Abstract
Key message
In Araucaria angustifolia, the seed scale is part of the ovule, the female gametophyte presents a monosporic origin and arises from a coenocytic tetrad, and the pollen tube presents a single axis.
Abstract
The seed cone of conifers has many informative features, and its ontogenetic data may help interpret relationships among function, development patterns, and homology among seed plants. We reported the seed cone development, from pollination to pre-fertilization, including seed scale, ovule ontogeny, and pollen tube growth in Araucaria angustifolia. The study was performed using light microscopy, scanning electron microscopy, and X-ray microcomputed tomography (μCT). During the pollination period, the ovule arises right after the seed scale has emerged. From that event to the pre-fertilization period takes about 14 months. Megasporogenesis occurs three weeks after ovule formation, producing a coenocytic tetrad. At the same time as the female gametophyte's first nuclear division begins, the pollen tube grows through the seed scale adaxial face. Until maturity, the megagametophyte goes through the free nuclei stage, cellularization stage, and cellular growth stage. Along its development, many pollen tubes develop in the nucellar tissue extending straight toward the female gametophyte. Our observations show that the seed scale came out of the same primordia of the ovule, agreeing with past studies that this structure is part of the ovule itself. The formation of a female gametophyte with a monosporic origin that arises from a coenocytic tetrad was described for the first time in conifers, and the three-dimensional reconstruction of the ovule revealed the presence of pollen tubes with only one axis and no branches, highlighting a new pattern of pollen tube growth in Araucariaceae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gymnosperms are a group of distantly related seed plants with a fossil record extending back to the mid-Devonian (Gerrienne et al. 2004). They differ from angiosperms because their ovule is entirely or partially exposed during the pollination stage, forming naked seeds. The main gymnosperm lineages include cycads, Ginkgo, conifers, and Gnetales (Ran et al. 2018) and present a wide variety of reproductive systems (Breygina et al. 2021), showing different ovule morphology, pollen structure, and pollen tube behavior (Biswas and Johri 1997).
The largest group of gymnosperms are the conifers which present two kinds of reproductive structures: pollen cones and seed cones (Farjon 2017). While pollen cones produce and disperse the pollen grains, the seed cones play a great variety of roles, such as producing the ovule, housing the fertilization process, and protecting and dispersing the seeds (Leslie 2011). According to Miller (1999), the seed cone structure has many informative features that allow extant families to be distinguished.
Araucaria is a conifer genus identified in the fossil record since the Jurassic (Panti et al. 2012), and all 19 species are recognized as living fossils. This genus presents two endemic species from South America, Araucaria araucana (Molina) K.Koch and Araucaria angustifolia (Bertol.) Kuntze (Dettmann and Clifford 2005) that have likely changed very little since the Jurassic (Farjon 2017). Araucaria belongs to Araucariaceae, a family primarily representing Southern Hemisphere distribution and comprising two other genera, Agathis and Wollemia (Farjon 2010). The most recent combined morphological and molecular phylogenetic analyses retrieved Agathis and Wollemia as a monophyletic group (i.e., agathioid clade), sister to Araucaria (Escapa and Catalano 2013).
The Araucaria seed cone is the largest of the conifers (Gleiser et al. 2019), and only the two South American species and one from Australia, together with several species of Pinus, produce seeds for human consumption (Farjon 2017).
The cones of Araucaria are known to bear a single and inverted ovule which is produced in bract/seed scale complexes. The comprehensive study of Burlingame (1913, 1914, 1915) analyzed several aspects of the reproductive cycle of A. angustifolia. Still, many aspects were not completely described, such as the seed scale, ovule development, and megasporogenesis process.
Therefore, many aspects of the embryonic ontogenesis of A. angustifolia were updated by Goeten et al. (2020), and Herting and Stützel (2020) described the A. araucana seed scale ontogeny discussing the homology of this structure. The overall pollination mechanism of Araucaria was analyzed, which includes wind pollination, the presence of non-saccate pollen, the absence of pollination drops, and extra-ovular pollen germination (e.g., Burlingame 1915; Haines et al. 1984; Owens et al. 1998), in which pollen grains laid on the seed scale, germinate and grow toward the seed cone axis straight to the micropyle.
