight history over the past 1–6 months, (2) food eaten during the previous month, (3) 13 symptoms which may have affected food intake over the previous 2 weeks and (4) current activity level and function. The above variables were scored (0–35) based on the abPG-SGA to assess for nutritional risk; a cutoff score ≥6 identified malnutrition risk [22].
Both weight and height were measured when sufficient clinic space was available (n = 35; 88 %). Weight was measured to the nearest 0.2 kg by a calibrated Seca Compact Digital Floor Scale III, model 899 (Seca Limited, Birmingham, UK). Height was measured to the nearest 0.5 cm with a collapsible ‘Leicester Height Measure’ stadiometer (CMS Weighing Equipment Limited, London, UK). Body Mass Index (BMI) was subsequently calculated (n = 35) and categorised according to the World Health Organisation 2006 criteria [23]. Percentage weight loss was calculated based on current measured weight and patient-reported values for weight at 1 month and 6 months previously. Performance status was assessed by the Eastern Co-operative Oncology Group (ECOG) (score 0–4) performance rating system [24].
Data analysis
Data analysis was performed using SPSS Version 22.0 (IBM Corporation, New York, USA). Independent sample t tests compared means between groups for normally distributed variables. The Mann-Whitney U test compared non-parametric variables between groups. Relationships between categorical variables were analysed by the chi-square test for independence. The chi-square test for goodness of fit compared the proportion of TSCs in the present sample with that in the US general population [3]. A two-sided P value of <0.05 was considered statistically significant.
Results
Patient characteristics
Ninety-six cancer patients were screened for study eligibility. Of those eligible to participate (n = 52), 40 agreed to participate and all subsequently completed the study (Fig. 1). The baseline characteristics of the study population are in Table 2. Median time since diagnosis was 93 days (IQR 48-209). Most were diagnosed within the last 4 months.
Prevalence and characteristics of taste and smell changes
Nearly half (n = 19, 48 %) (95 % CI, 32.5–62.5) reported some chemosensory abnormality, i.e. a complaint score >1; the median was 1 (IQR 0–5). This is statistically significantly higher than the prevalence of 0.49 % in the US general population (P < 0.001) [3]. Eleven participants reported taste changes alone and eight others changes in both senses. No one had loss of smell alone. Eleven graded their TSCs as ‘moderate’ or ‘severe’ and eight ‘mild’. The prevalence of reported TSCs (stratified) in relation to demographic and clinical data are described in Table 2.
Eleven females (n = 11/17) and eight males (n = 8/23) reported TSCs. They were noted mainly in those with a primary diagnosis of breast (n = 9/15) or prostate cancer (n = 5/15), which comprised most of the patient population. These differences by gender and primary diagnosis were not statistically significant (P > 0.05 for both). No significant differences were found between reported TSCs and age, extent of disease, previous cancer therapy, prior IV contrast, smoking status or BMI category (P > 0.05 for all).
There was variation in both the quality and intensity of TSCs reported. With regard to taste, 22 (55 %) had ‘bad taste’, nine ‘rarely’, nine ‘sometimes’ and four ‘often’. This was most frequently described as sour (n = 7). However, specific questions about each of the basic taste intensities revealed that sweet and salty were perceived as stronger by nine and seven, respectively. Of those, four also reported a stronger and four a weaker smell sensation. When asked to compare TSCs pre- and post-diagnosis, most reported no change in intensity of either (n = 24, 60 %) (95 % CI, 47.5–75.0). Those who described TSCs mainly characterised them as ‘distorted’ (n = 12), affecting meat (n = 4), vegetables (n = 3) and tea (n = 2).
Taste and smell changes, nutritional status and cluster symptoms
In this study cohort, ten breast (n = 10/15), two prostate (n = 2/15), four oesophageal (n = 4/6) and four ‘other’ (n = 4/4) cancer patients were at risk of malnutrition (abPG-SGA score ≥6). A trend towards higher prevalence of TSCs was observed in those at nutritional risk (n = 13/20 vs. n = 6/20; P = 0.057). For five, changes were mild, for seven moderate and for one severe.
Of the people who reported TSCs, seven reported eating less over the previous month, while four reported weight loss (WL) over the previous 2 weeks. The group mean percentage (%) WL was negligible (0.01 %WL, SD =/− 4.1) over the previous month and was unrelated to TSCs or BMI.
