Abstract
Purpose
The purpose of this review was to examine studies of interventions for the prevention and management of taste and smell alterations (TSA) experienced by adult oncology patients.
Methods
Articles published between 1993 and 2013 were identified by searching CINAHL, MEDLINE and Food Science & Technology Abstracts (FSTA) and were included if they were in English and focused on adult oncology patients. Only interventions within the scope of nursing practice were reviewed.
Results
Twelve articles were identified for inclusion. Four research groups examined zinc supplementation, with two claiming that zinc supplementation was an effective intervention and two claiming it had no effect on TSA. The remaining research groups examined eight other interventions, with varying results. Marinol, megestrol acetate and Synsepalum dulcificum interventions appear promising.
Conclusion
Based on this review, there does not yet appear to be an effective approach for preventing or managing TSA in adult oncology patients. Although some interventions show promise, further research is necessary to determine their efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taste dysfunction in patients living with cancer is a complex problem and is associated with alterations in smell [1]. Research, assessment and development of effective interventions will be necessary to offer supportive or preventive care. In this review, we summarize literature about the treatment and management of taste and smell alterations (TSA) in adult oncology patients.
TSAs are common and can be the result of the disease or treatment [2]. Mainly related to cell damage, TSAs can be the result of altered cell structure, receptor surface changes, interruption in neural coding or a decrease in the number of normal cells [3]. Hutton et al. found that 86 % of patients with advanced cancer reported chemosensory abnormality [4]. The most common complaints were persistent bad taste, taste distortion and heightened sensitivity to odours. Patients with severe chemosensory complaints showed lower energy intakes, higher weight loss and lower quality of life scores than patients with mild or moderate chemosensory complaints [4].
Food flavours and aromas enhance the feelings of satiety and pleasure from meals and are primary reinforcers of eating; sensory stimulation derived from food is important in patients with cancer, as other sources of gratification may be less available [5]. Alterations in taste are distressing for patients and can lead to food aversions, reduced food intake and nutritional deficits [2]. Anorexia is common among people who experience TSAs and related changes in food preference [6]. Treatment side effects that impair sensory organs affect the ability to interact with the environment, which can be distressing to patients [1]. Because smell and taste are linked, and both are required for the sensation of flavour, changes to a patient’s sense of smell can affect food flavour [2]. Although cancer is reported to be associated with impaired olfactory function, very few studies have directly evaluated this effect [5].
Patients rarely address taste alterations, and health care providers often consider them a side effect that is unavoidable [7]. In this summary, we identify gaps in knowledge, evaluate current evidence and formulate suggestions for research to identify and evaluate interventions for the prevention and management of TSA in adult oncology patients.
Methodology
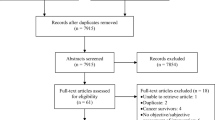
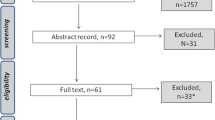
This state-of-the-art review [8] was based on a search of literature indexed in the Food Science & Technology Abstracts (FSTA), MEDLINE and CINAHL databases. The search terms: taste, smell, dysgeusia, chemosensory, neoplasm and cancer yielded 226 articles. English language studies about TSA published between 1993 and 2013 were selected for further evaluation (n = 42). The reference lists of these articles were reviewed for additional sources related to this topic, yielding an additional 37 articles. Only articles describing interventions within the scope of nursing practice were included. Of the 79 articles considered, 12 focused on TSA in adult oncology patients and included interventions that were within the scope of nursing practice and were thus included in this review (see Appendix Table 1).
Results
This review examines evidence on strategies for prevention and treatment of TSAs. Of the 12 included studies, six tested supportive TSA interventions [9–14], four tested interventions to prevent TSA [15–18] and two tested both supportive and preventive interventions [19, 20]. The primary end point of 11 studies was the effects of the intervention on TSA. The primary end point of the final study was the efficacy of an intervention on radiotherapy (RT)-induced mucositis; the effect of this intervention on TSA was a secondary end point [16]. Nine different interventions were examined.
