Abstract
Purpose
Taste and smell alterations (TSAs) are among the most frequent and troublesome symptoms reported by head and neck cancer (HNC) patients after treatment. Little is known about the relationship between TSAs and quality of life (QoL) among HNC patients. The aim of this study was to determine the effect of TSAs on overall QoL among tube-fed and orally fed HNC patients before treatment, at end of treatment and at 2.5-month follow-up.
Methods
Data were collected in a longitudinal study prior to treatment (n = 126), at end of treatment (n = 100) and at 2.5-month follow-up (n = 85). Chemosensory Complaint Score (CCS) and the University of Washington Quality of Life Questionnaire version 3 were used to assess TSAs and QoL, respectively. Generalized estimated equation modeling was used to estimate the effect of CCS on QoL.
Results
At end of treatment, QoL and CCS had declined for both tube-fed and orally fed patients and thereafter improved, but not to pre-treatment levels. Neither QoL nor CCS mean scores were different between the two groups at any time point. CCS was a significant predictor of overall QoL (β = −1.82, p < 0.0001), social-emotional (β = −1.76, p < 0.0001), physical (β = −1.12, p < 0.0001) and overall functions (β = −1.15, p < 0.0001) at a multivariate level. Taste was reported as an important symptom for both tube-fed and orally fed groups at end of treatment and follow-up.
Conclusions
TSAs are an important symptom and an independent predictor of QoL for both tube-fed and orally fed HNC patients. HNC patients need support to manage TSAs, regardless of the method of nutritional intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multimodal therapy (surgery/RT/CT) has been used over the last two decades to improve tumor control and survival among head and neck cancer (HNC) patients; however, this treatment approach significantly impacts patient quality of life [1]. Quality of life (QoL) can be defined as the ability of the individual to perform activities related to physical, mental, social and emotional well-being while reporting satisfaction with daily functions [2]. QoL has become an outcome as important as overall survival and disease-free survival to evaluate the success of the treatment [3, 4]. HNC treatment may alter functionality in activities of daily life [5], while treatment side effects (e.g., mucositis, taste and smell alterations, dry mouth, mouth sores, nausea and loss of appetite) reduce physical, emotional and social well-being [6–9] and have generally been shown to reduce QoL [10].
Taste and smell alterations (TSAs) are one of the most frequent and troublesome symptoms reported by cancer patients [7, 11]. TSAs vary in nature and severity and can be characterized as the total absence of taste or smell, reduced or increased sensitivity, distortion of normal taste and smell, presence of phantom tastes or odors and lingering bitter or metallic sensations [12]. TSAs are recognized as a nutrition impact symptom associated with reduced dietary intake, restricted food choice and weight loss among HNC patients [13]. When symptoms or tumor location interfere with appropriate oral intake during treatment, tube feeding is used to meet nutritional needs [14, 15]. The TSA experience of tube-fed HNC patients has not been documented.
A recent systematic review [16] showed that antineoplastic treatment modalities affect the prevalence of taste alterations as evaluated by both self-reports and clinical tests. Approximately half of the patients treated with only chemotherapy (chemo), two-thirds of the patients treated with radiotherapy (RT) and three quarters of the patients treated with combined chemo-RT experienced taste alterations [16]. High radiation dose and RT to the head and neck area specifically increase the risk of TSAs. Although taste loss improves 20–60 days upon completing RT, taste perception generally does not return to normal or near-normal levels even a year after RT therapy [16]. TSAs may persist up to 7 years, and a chronic reduced ability to taste may establish in one-third of patients [17, 18].
Several studies have investigated the effect of TSAs on HNC-specific QoL or feelings of depression. Baharvand et al. [19] found that all 22 HNC patients in their study developed clinically tested taste loss after RT and that QoL deteriorated significantly with all taste loss. Similarly, Kubrak et al. [13] observed a trend for self-reported TSAs as a predictor of reduced symptom-related QoL at a multivariate level [β = −5.0, 95 % confidence interval (CI) = −10.3; 0.2, p = 0.06] among RT-treated orally fed patients followed to 2.5 months post-treatment. Finally, in-depth interviews with 33 RT-treated HNC patients revealed that 90 % of patients experienced alterations in taste and attributed feelings of depression to the changes in their oral cavity [9].