Most (n = 32; 80 %) (95 % CI, 65–93) reported other symptoms over the previous 2 weeks. Of those recorded, fatigue (n = 19), dry mouth (n = 17), poor appetite (n = 17) and early satiety (n = 16) were most frequent (Fig. 2). The median number of these symptoms reported was 3 (IQR 1–5). Persons with any symptoms likely to affect nutritional status more often reported TSCs (n = 18/32; 56 %, P < 0.05). Specifically, those who reported dry mouth had more TSCs versus those who did not (n = 14/17 vs. n = 5/23, P < 0.01). TSCs were also significantly associated with early satiety (n = 12/16 vs. n = 7/24, P < 0.05) and fatigue (n = 13/19 vs. n = 6/21, P < 0.05). No significant associations were demonstrated between TSCs and the other symptoms described in Fig. 2.
Taste and smell changes and quality of life
Most (n = 14/19, P < 0.05) reported TSCs did not affect their quality of life. Of the five who did, anxiety about poor food variety was cited by three, a strong desire to ‘smell again’ by one and another reported being less sociable due to the TSCs.
Discussion
Taste and smell changes were reported by nearly half of treatment-naive cancer patients in this study. This is approximately 100 times greater than the prevalence of taste and smell impairment in the general population [3].
This is the first study conducted which characterises subjective TSCs prior to oncological treatment. Although one previous study did assess for the presence or absence of TSCs prior to cancer diagnosis using similar methods [11], there was no information on the characteristics of TSCs. In this study, TSCs were reported in people with recently diagnosed early disease even before treatment, which highlights the magnitude of perceived TSCs in a population not traditionally considered at risk.
There was heterogeneity in the TSCs observed. While most participants did not report any alterations in taste intensity pre- and post-diagnosis, for those who did, salt and sweet tastes were prominent and stronger post-diagnosis. Other work with objective and subjective measures have shown varied intensity changes in all basic tastes, both in early and advanced disease [2, 25]. With regard to smell, weaker smell sensation was most common, consistent with findings from objective measures in early breast cancer [15]. In most of the literature, taste and smell changes were assessed at the same time [2, 11, 26], as was undertaken here, and this is recommended for further studies.
Although not statistically significant, females were twice as likely to report TSCs. This finding was clinically significant and has also been found in an adequately powered post-treatment study [27]. Furthermore, normative data in the general population indicate that women of all ages have a better sense of smell than men [28]. Physiological variations in chemosensory perception or differences in men and women’s relationship to food may be responsible [10]. No hormonal cause has been identified. A possible explanation may be that many patients in this study belong to a generation where cooking and food preparation were traditionally female roles [10].
No significant relationship between age and TSCs was identified, similar to previous research [11, 29]. Other studies have suggested that younger patients are more likely to perceive TSCs; these used the same subjective measures as the present study [10, 12]. Since it is established that taste and smell in the general population are affected by age [30], a change in an older patient with an already reduced chemosensory ability may not be as evident as in younger individuals [12]. Insufficient sample size could explain insignificant age and gender results. Smoking status was not significantly associated with TSCs. Several objective and subjective cancer studies have corroborated this [12, 31], while one smaller study disagreed [10]. Given that only 18 % were current smokers, definitive conclusions cannot be reached due to the sample size.
Interestingly, half of the sample was at nutritional risk, although most had cancers not typically identified with this, i.e. breast and prostate cancer [32, 33]. TSCs were reported almost twice as often in breast compared with prostate cancer; however, no significant difference was observed between primary cancer site and TSCs. One study during treatment showed a significant association between TSCs and breast cancer [12], although this finding may be confounded by gender.
The majority at nutritional risk reported TSCs (n = 13/20). This neared statistical significance (P = 0.057) and is highly clinically relevant given that nearly all were due to receive future CT and/or RT, with both known to cause TSCs and weight loss [10]. Treatment would likely exacerbate the baseline TSCs and precipitate further nutritional status decline. This is supported by previous research [2, 34].
The TSCs noted did not exist in isolation. Dry mouth, early satiety and fatigue commonly co-occurred. This relationship has been previously identified in advanced cancer within the same symptom cluster [19]. It has been speculated [19] that these may share a common pathophysiology. On this basis, detection of dry mouth, early satiety or fatigue warrants automatic assessment of TSCs. In addition, effective treatment of TSCs might positively influence any or all of these three symptoms.