Pollen tube growth in the nucellus has only been precisely detailed in Agathis (Owens et al. 1995a), which indicated that pollen tubes meander and branch within the nucellar tissue, suggesting that besides the siphonogamic role, the pollen tube possesses a secondary haustorial function. Because of the lack of information about pollen tube growth in other species, this developmental pattern was generalized to Araucariaceae.
Although the study of reproductive biology of Araucariaceae has been increasing significatively in the last few decades, many questions remain. Thus, reinvestigating specific developmental processes, such as seed scale and ovule ontogeny, megasporogenesis and megagametogenesis, and pollen tube growth, is still necessary. Nevertheless, those ontogenetic data may aid in interpreting homologies among seed plants and help understand the relationships among function, developmental patterns, and the evolution of reproductive morphology.
Araucaria angustifolia, known as Brazilian Pine or Monkey Puzzles, is a Critically Endangered conifer (IBAMA 1992; Baillie et al. 2004), having lost 97% of its range in the last century due to human activities (Mantovani et al. 2004). A. angustifolia also plays an important role in the Atlantic Forest ecology, hosting many plant species in its canopy and providing shelter and nutritional resources to the wild fauna through its seed (Koch and Corrêa 2002).
In this article, we report the seed scale ontogeny, ovule formation, and development, including the megasporogenesis and megagametogenesis processes and pollen tube growth. Furthermore, given the difficulty of visualizing how the pollen tube grows inside the seed cone using traditional serial sections, a non-invasive approach using X-ray microcomputed tomography (µCT) was also performed to generate a high-resolution 3D model of the pollinated ovule. This data can be useful in enhancing breeding techniques and pollination success with fertilized cones and seed production and increases chances for successful management of the natural environment of this tree. Lastly, understanding the morphology of the reproductive structure in Araucaria is important to comprehend how reproductive strategies evolved in Araucariaceae.
Materials and methods
Plant material
The seed cones of A. angustifolia were obtained from three different plants of Nova Petropólis City, Rio Grande do Sul, Brazil. The collections were performed over 18 months (from June to November of the next year), starting at the beginning of the winter. From the first to the third month and from the seventh to the eighteenth months, the collections were made monthly, except that the fourth to sixth-month samples were taken weekly or every two weeks. The voucher specimens were deposited in the Federal University of Rio Grande do Sul's ICN herbarium under numbers 171957 and 171958.
Light microscopy (LM)
To prepare histological sections, the material was fixed in 1% glutaraldehyde and 4% formaldehyde in 0.1 M sodium phosphate buffer, pH 7.2 (McDowell and Trump 1976). Then it was washed in sodium phosphate buffer (0.1 M, pH 7.2) Gabriel (1982), dehydrated in an ethanol series (10–100%), and embedded in 2-hydroxyethyl – methacrylate (Gerrits and Smid 1983). Sections were cut 2–4 µm thick, using a Zeiss rotatory microtome, and stained with Toluidine Blue O 0.05%, pH 4.4 (O’Brien and McCully 1981). Photomicrographs were acquired under a bright field using a Leica DMR microscope and a Leica M165 FC stereomicroscope, with an AxioCam HRc digital camera and AxioVision LE (Carl Zeiss® Meditec AG, Oberkochen, Germany) software.
Chemical composition was detected using different histochemical tests (Supplementary material 1): IKI (Lugol’s solution) (Johansen 1940) to detect starch; Ruthenium Red (Johansen 1940) for polysaccharide acids and pectic acids; Alcian Blue 8GX (Lillie 1965) for mucopolysaccharides; Periodic Acid Schiff-PAS (Sass 1951) for total polysaccharides and Calcofluor White (Hughes and McCully 1975) for cellulose; Aniline Blue (Martin 1959) for callose.
Scanning electron microscopy (SEM)
For scanning electron microscopy (SEM), the samples were dehydrated in an acetone series and dried using the critical-point method (Gersterberger and Leins 1978) with BAL-TEC, CPD 030 equipment. The samples were then mounted onto stubs and coated with gold using a BAL-TEC SCD 050 sputtering device. Observations and electron micrographs were performed with a JEOL JSM 6060 microscope under 30 kV.