TSAs can be evaluated by self-reports or clinical tests; however, clinical tests are not able to capture dimensions such as flavor, food enjoyment or distortions of normal perception [6]. Previous studies exploring the impact of TSAs on QoL have not used a comprehensive self-report taste and smell assessment tool [1, 4, 20–23] in combination with a health-related QoL approach, which considers the person as a whole and is concerned with the level of satisfaction in other life spheres beyond the symptoms [24] (e.g., work, family, friends, spirituality and recreational environments). Since treatments and its side effects can affect multiple areas in the lives of patients [24], it is important to understand the impact of TSA on the overall level of satisfaction of the HNC patient. In addition, there are no published studies of TSAs experienced by tube-fed patients nor the impact of TSAs on the QoL of tube-fed patients. Thus, the aim of this study was to determine the effect of TSAs on overall QoL among tube-fed and orally fed HNC patients before treatment, at end of treatment and at 2.5-month follow-up, independent of age, gender, illness (tumor site and stage), treatment and lifestyle characteristics (smoking and alcohol).
Methods
Study design and clinical setting
This longitudinal study was conducted at the Cross Cancer Institute, the comprehensive treatment center for northern Alberta. Data were collected in two phases, between February 2007 and August 2009 and between February 2012 and June 2014. This paper presents the pooled data. Research procedures were in accord with the Alberta Cancer Research Ethics Committee. All patients provided written informed consent.
Sample size
At the baseline, a sample of 116 patients was required to achieve a power of 80 % with an effect size of 0.25 (small to medium) to study the association between QoL and taste and smell alterations as measured by Chemosensory Complaint Score (CCS). The level of significance was at a two-sided α level of 0.05.
Study population
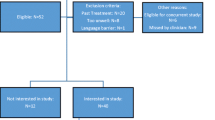
Inclusion criteria were as follows: older than 18 years, diagnosis of HNC (oral cavity, salivary glands, paranasal sinuses, oropharynx, nasopharynx, hypopharynx and larynx) with any histology and, at any stage, scheduled to receive curative-intent RT with or without concurrent CT or cetuximab. Patients with tumors of the lip and thyroid were excluded.
Patients received treatment according to the standard of care. The median total dose of radiotherapy was 60 Gy. Forty-two (26.2 %) participants were treated with surgery prior to chemo-RT. Planned chemotherapy included high-dose cisplatin (n = 48) or weekly carboplatin (n = 28); six patients were switched to carboplatin due to toxicity, and eight patients received cetuximab (Table 1).
Data collection
Data were collected prior to any treatment (baseline), on completion of RT or chemo-RT (end of treatment) and at 2.5-month follow-up. Seventy-one (44.4 %) patients completed all three study visits. Twenty patients (12.5 %) completed baseline and at least one of the other visits. Thirty-eight (23.7 %) patients completed only baseline, and 31 (19.4 %) patients did not have baseline information (Supplemental Table 2).
The mean timeframe between baseline and end of treatment assessments was 14.4 ± 5.8 and 16.5 ± 5.5 weeks for orally fed and tube-fed patients, respectively, whereas the mean time between end of treatment and 2.5-month follow-up was 8.4 ± 4.1 and 8.3 ± 3.3 weeks for orally fed and tube-fed patients, respectively. Variations in assessment time were due to travel constraints, holidays and patients’ convenience.
Individual characteristics and treatment
Patients’ main demographic characteristics, smoking status, alcohol consumption, the presence of feeding tube, tumor site and tumor stage and treatment (RT doses and schedule, and antineoplastic drugs administered) were extracted from medical records.
Energy and protein intake
Patients’ intake was recorded by a self-reported 3-day dietary record [25], a method that provides a valid and reliable estimate among cancer patients [26]. Food Processor II Nutrient Analysis Program™ (Esha Research, Salem, OR) was used to determine caloric and protein intake from the food records.
Taste and smell alterations
Self-perceived taste and smell functions were assessed through the taste and smell survey that quantifies the nature and severity of TSAs (Supplemental Table 1). Eight items of the taste and smell survey assess taste, and six evaluate smell perception. The final score, the Chemosensory Complaint Score (CCS) (range 0–16, higher score indicates greater severity of complaint), is the sum of the Taste Complaint Score (range 0–10) and the Smell Complaint Score (range 0–6) [27]. Open-ended questions allow patients to indicate how their altered sense of taste/smell has impacted their quality of life.