Taste and smell changes did not significantly affect quality of life. Although research has identified a link between them, studies were mostly in advanced cancer [2] or during treatment [27]. It has been hypothesised that the confluence of multiple symptoms may affect wellbeing [27]. People with advanced cancer experience a median of 11 symptoms [35]. The median number of nutrition impact symptoms in this study, predominantly composed of patients with early disease, was three. This may explain the discrepancy in quality of life between the present study and other literature.
This study had several strengths. Firstly, the sample was consecutive and representative of the current cancer prevalence in the UK and Ireland by age and gender [36, 37]. Breast and prostate cancer are also the two mostly commonly diagnosed cancers in the USA, UK and Ireland (non-melanoma skin cancers excluded) [36–38]. Consequently, results are clinically applicable. Secondly, statistically significant results were found, despite a small sample size. This reinforces the magnitude of the relationships between the variables identified. The questionnaires employed have been used in other studies on TSCs in cancer [2, 10, 11], which facilitates direct comparison with the current findings. Finally, all participants completed the study. This supports the feasibility of this methodology for future work.
Limitations included recruitment by convenience sampling dictated by the specialty areas of the clinical teams. This limited the sample size and range of cancer types. Most participants had a good performance status and early-stage cancer. More debilitated patients were therefore not represented. Furthermore, social desirability and acquiescence biases may have occurred [39]. Due to incomplete electronic medical record information, medication data were unavailable for 73 % and cannot be excluded as a potential confounder. There is variability in the literature with regard to the impact of medication on taste and smell; one study reported that concurrent medications predict TSCs [12], while another found a weak or insignificant correlation with TSCs [40]. Finally, changes in physical activity post-diagnosis were not measured. Reduced physical activity can mask the effects of reduced dietary intake. Although this may prevent overt weight loss, as observed in this group, it will not avert changes in body composition, i.e. loss of lean body mass [41]. This can negatively impact health and treatment outcomes.
There are several possible explanations for the observations about characterisation of TSCs. There may be an individual predisposition to TSCs [42]. It was noted that people had difficulty articulating their experiences, similar to a previous study [12]. Finally, methodological variations (i.e. subjective vs. objective), cancer type and study duration may have contributed.
The present observations have important clinical implications. Clinicians should consider screening for TSCs at diagnosis with a common terminology. Early recognition may mitigate malnutrition, particularly if RT or CT is planned [1]. Furthermore, given the limited therapeutic strategies for cancer TSCs [43], new treatments are needed. The development of evidence-based practice guidelines is important.
Future longitudinal research should assess the prevalence of TSCs pre- and post-cancer treatment. The pathophysiology and characteristics of perceived TSCs also need further investigation. Validation of the ‘Taste and Smell Survey’ [21] in this cohort is warranted. Quantitative dietary intake data should evaluate the relationship between diet and TSCs. This could determine whether improved chemosensation can increase food intake [2]. Current management strategies such as altering food choice and saliva substitutes require systematic evaluation. Finally, research is needed to clarify the clinical relevance of TSCs symptom clusters.
Conclusions
Subjective TSCs preceded CT or RT in almost half of patients with non-H&N solid tumours, notably stronger sweet and salt tastes. They were most often reported by females and those with breast cancer. The participants were representative of the UK and Irish cancer population by age and gender. Half of the sample was at risk of malnutrition and most of them reported TSCs. TSCs were significantly associated with dry mouth, early satiety and fatigue and may be part of a symptom cluster. Future research and clinical guidelines which incorporate a common terminology for the assessment, diagnosis and management of TSCs in cancer populations are needed.