X-ray microcomputed tomography (μCT)
The seed cone in the early stage of development was collected and fixed in 1% glutaraldehyde and 4% formaldehyde in 0.1 M sodium phosphate buffer, pH 7.2 over 72 h (McDowell and Trump 1976), and washed in 0.1 M sodium phosphate buffer, pH 7.2. After washing, the sample was dehydrated in an acetone series and dried using the critical-point method (Gersterberger and Leins 1978) with BAL-TEC, CPD 030 equipment.
For the μCT analyses, the material was mounted in a plastic sample holder, and the scanning was performed in SkyScan 1172 (Bruker-microCT, Kontich, Belgium). Source voltage and current were 40 kV and 250 μA, respectively, and no filter was applied. Exposure time was 1700 ms, resulting in 2657 slices with 2.06 μm voxel size. To perform the 3D reconstruction and virtual sectioning, the open-source FIJI, a distribution of ImageJ (Schindelin et al. 2012), was used. The Segmentation plug-in was used to create binary masks corresponding to different structures in the seed cone (Schindelin et al. 2012). A detailed description of the image treatment and the three-dimensional reconstructions is given by (Nogueira et al. 2017, 2019; Palombini et al. 2020).
Results
Morphology of the Seed Cone development
At the beginning of the winter, the seed cone was in the early stage of development and presented a plagiotropic position in the tree branch (Fig. 1a). The seed cone is composed of the cone axis and bracts that are helically arranged (Fig. 1b, d). The bract presents an ensiform shape in outline and does not have any photosynthetic portion. At this moment, the seed cone was nested and completely covered by the regular leaves.
Stages of development of Araucaria angustifolia seed cone. a Regular leaves involve seed cone in the early stage of development. b Transversal section of seed cone in the early stage of development showing the cone axis and bracts. c–e Stages of seed cone exposure by the regular leaves and subtending leaves. f Seed cone in an advanced stage of development. g Detail of adaxial view of the bract with a photosynthetic distal portion and a fertile proximal portion. Legend: Regular leaves (rl), cone axis (ca), bracts (br), subtending leaves (sl), distal portion (dp), proximal portion (pp)
At the end of the winter, the seed cone started to emerge from the regular leaves and subtending leaves (Fig. 1c), and in the early spring, the seed cone was completely free to receive the pollen grains in the pollination period (Fig. 1d, e).
From the spring to the following spring, the seed cone keeps growing and developing its seed cone structures, preparing for the fertilization event, which occurs 14 months after the pollination period (Fig. 1f). From the pollination onwards, the distal portion of each bract is acuminated and green, and the median and the proximal portion, where the ovule develops, remains non-photosynthetic (Fig. 1g).
Supplementary material 2 indicates the sample dates and the sample size related to the reproductive stages of A. angustifolia.
Ovule and pollen tube anatomy development
In the early spring, the seed scale primordium arose in the adaxial and axillary portion of the bract (Fig. 2a). Right after the seed scale formation, the nucellus primordium emerged in the most proximal portion of the seed scale being oriented along its longitudinal axis (Fig. 2b). At this moment, it was possible to observe that the distal portion of the seed scale is detached from the bract, and its proximal portion is fused to it. Some days later, the seed scale elongated toward the distal portion of the bract (Fig. 2c). Subsequently, the integument primordium arose as a slight bulge evidencing the micropyle orientation toward the cone axis (Fig. 2d). The seed scale and the ovule kept developing in the following weeks and increased in length and width (Fig. 2e).
Longitudinal section of the seed cone unit of Araucaria angustifolia and its stages of development in light microscopy. a Formation of the seed scale in the proximal portion (pp) of the bract. b Nucellus initiation and c enlargement of seed scale. d Integument formation and e, f seed cone unit growth and development of the third bulge (tb). g Nucellus with megaspore mother cell (mmc), h nucellus with the dyad, and i with coenocytic tetrads. Legend: Bract (br), seed scale (sc), nucellus (nc), integument (in), megaspore mother cell (mmc), the dyad (dy), tetrad (te)
Within three weeks from the integument formation, the nucellus elongated and protruded through the micropyle towards the cone axis. A third bulge of cells appeared distally to the integument (Fig. 2f). At this moment, the megaspore mother cell (MMC) differentiated within the nucellus, presenting an elongated shape and a central nucleus (Fig. 2g). The first meiotic division occurs, forming the dyad with a thick cell wall, however, no cell cross-wall isolates its nuclei (Fig. 2h). The dyad immediately enters in the second meiotic division producing a coenocytic tetrad of megaspores with a thick and pectic megaspore cell wall and no internal cross-wall isolating these nuclei (Fig. 2i). No callose compound was found in the megaspore wall during megasporogenesis.