Quality of life
QoL was assessed by the University of Washington Quality of Life version 3 (UW-QoL v3) [28]. The UW-QoL v3 is one of the most commonly used health-related QoL questionnaires in head and neck oncology. This HNC-specific QoL questionnaire is brief, self-administered and detailed enough to identify small changes [29] and has an internal consistency score of 0.85 [28]. The questionnaire contains 10 domains exploring pain, appearance, activity, recreation, swallowing, chewing, speech, shoulder, taste and saliva with scoring scaled in equal stages from 0 to 100 (higher scores indicate better status). The UW-QoL also asks patients to identify the three most important domains during the past 7 days. Patient-perceived clinical distress for taste and the need for intervention was evaluated through the identification of “trigger criteria,” which occurs when a patient’s evaluation of the taste domain is “I cannot taste any food” or “I can taste some food” and indicates taste as an important domain during the past 7 days [28].
Composite scores can be calculated to represent both the physical function and the social-emotional function of patients [28]. Additionally, overall function and overall QoL are assessed through this tool. Composite scores for health-related QoL dimensions were computed as follows: physical function as the average of chewing, swallowing, speech, saliva and appearance domain scores (excluding taste); social-emotional function as the average of pain, activity, recreation and shoulder domain scores; and overall function as the average of all domains scores (pain, appearance, activity, recreation, swallowing, chewing, speech, shoulder and saliva), except taste. Scores for physical, social-emotional and overall functions range from 0–100, with higher scores indicating better condition [28]. The global question of overall QoL has 6 possible responses scored as 0 (very poor), 20 (poor), 40 (fair), 60 (good), 80 (very good) and 100 (outstanding) [28].
Statistical analysis
Descriptive statistics were adopted: Continuous variables were expressed as averages and confidence intervals (CI) at 95 %, mean and standard deviation or median and interquartile range (IQR), while categorical variables were summarized as sums and percentages. The Chi-squared test with Yates’ correction (or Fisher’s exact test) and t test or Mann–Whitney U test were used for comparisons between categorical variables. Descriptive data were presented for all patients included in the study (n = 160), those tube-fed (n = 44) and those orally fed (n = 116).
Data presentation of overall QoL was stratified into tube-fed and orally fed groups since significant differences were found between the two groups in tumor site, tumor stage and treatment. In addition, Terrell et al. [30] showed the feeding tube to have the most negative impact on QoL compared to 13 different demographic and clinical characteristics.
Generalized estimated equation (GEE) modeling was used to estimate the effects of CCS on overall QoL, social-emotional function, physical function and overall function to allow simultaneous modeling of data with repeated measurements [31] and provide robust standard errors [32, 33]. For our analysis, GEE was used to calculate the magnitude of change (i.e., β coefficients) of the study variables over time. For GEE analysis of physical and overall functions, we excluded the taste domain of the UW-QOL instrument because CCS was included as an independent variable.
Results
Study population
One hundred and sixty patients consented to participate, and about a quarter were tube-fed. Data at baseline, end of treatment and 2.5-month follow-up were available for 126, 100 and 85 patients, respectively (Supplemental Table 2).
Most patients were male (n = 126, 78.8 %), and the mean age was 58.9 years. Three quarters of patients were current or former smokers, and almost 70 % (n = 107) consumed alcohol at baseline (Table 1). Eighty-eight patients (55.0 %) had a pharynx neoplasm. One hundred and twenty-four (80 %) patients had an advanced tumor stage (T3/T4). RT ± surgery (n = 57, 49.1 %) was the main therapy in orally fed patients, whereas the majority of tube-fed patients (n = 37, 84.1 %) received chemo-RT ± surgery (Table 1).
All tube-fed patients continued to have oral intake in the form of clear liquids and supplements during the three study time points. There were no differences in caloric or protein intake, standardized by body weight, between orally fed and tube-fed patients at any time point (Table 1).
Most important issues in tube-fed and orally fed patients over the time
Patients attributed a different importance to the symptoms evaluated through the UW-QoL over time (Table 2). At baseline, swallowing (n = 51.6 %) and pain (n = 36, 37.9 %) were reported as the most important symptom during the past 7 days by tube-fed and orally fed patients, respectively. In both groups, taste was ranked as the least relevant problem; only five orally fed patients and one tube-fed patient reported taste alterations among the three most important symptoms during the past 7 days.
At end of treatment, swallowing became the most important symptom for both tube-fed (n = 24, 82.7 %) and orally fed patients (n = 49, 69 %). Similarly, taste increased in importance in both groups and was ranked as the third and fourth most important symptom by tube-fed (n = 12, 41.4 %) and orally fed (n = 23, 32.4 %) patients, respectively. Overall, 10 patients (4 tube-fed and 6 orally fed) chose more than 3 symptoms as important at this time point.