References
Brisbois TD, Hutton JL, Baracos VE, Wismer WV (2006) Taste and smell abnormalities as an independent cause of failure of food intake in patients with advanced cancer-an argument for the application of sensory science. J Palliat Care 22:111–114
Hutton JL, Baracos VE, Wismer WV (2007) Chemosensory dysfunction is a primary factor in the evolution of declining nutritional status and quality of life in patients with advanced cancer. J Pain Symptom Manag 33:156–165
Hoffman HJ, Cruickshanks KJ, Davis B (2009) Perspectives on population-based epidemiological studies of olfactory and taste impairment. Ann N Y Acad Sci 1170:514–530
Henkin RI, Levy LM, Fordyce A (2013) Taste and smell function in chronic disease: a review of clinical and biochemical evaluations of taste and smell dysfunction in over 5000 patients at The Taste and Smell Clinic in Washington. DC Am J Otolaryngol 34:477–489
Landis BN, Beutner D, Frasnelli J, Hüttenbrink KB, Hummel T (2005) Gustatory function in chronic inflammatory middle ear diseases. Laryngoscope 115:1124–1127
Zabernigg A, Gamper EM, Giesinger JM, Rumpold G, Kemmler G, Gattringer K, Sperner-Unterweger B, Holzner B (2010) Taste alterations in cancer patients receiving chemotherapy: a neglected side effect? Oncologist 15:913–920
Ovesen L, Hannibal J, Sørensen M, Allingstrup L (1991) Food intake, eating-related complaints, and smell and taste sensations in patients with cancer of the lung, ovary and breast undergoing chemotherapy. Clin Nutr 10:336–341
Baharvand M, Shoaleh Saadi N, Barakian R, Moghaddam EJ (2013) Taste alteration and impact on quality of life after head and neck radiotherapy. J Oral Pathol Med 42:106–112
Leyrer CM, Chan MD, Peiffer AM, Horne E, Harmon M, Carter AF, Hinson WH, Mirlohi S, Duncan SE, Dietrich AM, GJ L (2014) Taste and smell disturbances after brain irradiation: a dose-volume histogram analysis of a prospective observational study. Pract Radiat Oncol 4:130–135
McGreevy J, Orrevall Y, Belqaid K, Wismer W, Tishelman C, BM B (2014) Characteristics of taste and smell alterations reported by patients after starting treatment for lung cancer. Support Care Cancer 22:2635–2644
Belqaid K, Orrevall Y, McGreevy J, Månsson-Brahme E, Wismer W, Tishelman C, BM B (2014) Self-reported taste and smell alterations in patients under investigation for lung cancer. Acta Oncol 53:1405–1412
Bernhardson BM, Tishelman C, LE R (2008) Self-reported taste and smell changes during cancer chemotherapy. Support Care Cancer 16:275–283
Gamper EM, Zabernigg A, Wintner LM, Giesinger JM, Oberguggenberger A, Kemmler G, Sperner-Unterweger B, Holzner B (2012) Coming to your senses: detecting taste and smell alterations in chemotherapy patients. A systematic review. J Pain Symptom Manage 44:880–895
Johnson FM (2001) Alterations in taste sensation: a case presentation of a patient with end-stage pancreatic cancer. Cancer Nurs 24:149–155
Lehrer S, Levine E, Bloomer WD (1985) Abnormally diminished sense of smell in women with oestrogen receptor positive breast cancer. Lancet 2:333
Comeau TB, Epstein JB, Migas C (2001) Taste and smell dysfunction in patients receiving chemotherapy: a review of current knowledge. Support Care Cancer 9:575–580
Mattes R, Arnold C, Boraas M (1987) Learned food aversion among cancer chemotherapy patients. Cancer 60:2576–2580
Ottery FD (1994) Cancer cachexia: prevention, early diagnosis, and management. Cancer Pract 2:123–131
Walsh D, Rybicki L (2006) Symptom clustering in advanced cancer. Support Care Cancer 14:831–836
Komurcu S, Nelson KA, Walsh D, Ford RB, LA R (2002) Gastrointestinal symptoms among inpatients with advanced cancer. Am J Hosp Palliat Care 19:351–355
Heald AE, Pieper CF, Schiffman SS (1998) Taste and smell complaints in HIV-infected patients AIDS 12: 1667–1674
Gabrielson DK, Scaffidi D, Leung E, Stoyanoff L, Robinson J, Nisenbaum R, Brezden-Masley C, PB D (2013) Use of an abridged scored Patient-Generated Subjective Global Assessment (abPG-SGA) as a nutritional screening tool for cancer patients in an outpatient setting. Nutr Cancer 65:234–239
World Health Organisation. BMI Classification 2000. (Internet: http://www.who.int/bmi/index.jsp?introPage=intro_3.html) (accessed 13 April 2015)