Zinc supplementation
Zinc sulfate was the only intervention included in multiple trials. The authors of these studies reported conflicting results. The specific mechanism of zinc in taste perception is unknown. As a growth factor and component of the salivary enzyme carbonic anhydrase VI, zinc contributes to sensory stem cell stimulation [11]. In the first study, zinc sulfate (45 mg po tid) appeared to provide benefit to patients [12]. Participants were given zinc sulfate tablets or placebo three times daily after meals starting with the onset of perceived TSA. This regime was followed during the course of external radiotherapy (ERT) and up to 1 month after ERT termination. Researchers assessed taste acuity by measuring detection and recognition thresholds using a standard three-stimulus drop technique for four taste qualities (salty, sweet, sour and bitter assessed using aqueous solutions of NaCl, saccharose, HCl and urea, respectively). In this study, the intervention was considered effective because group threshold differences for bitter detection and for salt recognition during ERT, as well as salt, sweet and sour recognition at the end of ERT, were significantly lower than the control group, indicating higher taste acuity. The secondary end points (decline and recovery of taste acuity) favoured the zinc group, but the differences were not statistically significant. Zinc sulfate was well tolerated.
A second research group explored the usefulness of IV zinc (0.33 mg daily) during chemotherapy as a strategy for preventing TSA [18]. In this study, participants in the experimental group were given parenteral zinc, administered with chemotherapy. Taste disorder was evaluated using an electrogustometer. An increase in taste threshold was defined as the indicator of an adverse alteration in taste. Taste threshold was measured in two different nerve areas before and after day three and after 1, 2 and 4 weeks of chemotherapy. The authors reported a statistically significant change in the taste threshold between the experimental and control group at 2 and 4 weeks (p < 0.05), in the chorda tympani nerve area. Differences were noted in the glossopharyngeal nerve area at 2 weeks, but were not statistically significant, and there was no difference in this nerve area at 4 weeks. The authors concluded IV zinc may prevent TSA.
In the third study, participants in the experimental group (n = 61) were given zinc sulfate tablets (45 mg) three times daily [20]. Their scores on a previously validated taste questionnaire and patient-reported taste alterations were used as measurement tools. The zinc sulfate/placebo was started within 7 days of the initiation of RT and was given for 4 weeks after RT completion, for a total treatment period of 2 months. Zinc sulfate administration reduced the median interval to the appearance of TSA (p = 0.09), but did not improve post-treatment time to taste recovery compared to the placebo group (p = 0.16). In this study, zinc sulfate administration prior to therapy appears to have had a negative effect on the interval to taste recovery and the authors recommended that zinc sulfate not be used to treat or prevent TSA.
The most recent trial comparing zinc with placebo for chemotherapy-related TSA revealed no significant difference between the use of oral zinc sulfate (220 mg bid) and placebo [10]. To measure improvement of TSA, a zero-to-100 scale was used. There was no statistically significant improvement in loss or distortion of taste or smell with the addition of zinc. There was a trend toward loss of taste improvement over time in both groups and a non-significant worsening in loss of smell over time in the zinc group. These results were consistent with other studies that failed to demonstrate prevention of TSA with similar doses of zinc.
The studies in favour of zinc supplementation used validated objective measurement tools and compensated for smaller sample sizes with appropriate statistical analyses [12, 18]. It is worth noting that all participants were given standardized meals and age was identified as a possible confounding variable [18]. Alternatively, the studies that did not support the use of zinc sulfate had relatively larger samples and relied on subjective measures to determine group differences [10, 20]. Objective evaluation of taste and smell testing was not used, but patient report is considered the gold standard in clinical care [10, 20]. The route of zinc administration and dosing varied, which may have contributed to the conflicting results.
Megestrol acetate
Megestrol acetate (480 mg daily) was administered daily to weight-losing patients with advanced cancer experiencing TSA [19]. Megestrol acetate acts predominantly as a potent agonist of progesterone receptors to exert its effects [21]. Subjective taste and smell were assessed at baseline and at the 1-, 2- and 3-month points using a non-validated brief questionnaire scored from one to five, according to the degree of the loss or change for each parameter. Using this instrument, significant improvements were seen in loss of taste (p = 0.000) and smell qualities (p = 0.02) in the megestrol acetate (MA) group compared to the placebo group after 3 months. Although megestrol acetate did have a statistically significant positive effect on TSA, there were several methodological problems with this study. First, the authors changed the tool used for assessment of TSA part way through the study due to lack of patient compliance/dissatisfaction [19]. In addition, the groups were heterogeneous (within and between) in relation to nutritional supports (p = 0.001, 0.01), age (p = 0.02) and primary tumour site (p = 0.03). Therefore, it is difficult to exclude the possibility of confounding variables affecting the outcome.