At 2.5-month follow-up, the most distressing symptom for tube-fed patients continued to be swallowing (n = 14, 58.3 %), whereas orally fed patients ranked saliva (n = 35, 57.4 %) as the most distressing symptom. Taste continued to be the third and fourth most important symptom for tube-fed and orally fed patients, respectively.
Overall quality of life, Chemosensory Complaint Score and taste trigger criteria for tube-fed and orally fed patients over time
No significant differences between orally fed and tube-fed groups were observed for overall QoL and CCS mean scores at any of the study time points. Overall QoL scores declined from baseline to end of treatment for both tube-fed and orally fed patients and were improved at 2.5-month follow-up but not to pre-treatment levels (Table 3). At baseline, 75 % of patients (20/31 tube-fed and 74/95 orally fed) reported a good or higher (score ≥60) QoL, and only 9 patients (3 tube-fed and 6 orally fed) reported a poor or lower (score ≤20) QoL. At end of treatment, less than half of patients rated their overall QoL as good or higher, while one in five patients (5 tube-fed and 15 orally fed) reported a poor or lower QoL. At 2.5-month follow-up, 54 (63.5 %) patients (12/24 tube-fed and 42/61 orally fed) reported a good or higher overall QoL, but 10 (12 %) patients continued to report a poor (score = 20) QoL. None reported a very poor (score = 0) QoL.
At end of treatment, no patients rated their overall QoL as outstanding (score = 100) and none of the tube-fed patients rated their QoL as outstanding at any time point. The proportion of tube-fed patients with a good or higher overall QoL was always lower than that of orally fed patients at any time point [64.5 vs 77.9 % (20/31 vs 74/95) at baseline, 44.8 vs 49.2 % (13/23 vs 35/71) at end of treatment, 50.0 vs 69.4 % (12/24 vs 42/61) at 2.5-month follow-up].
The pattern for CCS was similar to overall QoL scores. CCS increased from baseline after the completion of treatment in both the tube-fed group (8.0 ± 2.3 vs 0.2 ± 0.2 at baseline) and orally fed group (8.7 ± 0.7 vs 1.5 ± 0.5 at baseline) with a reduction in complaints at 2.5-month follow-up (6.4 ± 1.6 in tube-fed and 6.0 ± 0.7 in orally fed) but not to baseline levels.
At the end of treatment, 35 (49.3 %) orally fed patients reached trigger criteria on the UW-QoL taste domain. These patients also had higher Taste Complaint Scores than orally fed patients without trigger criteria (8.4 ± 1.4 vs 6.2 ± 2.8, p = .003). Similarly, 18 (62.1 %) tube-fed patients had trigger criteria for the taste domain and higher Taste Complaint Scores and CCS scores compared to tube-fed patients without trigger criteria (8.3 ± 1.6 vs 4.1 ± 3.5, p = .005 and 10.2 ± 2.4 vs 5.7 ± 4.5, p = .022, respectively). No differences were found in Smell Complaint Scores at the end of treatment or in any of the scores at baseline and at 2.5-month follow-up between patients with trigger criteria compared to patients without, for both orally fed and tube-fed patients.
Many orally fed and some tube-fed participants described the impact of altered taste/smell on their quality of life as a “reduced enjoyment of food” and as “less rewarding” at both end of treatment and 2.5-month post-treatment. Participants also indicated that the alterations resulted in a lack of appetite and lack of desire to eat at these time points.
Factors affecting quality of life
GEE was used to estimate the impact of self-perceived taste and smell functions, treatment, tube feeding and six clinical and demographic variables on overall QoL. Table 4 presents multivariate results for overall QoL for both tube-fed and orally fed patients. CCS was a significant predictor of overall QoL in the univariate analysis (β = −1.685, CI: −2.25; −1.12, p < 0.0001). The β coefficient from multivariate analysis revealed that each unit increase in CCS resulted in a decrease in overall QoL of 1.82.
Tumor characteristics such as site and stage were associated with QoL (Table 4). Age was an independent predictor of overall QoL. Compared to current smokers, patients who had never smoked had a significantly better overall QoL.
Table 5 shows the GEE results for social-emotional function, physical function and overall function. After adjusting for age, gender, smoking status, alcohol consumption, tumor site, treatment and tube feeding, CCS was a significant independent predictor of social-emotional function (β = −1.76, p < 0.0001), physical function (β = −1.12, p = < 0.0001) and overall function (β = −1.15, p = <0.0001).