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, PP C (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Steinbach S, Hundt W, Zahnert T, Berktold S, Böhner C, Gottschalk N, Hamann M, Kriner M, Heinrich P, Schmalfeldt B, Harbeck N (2010) Gustatory and olfactory function in breast cancer patients. Support Care Cancer 18:707–713
Harris AM, Griffin SM (2003) Postoperative taste and smell deficit after upper gastrointestinal cancer surgery-an unreported complication. J Surg Oncol 82:147–150 discussion 150-152
Bernhardson BM, Tishelman C, Rutqvist LE (2009) Taste and smell changes in patients receiving cancer chemotherapy: distress, impact on daily life, and self-care strategies. Cancer Nurs 32:45–54
Hummel T, Kobal G, Gudziol H, Mackay-Sim A (2007) Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 264:237–243
Maes A, Huygh I, Weltens C, Vandevelde G, Delaere P, Evers G, den Bogaert W V (2002) De Gustibus: time scale of loss and recovery of tastes caused by radiotherapy. Radiother Oncol 63:195–201
Schiffman SS (1997) Taste and smell losses in normal aging and disease. JAMA 278:1357–1362
Konstantinidis I, Chatziavramidis A, Printza A, Metaxas S, Constantinidis J (2010) Effects of smoking on taste: assessment with contact endoscopy and taste strips. Laryngoscope 120:1958–1963
Bering T, Mauricio SF, Silva JB, MI C (2014) Nutritional and metabolic status of breast cancer women. Nutr Hosp 31:751–758
Hebuterne X, Lemarie E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F (2014) Prevalence of malnutrition and current use of nutrition support in patients with cancer. J Parenter Enter Nutr 38:196–204
Brisbois TD, de Kock IH, Watanabe SM, Baracos VE, Wismer WV (2011) Characterization of chemosensory alterations in advanced cancer reveals specific chemosensory phenotypes impacting dietary intake and quality of life. J Pain Symptom Manag 41:673–683
Walsh D, Donnelly S, Rybicki L (2000) The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer 8:175–179
National Cancer Registry Ireland, Cancer in Ireland 1994–2012: annual report of the National Cancer Registry, Cork, Ireland, 2014
Cancer Research UK. Cancer Incidence Statistics 2011. (Internet: http://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence) (accessed 11 August 2015)
American Cancer Society. Cancer Facts & Figures 2015. (Internet: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf) (accessed 11 August 2015)
Bowling A (2005) Mode of questionnaire administration can have serious effects on data quality. J Public Health (Oxf) 27:281–291
Alt-Epping B, Nejad RK, Jung K, Gross U, Nauck F (2012) Symptoms of the oral cavity and their association with local microbiological and clinical findings-a prospective survey in palliative care. Support Care Cancer 20:531–537
Aapro M, Arends J, Bozzetti F, Fearon K, Grunberg SM, Herrstedt J, Hopkinson J, Jacquelin-Ravel N, Jatoi A, Kaasa S, Strasser F (2014) Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol 25:1492–1499
Bernhardson BM, Tishelman C, LE R (2009) Olfactory changes among patients receiving chemotherapy. Eur J Oncol Nurs 13:9–15
Thorne T, Olson K, Wismer W (2015) A state-of-the-art review of the management and treatment of taste and smell alterations in adult oncology patients. Support Care Cancer 23:2843–2851
Acknowledgments
The authors acknowledge the patients and staff at St. Luke’s Radiation Oncology Network, St. James’s Hospital and Our Lady’s Hospice and Care Services for their support. Particular thanks to Aine Breen, Mary Cunningham, Aoife Gorham, Anita O’Donovan, Anne O’Hara and Laoise Ryan for their help with the logistical planning of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval was obtained from the Research Ethics Committee at St. Luke’s Radiation Oncology Network and the joint St. James’s Hospital/Adelaide and Meath Hospital, Dublin Research Ethics Committee.
Conflict of interest
None
Disclosures
None
Rights and permissions
About this article
Cite this article
Spotten, L., Corish, C., Lorton, C. et al. Subjective taste and smell changes in treatment-naive people with solid tumours. Support Care Cancer 24, 3201–3208 (2016). https://doi.org/10.1007/s00520-016-3133-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3133-2