Oral glutamine
Cancer patients receiving taxane-based chemotherapy for the first time were given oral glutamine in an attempt to prevent TSA [17]. Glutamine is vital in nitrogen transfer between tissues and synthesis of RNA, DNA, and some neurotransmitters. Patients were randomized to receive oral glutamine (30 g daily) or placebo (maltodextrin) from day one of chemotherapy. Dysgeusia was measured with a visual analogue scale. Objective taste (sour, sweet, salty, bitter recognition) and toxicity were also assessed. Daily dysgeusia scores did not differ between the groups. These results may have been due to the relatively small sample, with 11 of 41 participants not completing the trial. Oral glutamine was not effective in reducing TSA.
Amifostine
Amifostine is a thiophosphate prodrug, which protects normal organs and tissues from oxidative damage [3, 22]. A prospective follow-up study of more than 5 years was performed to determine the preventive effect of amifostine on long-term chemo-radiotherapy-associated toxicities, including TSA [15]. Taste was measured using an electrogustometer. Altered taste was reported by 33 % of patients. Compared to the control group, loss of taste was significantly lower in the amifostine group (0.381 versus 0.464, p = 0.04). Amifostine administration was beneficial in reducing TSA up to 1 year after treatment, but showed no benefits after that time point. The large treatment effect and trial duration lend credibility to this study. Unfortunately, the non-randomized design and the specific treatment modalities limit the generalizability of the conclusions [15].
Self-care intervention and patient strategies to manage taste and smell alterations
Each of the 42 patients participating in the study was given a taste change suggestion sheet and an educational intervention [11]. Examples of items on the suggestion sheet included adding more/using less seasoning, eating bland foods, avoiding spicy foods and sucking on hard candy. The taste change survey developed for this study, based on the results of a large study of 254 patients undergoing chemotherapy, was used to capture the nature of taste changes, presence of other symptoms and satisfaction with the suggested strategies. Two open-ended questions elicited foods patients avoided and additional strategies used to manage taste and smell changes. Initially, patients were eligible to participate if their diagnosis was lymphoma, breast, lung or ovarian cancer and if they were receiving doxorubicin, carboplatin, cisplatin or cyclophosphamide. The authors felt the strict inclusion criteria affected accrual, and ultimately, any patient with cancer and undergoing chemotherapy was included. Relationships were found between types of taste changes experienced and the helpfulness of particular strategies. For example, patients bothered by a bitter taste reported avoiding beef and eating smaller meals more frequently to be more beneficial than patients not bothered by bitter taste. Use of a taste change suggestion sheet encouraged self-care and provided strategies for managing taste changes that were helpful for the majority of participants. Although the study was designed to measure patient responses pre- and post-intervention, no significant differences were found between time points [11]. The 2-week study interval may have been too short to determine changes over time. It is also possible that the suggestion sheet was confusing for patients, as it included negative and positive strategies. In addition to this, the authors’ instruments were not validated and may not have been sufficiently sensitive to capture changes in patient experiences. The results of this study were also restricted by the small sample size and lack of comparison to a standard of care group.
Dietary counselling and flavour enhancement
The experimental group was given 13 bottles of various food flavours and educated on their ability to counteract unpleasant tastes such as bitter and compensate for TSA [13]. The flavours used were familiar, versatile, natural, sodium free and non-allergenic (contain no protein). Patients were encouraged to masticate their food well to increase salivation and release odorous compounds. Pushing food against the soft palate, which has many taste buds, was also encouraged. Meal planning instruction was given, with emphasis on textures and temperatures. Subjects in both groups were instructed to avoid certain foods if they had a dry or sore mouth (citrus, spicy). Taste and odour assessments were measured using a taste and smell questionnaire, taste threshold testing and olfactory threshold testing. Detection and recognition thresholds were determined using an ascending forced-choice method of limits. The protocol encouraged subjects to amplify the flavour of their foods individually, in order to compensate for TSA. Control subjects received nutritional information only. Two taste/odour measures demonstrated a statistically significant difference between groups [13]. For sucrose detection thresholds, the experimental group had significantly higher thresholds than the control group across all time points (p 0.01). Phenethyl alcohol (odour) detection thresholds were also lower in the experimental group across all time points (p 0.05). The data collected in the taste and smell questionnaire showed a significant interaction between the experimental group and time point in relation to self-reported ability to taste (p < 0.05). At 1 and 3 months, a negligible difference was seen between groups. However, by the 8-month point, 78 % of the experimental group reported a good to excellent ability to taste, compared to 50 % in the control group. The same trend was seen for perceived ability to smell, although it was not statistically significant. At the 8-month point, there were no substantial differences between the experimental and control groups’ ability to taste, but there were significant differences in perception of ability. High attrition rates were noted in both groups, and the results of this study may not be generalizable to elderly cancer patients, as those who dropped out were older and in poorer health [13]. The authors collected subjective and objective data at multiple assessment points, confirming the combination of flavour enhancement, chemosensory education and nutritional information may have a positive effect on TSA.