Discussion
This is the first study to determine the impact of TSAs on overall QoL among HNC patients using self-report measures of both. We found TSAs to be a significant independent predictor of overall QoL, social-emotional function, physical function and overall function after adjusting for age, gender, tumor stage, tumor site, treatment, smoking, alcohol consumption and tube feeding. These findings add new evidence to the field of QoL in two ways. First, we illustrate an association between self-reported TSAs in HNC patients and their overall QoL, confirming Baharvand and colleagues’ observations [19] of a significant deterioration in QoL associated with clinically evaluated chemo/radiotherapy-induced taste loss. Second, we determined TSAs to be an independent predictor of QoL (GEE analysis) and identified TSAs as important issues for tube-fed patients as well as for patients capable of oral intake at both end of treatment and 2.5-month follow-up.
We observed that greater TSAs were associated with reduced social-emotional function, physical function and overall function, which may limit participation in social and recreational activities. Previous qualitative studies describe frustration and disappointment with eating as common experiences among HNC patients that limit participation in social and recreational activities with friends and family [34, 35]. Although the precise mechanism by which TSAs affect QoL is likely multifactorial, our study and others suggest that the primary factor may be the loss of the social enjoyment of food with limitations in communal and recreational activities.
For data collection, we used two self-report tools, the best method to capture subjective and individual dimensions such as food enjoyment [6]. The taste and smell survey has been used in a variety of disease settings, while the UW-QoL questionnaire is specific to patients with head and neck cancer to capture subjective dimensions of health-related QoL. Consistent with a recent recommendation that identified taste alterations as a patient-reported HN-specific core symptom [36], we believe that self-report tools are the method of choice to assess TSAs and their impact on QoL.
Overall QoL changed over the three study time points as previously shown by a prospective study [37]. The end of treatment time point was the worst period with the lowest QoL scores reported by both tube-fed and orally fed groups, and with 20 % of all patients reporting a poor or lower QoL. However, QoL was poorer for tube-fed patients compared to orally fed as the proportion of tube-fed patients with a good or higher QoL was always lower than orally fed patients at any time point. Moreover, we found that none of tube-fed patients rated their QoL as outstanding at any time point. CCS predicted overall QoL after adjusting for tube feeding. Although different treatments were given to orally fed and tube-fed patients, we adjusted for type of treatment in our analyses and the proportion of surgery-treated patients was similar in tube-fed and orally fed groups. Future studies with larger sample sizes will facilitate evaluation of type of surgery on QoL. We observed a significant association between early-stage disease and better QoL that is consistent with other studies [38].
CCS trended similarly with QoL scores. Taste perception and smell perception were significantly impaired after the completion of treatment with only a partial recovery at 2.5-month follow-up as highlighted in previous papers [4, 39] that show taste pre-treatment levels not yet recovered 1 year after treatment. This trend is confirmed by the issues reported by patients as the most important during the past 7 days. At baseline, the taste domain was ranked as the least important issue by all patients, but at end of treatment, it was ranked third and fourth most important by tube-fed and orally fed patients, respectively, and this ranking was maintained at 2.5-month follow-up.
Our study investigated the effect of the main demographic and clinical variables on QoL. Having never smoked was associated with a better QoL. These findings confirm Duffy and colleagues’ pilot study results [40] that found smoking negatively associated with five scales of the QoL Short Form Health Survey (SF-36V), including general health among HNC patients. Further studies are warranted to explore the different predictors of QoL in these two populations.
Similar to other studies of HNC populations [39, 41], participants of this study were predominantly male; in North America, the ratio of HNC of males to females can be greater than 2:1 [42, 43]. We observed that gender was not a significant predictor of overall QoL. Females may respond differently to symptoms [38]; however, the effect of treatment should not differentially affect taste function [19]. Comorbidity was not adjusted in the GEE modeling; however, comorbidity alone has been shown not to affect QoL indices [44].
Patient recruitment and retention can be challenging in oncology studies. To reduce burden, patients were invited to take part in all time points and were allowed to enter the study at any time point. This strategy increased recruitment rate, but resulted in some missing data at baseline. The main reasons for missing data at the second and third assessments were fatigue, feeling unwell and missed appointments. A comparison of the patients who continued in the study and those who withdrew their consent after the first assessment did not show any difference for overall QoL or CCS.