Subsequently, the seed cone unit develops even more (Fig. 3a). The nucellus elongates, the same as the megaspore in its interior, and the nucellar cells adjacent to the megaspore appear compressed by its growth. The megaspore presented a dense cytoplasm and bore one prominent and viable nucleus (Fig. 3b) and three degenerative nuclei, which underwent nuclear shrinkage (Fig. 3c).
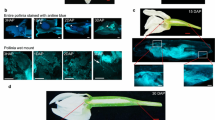
Seed cone development of Araucaria angustifolia shows the ovule's longitudinal section in light microscopy and pollen tube growth in scanning electron microscopy. a Ovule general overview in the megasporogenesis phase. b Coenocytic megaspore showing viable nucleus (n) and in (c) degenerative nuclei (dn). d, e Four-nucleus phase of the megagametophyte (mg), with megaspore wall (mw), nucleus (nu) and in (e) second nucleus detail. (f) Overview of seed scale adaxial face showing pollen tube growth (g) toward micropyle. h Coenocytic megagametophyte in 16 nuclei phase and i nuclei detail. j, k ovule with pollen tubes at the micropyle and l pollen tube nuclei (tn) detail. m Nucellar tip cells with starch grains. Legend: Megaspore (m), Bract (br), integument (in), micropyle (mi), pollen tube (pt), starch grains (sg)
Some days later, the female gametophyte expands and undergoes megagametogenesis presenting four conspicuous nuclei with prominent nucleoli, two at the micropylar position and the other two in the central position. Figure 3d and e show two of the four nuclei. The female gametophyte wall is thin and stained purple with Toluidine Blue O. During the same period, the pollen tube was visible in the seed cone unit’s adaxial face elongating toward the cone axis, straight to the micropyle (Fig. 3f, g). The female gametophyte kept in development, and after two weeks, it bore more than 16 free large nuclei. However, no mitotic divisions were observed. Figure 3h and i show two of the 16 nuclei. At the end of the spring, many pollen tubes penetrated the micropyle (Fig. 3j, k), and each pollen tube contained many nuclei (Fig. 3l). In this stage, the lugol reaction also evidenced the presence of starch grains in the nucellar tip (Fig. 3m).
Throughout the summer, the female gametophyte kept growing and forming hundreds of free nuclei. The nuclei present a conspicuous nucleolus and are inserted in a thin layer of cytoplasm, which revest the inner wall of the coenocytic female gametophyte (Fig. 4a, b). The growth of the female gametophyte obliterates the nucellar cells surrounding it. However, the surrounding cells that remain intact present a denser cytoplasm when compared to the other nucellar cells (Fig. 4c).
Longitudinal section of the megagametophyte and pollen tube of Araucaria angustifolia in light microscopy. a Coenocytic megagametophyte overview, with b cytoplasmatic layer, and c nucellar cells detail. d Ovule with deeply inserted pollen tubes and e pollen tube tip detail. Histochemical tests in the pollen tube wall (pw) indicated f cellulose (Calcofluor White), g polysaccharide (Periodic Acid Schiff-PAS), and h mucopolysaccharide (Alcian Blue 8GX). i–k Different archegonium development phases. i Early development shows the archegonial initial cell. The sequential sections of subsequent development indicate j the central cell (cc) and k the primary neck cell (pn). Legend: Cytoplasmatic layer (ci), obliterate nucellar cells (on), nucellar cells (nu), megagametophyte (mg), tube nuclei (tn), jacket cells (jc)
The pollen tube penetrates deeply toward the female gametophyte (Fig. 4d), and near its tip, there are many nuclei (Fig. 4e). Histochemical tests indicated that the pollen tube wall is compounded by cellulose, polysaccharide, and mucopolysaccharide (Fig. 4 f–h). The pollen tube wall did not stain for callose, acidic polysaccharide, and acidic pectin.