Bethanechol
The authors performed a secondary analysis of data on 36 patients to evaluate the effect of bethanechol on salivary gland dysfunction in head and neck cancer patients receiving RT. Because bethanechol has been shown to stimulate saliva production, the authors hypothesized that it would prevent taste loss [16]. Oral bethanechol (25 mg) was administered thrice daily from the start to the completion of RT to reduce RT-induced mucositis, candidiasis and TSA. Although not statistically significant, almost 70 % of the bethanechol group compared to 95 % of those in the placebo group (artificial saliva) reported taste loss during RT. There was no difference between the two groups in the time course development of taste loss. The authors concluded that bethanechol did not prevent RT-associated taste loss, mucositis or candidiasis. Daily timing of bethanechol with respect to RT was not standardized, and due to the different formulations of the experimental and placebo products, it was not possible for the study to be double blinded. The study should be repeated with a larger sample size in order to evaluate possible benefits of bethanechol.
Synsepalum dulcificum (miracle fruit)
Miraculin, a protein in the fruit S. dulcificum, binds to taste receptors in an acidic environment to generate a sweet sensation, thus improving the palatability of foods by masking some unpleasant tastes for a short duration [14]. The study consisted of a convenience sample of eight patients and used a crossover design. Miracle fruit or a placebo of dried cranberries was consumed immediately prior to meals for 2 weeks. Both formulations were packaged similarly by the pharmacy. Participants recorded food and drink intake in daily food diaries and rated taste changes with each food as the same, better or worse than their previous experience. Participants were also asked to record if the changes lasted throughout the meal. In addition to individual food choices, patients were asked to trial common sour- or bitter-tasting foods such as lemons or grapefruit. Study participants who ingested miraculin before meals reported positive taste changes and some reported increased food intake compared to the placebo group. The sample size was too small to be analysed statistically, but the results are supportive of the hypothesis that miracle fruit is able to reduce the impact of TSA and make foods more palatable.
Marinol
This was a proof-of-principle study and was intended to provide direction for future trials [9]. Adult patients with advanced cancer experiencing TSA were given marinol (delta-9-tetrahydrocannabinol (THC) 2.5 mg) or a placebo twice daily, to determine its effect on TSA. THC increases motivation to eat energy dense foods and enhances food enjoyment via endocannabinoid receptors that stimulate the orosensory reward pathway. Multiple tools of assessment were used, including a taste and smell survey, 3-day food record, appetite (SLIM) and macronutrient preference assessments, quality of life questionnaire (FAACT), Edmonton Symptom Assessment Scale (ESAS) and an interview. After 14 days, the patients taking marinol had increased protein intake as a proportion of total calories compared to those on placebo. Chemosensory perception (p < 0.001 to 0.026), appetite (p = 0.05), relaxation (p = 0.045) and quality of sleep (P = 0.025) also showed statistically significant improved responses to marinol. Patients taking marinol were two times more likely to report “better taste” than placebo (p = 0.026). Marinol shows promise and may be useful in treating TSA and increasing oral intake. The number of assessment tools reduced the potential for bias. Potential limitations were the sample size and the short duration of the trial. These results are promising and warrant further investigation.
Discussion
The articles included in this review are an indication of the growing interest in interventions for the prevention and treatment of TSA in the adult oncology patients. Some methodological issues limited the study findings and thus reduced the ability to compare study results. First, most of the studies in this review had small samples drawn from populations where TSAs were most common, limiting generalizability. The samples of the randomized control trials (RCT) included in this review ranged from 12 to 107 participants [9, 10, 12, 13, 16–20]. The remaining studies had samples ranging from 8 to 851 participants [11, 14, 15]. Inadequate sample size obscures differences between groups that may exist. Power calculations were only reported for two studies, one at 85 % [10] and the other at 90 % [20]. Power was not relevant in the trial by Brisbois et al., as it was a proof-of-principle pilot trial [9], and power was not discussed in the remaining studies [12, 13, 16–19]. Second, generalizability was reduced by the study of specific populations; four studies included only individuals with head and neck cancer [12, 15, 16, 20], and another study limited recruitment to patients receiving docetaxel and paclitaxel [17]. Only three of the studies in this review were representative of the general cancer population and various treatment regimes [9, 10, 14]. Although there are merits to both approaches, generalizability is compromised with a homogeneous sample.