While the UW-QoL v3 is brief and HNC-specific [29], it is considered to be a health-related QoL tool that captures the subjective well-being of HNC patients [28] as they rate their own overall quality of life considering all factors that affect their enjoyment of life in addition to physical and mental symptoms. “Mood” and “anxiety” items have been added in the development of the UW-QOL v4 [28] and should be used in future studies to assess the QoL of HNC patients.
In summary, we found TSAs to be a significant independent predictor of overall QoL, social-emotional function, physical function and overall function. TSAs are one of the clusters of nutrition impact symptoms with clinical consequences, such as restricted food choice and decreased dietary intake, nutritional status and food enjoyment [39]. Our aim was to determine the impact of TSAs on QoL among HNC patients, and we found that they are an independent predictor of QoL also when oral intake is restricted.
These findings highlight the importance of screening all HNC patients for self-reported TSAs [36] even when they are not exclusively dependent on oral food intake [45]. Moreover, new treatment-support pathways must be developed for tube-fed patients; current TSAs symptom management is focused on the provision of food choice and eating suggestions to orally fed patients, while a different approach is required to fully address TSAs experienced by tube-fed patients. A comprehensive self-report tool for TSAs in addition to a routine QoL questionnaire can help healthcare professionals identify the nature and severity of the taste and smell alterations and choose the best strategy for reducing their impact on QoL.
References
de Graeff, A., de Leeuw, J. R. J., Ros, W. J. G., Hordijk, G. J., Blijham, G. H., & Winnubst, J. A. M. (2000). Pretreatment factors predicting quality of life after treatment for head and neck cancer. Head and Neck, 22(4), 398–407.
Epstein, J., Robertson, M., Emerton, S., Phillips, N., & Stevenson-Moore, P. (2001). Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head and Neck, 23(5), 389–398.
Bonnetain, F. (2010). Health related quality of life and endpoints in oncology. Cancer Radiotherapie, 14(6–7), 515–518.
Rampling, T., King, H., Mais, K., Humphris, G., Swindell, R., Sykes, A., & Slevin, N. (2003). Quality of life measurement in the head and neck cancer radiotherapy clinic: Is it feasible and worthwhile? Clinical Oncology, 15(4), 205–210.
Epstein, J. B., Emerton, S., Kolbinson, D. A., Le, N. D., Phillips, N., Stevenson-Moore, P., & Osoba, D. (1999). Quality of life and oral function following radiotherapy for head and neck cancer. Head and Neck, 21, 1–11.
Brisbois, T. D., de Kock, I. H., Watanabe, S. M., Baracos, V. E., & Wismer, W. V. (2011). Characterization of chemosensory alterations in advanced cancer reveals specific chemosensory phenotypes impacting dietary intake and quality of life. Journal of Pain and Symptom Management, 41(4), 673–683.
Bernhardson, B. M., Tishelman, C., & Rutqvist, L. E. (2008). Self-reported taste and smell changes during cancer chemotherapy. Supportive Care in Cancer, 16(3), 275–283.
Hutton, J. L., Baracos, V. E., & Wismer, W. V. (2007). Chemosensory dysfunction is a primary factor in the evolution of declining nutritional status and quality of life in patients with advanced cancer. Journal of Pain and Symptom Management, 33(2), 156–165.
Rose-Ped, A., Bellm, L., Epstein, J., Trotti, A., Gwede, C., & Fuchs, H. (2002). Complications of radiation therapy for head and neck cancers: The patient’s perspective. Cancer Nursing, 25(6), 461–467.
Bernhardson, B. M., Olson, K., Baracos, V. E., & Wismer, W. V. (2012). Reframing eating during chemotherapy in cancer patients with chemosensory alterations. European Journal of Oncology Nursing, 16, 483–490.
Bansal, M., Mohanti, B. K., Shah, N., Chaudhry, R., Bahadur, S., & Shukla, N. K. (2004). Radiation related morbidities and their impact on quality of life in head and neck cancer patients receiving radical radiotherapy. Quality of Life Research, 13(2), 481–488.
Hong, J. H., Omur-Ozbek, P., Stanek, B. T., Dietrich, A. M., Duncan, S. E., Lee, Y. W., & Lesser, G. (2009). Taste and odor abnormalities in cancer patients. The Journal of Supportive Oncology, 7(2), 58–65.
Kubrak, C., Olson, K., Jha, N., Scrimger, R., Parliament, M., McCargar, L., et al. (2012). Clinical determinants of weight loss in patients receiving radiation and chemoirradiation for head and neck cancer: A prospective longitudinal view. Head and Neck, 35(5), 695–703.
Nugent, B., Lewis, S., & O’Sullivan, J. M. (2013). Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database of Systematic Reviews, 1, 007904.
Cheng, S. S., Terrell, J. E., Bradford, C. R., Ronis, D. L., Fowler, K. E., Prince, M. E., et al. (2006). Variables associated with feeding tube placement in head and neck cancer. Archives of Otolaryngology—Head and Neck Surgery, 132(6), 655–661.
Hovan, A. J., Williams, P. M., Stevenson-Moore, P., Wahlin, Y. B., Ohrn, K. E., Elting, L. S., et al. (2010). A systematic review of dysgeusia induced by cancer therapies. Supportive Care in Cancer, 18(8), 1081–1087.
Redda, M. G. R., & Allis, S. (2006). Radiotherapy-induced taste impairment. Cancer Treatment Reviews, 32, 541–547.
Vissink, A., Jansma, J., Spijkervet, F. K. L., Burlage, F. R., & Coppes, R. P. (2003). Oral sequelae of head and neck radiotherapy. Critical Reviews in Oral Biology and Medicine, 14(3), 199–212.
Baharvand, M., Shoalehsaadi, N., Barakian, R., & Jalali Moghaddam, E. (2012). Taste alteration and impact on quality of life after head and neck radiotherapy. Journal of Oral Pathology and Medicine, 42(1), 106–112.
Fang, F. M., Chien, C. Y., Tsai, W. L., Chen, H. C., Hsu, H. C., Lui, C. C., et al. (2008). Quality of life and survival outcome for patients with nasopharyngeal carcinoma receiving three-dimensional conformal radiotherapy vs. intensity-modulated radiotherapy—A longitudinal study. International Journal of Radiation Oncology Biology Physics, 72(2), 356–364.
Chencharick, J. D., & Mossman, K. L. (1983). Nutritional consequences of the radiotherapy of head and neck cancer. Cancer, 51, 811–815.
List, M., Stracks, J., Colangelo, L., Butler, P., Ganzenko, N., Lundy, D., et al. (2000). How do head and neck cancer patients prioritize treatment outcomes before initiating treatment? Journal of Clinical Oncology, 18(4), 877–884.
Mowry, S. E., LoTempio, M. M., Sadeghi, A., Wang, K. H., & Wang, M. B. (2006). Quality of life outcomes in laryngeal and oropharyngeal cancer patients after chemoradiation. Otolaryngology—Head and Neck Surgery, 135(4), 565–570.
Friedman, M. L. (1997). Basic issues and challenges. In Improving the quality of life: A holistic scientific strategy (pp. 4–17). Westport, CT: Praeger Publishers.
Gibson, R. S. (1990). Principles of nutritional assessment. Oxford, UK: Oxford University Press.
Bruera, E., Chadwick, S., Cowan, L., Drebit, D., Hanson, J., MacDonald, N., et al. (1986). Caloric intake assessment in advanced cancer patients: comparison of three methods. Cancer Treatment Reports, 70(8), 981–983.
Heald, A. E., Pieper, C. F., & Schiffman, S. S. (1998). Taste and smell complaints in HIV-infected patients. AIDS, 12(13), 1667–1674.
Rogers, S. N., & Lowe, D. (2010). The University of Washington Quality of Life Scale. In V. R. Preedy & R. R. Watson (Eds.), Handbook of disease burdens and quality of life measures (p. 101). New York, USA: Springer Science+Business MEDIA LLC.
Hassan, S. J., & Weymuller, E. A. (1993). Assessment of quality of life in head and neck cancer patients. Head and Neck, 15(6), 485–496.
Terrell, J. E., Ronis, D. L., Fowler, K. E., Bradford, C. R., Chepeha, D. B., Prince, M. E., et al. (2004). Clinical predictors of quality of life in patients with head and neck cancer. Archives of Otolaryngology—Head and Neck Surgery, 130(4), 401–408.
Diggle, P., Heagerty, P., Liang, K., & Zeger, S. (2002). Analysis of longitudinal data (2nd ed.). Oxford: Oxford University Press.
Liang, K., & Zeger, S. L. (1986). Longitudinal data analysis using generalized linear models. Biometrika, 73(1), 13–22.
Zeger, S. L., & Liang, K. (1986). Longitudinal data analysis for discrete and continuous outcomes. Biometrics, 42(1), 121–130.