Blinding is a common strategy used to increase the internal validity of a study. Patients were double blinded in five of the RCT studies [9, 10, 12, 17, 20] in this review and were blinded to treatment in one study [19]. It was unclear if the patients were blinded in the trial by Yamagata et al. [18]. The patients were not blinded in the remaining two RCTs [13, 16], and thus, participants’ responses may have been influenced by their knowledge of whether they were receiving the experimental treatment.
Factors such as study design, dose variations, measurement approaches and primary end points influence whether study results can be compared. The studies included in this review used a variety of tools to assess TSA. Although nine of the studies in this review were randomized control trials [9, 10, 12, 13, 16–20], the variables studied and measures used were not consistent. The remaining studies included a prospective follow-up study [15], and two studies that used quasi-experimental designs with reported study lengths ranging from 22 days to 8 months [11, 14].
We recommend that future researchers use an RCT design with an adequate sample size and collect data using both validated objective assessments and validated patient-reported outcomes. This approach would ensure that the data includes a more comprehensive view of participants’ experiences of TSA. Potential confounders such as oral rinses, nutritional supplements and variation in dosing should be controlled in the analysis. Unfortunately, there are few studies on the pathophysiological features of TSA. Multidisciplinary research teams are strongly urged to design their studies in ways that make it possible to explore these underlying mechanisms. These teams should also explore the importance of oral assessment and the impact of oral hygiene on TSAs and study outcomes. Based on the findings of this review, larger trials evaluating the efficacy of marinol, megestrol acetate and S. dulcificum as treatments of TSA are warranted.
Selection of a single intervention to alleviate TSAs is limited by our lack of knowledge of the precise mechanisms causing TSAs. Suggested mechanisms include treatment-associated damage to receptor cells and interference with neuronal activities, bitter and metallic sensations from antineoplastic and other drugs, oral mucositis, reduced taste cell regeneration due to zinc depletion and unpleasant sensations generated by oral health issues such as lack of oral hygiene and infections [1, 23, 24]. Further, sensory perception of a food is influenced by all of its sensory properties, including texture, appearance, and food presentation [25].
This review has several implications for practice and education. Nurse practitioners are well established in oncology care in Alberta, but the scope and availability of nurse practitioners varies among other jurisdictions. In some settings where nurse practitioners are not available, ordering these medications would be the responsibility of the attending physician. Regardless of who prescribes, decisions regarding interventions should be made in close collaboration with all members of the health care team. Nurses should be educated about TSA and taught simple strategies for assessment that could be included in routine patient care, such as routinely asking patients if they have noticed changes in taste or smell. Assessment of TSA is critical because it may also be linked to other common problems such as weight loss. If there are significant changes in taste and smell, patients should be referred to a dietician for further evaluation and possible diet modifications. The multidisciplinary team should also be encouraged to advise patients of the potential for TSAs, as distress may be worse if such changes are unexpected [6].
Limitations
This state-of-the-science review was limited to research published in the last 20 years, which may have had an impact on the interventions included and the conclusions that were drawn. This review was also limited to interventions that were within the scope of professional nursing practice and, thus, excluded strategies such as limiting the size of the field during radiation therapy.
Conclusions
The purpose of the review was to provide a comprehensive summary of studies on therapies and supportive strategies that nurses could use to prevent or manage TSA experienced by adult oncology patients. Nine interventions were identified and examined. The most promising interventions are marinol, megestrol acetate and S. dulcificum, but future research with larger samples are needed to confirm their efficacy. In the meantime, nurses should ensure that patients are aware of the potential for TSA and should include assessment of TSA in their routine practice, given the potential links to other adverse patient outcomes such as weight loss.