McQuestion, M., Fitch, M., & Howell, D. (2010). The changed meaning of food: Physical, social and emotional loss for patients having received radiation treatment for head and neck cancer. European Journal of Oncology Nursing, 15(2), 145–151.
Larsson, M., Hadelin, B., & Athlin, E. (2003). Lived experiences of eating problems for patients with head and neck cancer during radiotherapy. Journal of Clinical Nursing, 12, 562–570.
Chera, B. S., Eisbruch, A., Murphy, B. A., Ridge, J. A., Gavin, P., Reeve, B. B., et al. (2014). Recommended patient-reported core set of symptoms to measure in head and neck cancer treatment trials. Journal of the National Cancer Institute, 106(7), JU127.
Melo Filho, M. R., Rocha, B. A., Pires, M. B. O., Fonseca, E. S., Freitas, E. M., Martelli Junior, H., & Santos, F. B. G. (2013). Quality of life of patients with head and neck cancer. Brazilian Journal of Otorhinolaryngology, 79(1), 82–88.
Hammerlid, E., Bjordal, K., Ahlner-Elmqvist, M., Boysen, M., Evensen, J., Biorklund, A., et al. (2001). A prospective study of quality of life in head and neck cancer patients. Part I: At diagnosis. Laryngoscope, 111(4), 669–680.
Molassiotis, A., & Rogers, M. (2012). Symptom experience and regaining normality in the first year following a diagnosis of head and neck cancer: A qualitative longitudinal study. Palliative and Supportive Care, 10(3), 197–204.
Duffy, S. A., Terrell, J. E., Valenstein, M., Ronis, D. L., Copeland, L. A., & Connors, M. (2002). Effect of smoking, alcohol, and depression on the quality of life of head and neck cancer patients. General Hospital Psychiatry, 24(3), 140–147.
Dooks, P., McQuestion, M., Goldstein, D., & Molassiotis, A. (2012). Experiences of patients with laryngectomies as they reintegrate into their community. Supportive Care in Cancer, 20(3), 489–498.
Siegel, R., Ma, J., Zou, Z., & Jemal, A. (2014). Cancer statistics, 2014. CA: A Cancer Journal for Clinicians, 64, 9–29.
Canadian Cancer Society’s Advisory Committee on Cancer Statistics. (2014). Canadian cancer statistics 2014. Toronto, ON: Canadian Cancer Society.
Gourin, C. G., Boyce, B. J., Vaught, C. C., Burkhead, L. M., & Podolsky, R. H. (2009). Effect of comorbidity on post-treatment quality of life scores in patients with head and neck squamous cell carcinoma. Laryngoscope, 119(5), 907–914.
Wickham, R. S., Rehwaldt, M., Kefer, C., Shott, S., Abbas, K., Glynn-Tucker, E., et al. (1999). Taste changes experienced by patients receiving chemotherapy. Oncology Nursing Forum, 26(4), 697–706.
Acknowledgments
The authors thank Asifa Mawani for her help with data collection, Danielle Wiese, Michelle Wong and Lindsay Gervais for their support with data entry and food records analysis and financial support from the Canadian Institutes of Health Research (CIHR) and Alberta Cancer Foundation (ACF) (WW), Secretaría de Educación Pública (SEP), the Government of Mexico and Consejo Nacional de Ciencia y Tecnología (CONACyT Mexico) (MAC).
Author contributions
Mirey Alvarez-Camacho, Silvia Gonella and Sunita Ghosh contributed to conception and design; Wendy V. Wismer financially supported the article; Rufus A. Scrimger and Karen P. Chu contributed to provision of study materials or patients; Mirey Alvarez-Camacho and Catherine Kubrak contributed to collection and assembly of data; Mirey Alvarez-Camacho, Silvia Gonella and Sunita Ghosh analyzed and interpreted the data; Mirey Alvarez Camacho, Silvia Gonella and Wendy Wismer wrote the manuscript ; Mirey Alvarez Camacho, Silvia Gonella, Sunita Ghosh, Catherine Kubrak, Rufus A. Scrimger, Karen P. Chu and Wendy V. Wismer finally approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alvarez-Camacho, M., Gonella, S., Ghosh, S. et al. The impact of taste and smell alterations on quality of life in head and neck cancer patients. Qual Life Res 25, 1495–1504 (2016). https://doi.org/10.1007/s11136-015-1185-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-015-1185-2