References
McLaughlin L, Mahon SM (2012) Understanding taste dysfunction in patients with cancer. Clin J Oncol Nurs 16(2):171–178
Ravasco P (2005) Aspects of taste and compliance in patients with cancer. Eur J Oncol Nurs 9:S84–S91
Hovan AJ, Williams MP, Stevenson-Moore P, et al (2010) A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer 18:1081–1087
Hutton JL, Baracos VE, Wismer WV (2007) Chemosensory dysfunction is a primary factor in the evolution of declining nutritional status and quality of life in patients with advanced cancer. J Pain Symptom Manag 33(2):156–165
Yakirevitch A, Bercovici M, Migirov L, et al (2006) Olfactory function in oncologic hospice patients. J Palliat Med 9(1):57–60
Mahmoud FA, Aktas A, Walsh D, et al (2011) A pilot study of taste changes among hospice inpatients with advanced cancer. Am J Hosp Palliat Med 28(7):487–492
Zabernigg A, Gamper E, Giesinger JM, et al (2010) Taste alterations in cancer patients receiving chemotherapy: a neglected side effect? Oncologist 15(8):913–920
Grant MJ, Booth A (2009) A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libr J 26:91–108
Brisbois TD, de Kock IH, Watanabe SM, et al (2011) Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double blind, placebo-controlled pilot trial. Ann Oncol 22:2086–2093
Lyckholm L, Heddinger SP, Parker G, et al (2012) A randomized, placebo controlled trial of oral zinc for chemotherapy-related taste and smell disorders. J Pain Palliat Care Pharmacother 26(2):111–114
Rehwaldt M, Wickham R, Purl S, et al (2009) Self-care strategies to cope with taste changes after chemotherapy. Oncol Nurs Forum 36(2):E47–E56
Ripamonti C, Zecca E, Brunelli C, et al (1998) A randomized, controlled clinical trial to evaluate the effects of zinc sulfate on cancer patients with taste alterations caused by head and neck irradiation. Cancer 82(10):1938–1945
Schiffman SS, Sattely-Miller EA, Taylor EL, et al (2007) Combination of flavor enhancement and chemosensory education improves nutritional status in older cancer patients. J Nutr Health Aging 11(5):439–454
Wilken MK, Satiroff BA (2012) Pilot study of “miracle fruit” to improve food palatability for patients receiving chemotherapy. Clin J Oncol Nurs 16(5):E173–E177
Buntzel J, Glatzel M, Mucke R, et al (2007) Influence of amifostine on late radiation-toxicity in head and neck cancer: a follow up study. Anticancer Res 27:1953–1956
Jham BC, Chen H, Carvalho AL, et al (2009) A randomized phase III prospective trial of bethanechol to prevent mucositis, candidiasis, and taste loss in patients with head and neck cancer undergoing radiotherapy: a secondary analysis. J Oral Sci 51(4):565–572
Strasser F, Demmer R, Böhme C, et al (2008) Prevention of docetaxel- or paclitaxel-associated taste alterations in cancer patients with oral glutamine: a randomized, placebo-controlled, double blind study. Oncologist 13(3):337–346
Yamagata T, Nakamura Y, Yamagata Y, et al (2003) The pilot trial of the prevention of the increase in electrical taste thresholds by zinc containing fluid infusion during chemotherapy to treat primary lung cancer. J Exp Clin Cancer Res 22(4):557–563
Erkurt E, Erkisi M, Tunali C (2000) Supportive treatment in weight-losing cancer patients due to the additive adverse effects of radiation treatment and/or chemotherapy. J Exp Clin Cancer Res 19(4):431–439
Halyard MY et al (2007) Does zinc sulfate prevent therapy-induced taste alterations in head and neck cancer patients? Results of phase III double blind, placebo-controlled trial from the North Central Cancer Treatment Group (N01C4). Int J Radiat Oncol Biol Phys 67(5):1318–1322
Teulings FA, van Gilse HA, Henkelman MS, et al (1980) Estrogen, androgen, glucocorticoid, and progesterone receptors in progestin-induced regression of human breast cancer. Cancer Res 40(7):2557–2561
Kouvaris JR, Kouloulias VE, Vlahos LJ (2007) Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist 12(6):738–747
Hong JH, Omur-Ozbek P, Stanek BT, et al (2009) Taste and odor abnormalities in cancer patients. J Support Oncol 7(2):58–65
Epstein JB, Thariat J, Bensadoun RJ, et al (2012) Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin 62(6):400–422
Lawless HT, Heymann H (2010) Sensory evaluation of food: principles and practices, 2nd edn. Chapman & Hall, New York, p. 596
Conflict of interest
The authors declare that they have no competing interests. The authors have full control of all primary data and permit the journal to review the data if requested.
Author information
Authors and Affiliations
Corresponding author
Appendix A
Appendix A
Rights and permissions
About this article
Cite this article
Thorne, T., Olson, K. & Wismer, W. A state-of-the-art review of the management and treatment of taste and smell alterations in adult oncology patients. Support Care Cancer 23, 2843–2851 (2015). https://doi.org/10.1007/s00520-015-2827-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2827-1