The female gametophyte and the pollen tubes undergo a few changes from the middle of summer to the middle of winter of the next year. After this period, the female gametophyte restarts its development and reaches the cellularized phase.
The female gametophyte in the micropylar pole develops about twelve archegonia. At the beginning of the development, the archegonium presents the archegonial initial differentiated at the micropylar end. The female gametophyte cells adjacent to the archegonial initial are differentiated as jacket cells with darkly stained cytoplasm (Fig. 4i). Subsequently, a periclinal division of the archegonial initial forms the central cell (lower cell) (Fig. 4j) and primary neck cell (upper cell) (Fig. 4k). Figure 4j and k represent sequential sections of the archegonium.
In the female gametophyte at maturity (Fig. 5a–c), anticlinal divisions in the primary neck cell form a tier composed of 13 neck cells which form a central channel (Fig. 5d). In this phase, the neck cells and the jacket cells contain large amounts of starch grains in their cytoplasm (Fig. 5e) and the egg cell have already been formed from the central cell (Fig. 5a, c). At this moment, the pollen tube arrives at the female gametophyte, preparing for fertilization (Fig. 5a).
Longitudinal section of the ovule and archegonia detail of Araucaria angustifolia in light microscopy. a Pollen tube approach into megagametophyte with mature archegonium. b Starch grains detection in nucellar cells next to megagametophyte and archegonium. c Mature archegonia showing neck cells, jacket cells, and egg cell. d Archegonia with an oblique view of neck cells and central channel. e Starch grains detection in the neck cells of archegonium with Lugol. Legend: Pollen tube (pt), megagametophyte (mg), neck cells (nc), jacket cells (jc), egg cell (eg), nucellar cells (nu), archegonium (ac), central channel (cc), starch grains (sg)
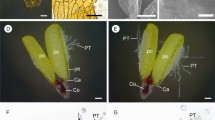
The three-dimensional reconstruction of the ovule shows the elements that were segmented in a way to discern its components: the most external tissue, the integuments, and inside the nucellus (Fig. 6a). The integuments and the nucellus are shown with transparency and are possible see the female gametophyte with several pollen tubes nearby (Fig. 6b). Figure 6c shows the 3D reconstruction of the nucellus, and by the transparency of the nucellus, Fig. 6d shows the perspective of the female gametophyte and the pollen tubes. μCT reconstruction shows that many pollen tubes grow through the nucellar tissue. They border each other, but each presents only one axis that extends straight toward the female gametophyte (Fig. 6e).
µCT 3D reconstruction of the ovule of Araucaria angustifolia. a Lateral view of the ovule with the integument and nucellus. b Frontal view of the ovule using integument transparency. Note the nucellus, the megagametophyte (in red), and the micropylar pollen tubes (yellow, orange, green, purple, and pink). c Frontal view of the nucellus. d Frontal view of the nucellus with transparency. Note the megagametophyte and micropylar pollen tubes. e Frontal view of the megagametophyte and the pollen tubes. Legend: integument (pale pink), nucellus (pale purple), megagametophyte (red), pollen tubes (yellow, orange, green, purple, pink, and light blue)
The process from pollination to pre-fertilization takes about fourteen months. In the first and second months, the pollen grains arrive in the seed cone, and the pollen tube germinates. The pollen tubes and unfertilized female gametophyte remain for about 4–5 months in a dormancy period, and after that, it takes about 3–4 months to complete their development (Fig. 7).
Seed cone unit development of Araucaria angustifolia. The cone unit from 1 to 5 develops in the first year of the reproductive cycle. The cone unit represented from 6 to 7 develop in the second year of the reproductive cycle. Brown: cone axis; Green: bract; Light Brown: seed scale (funiculus); Purple: nucellus; Beige: integument; Grey: third bulge; Red: Female Gametophyte; Yellow: Archegonia
Discussion
Ovule development
The identification of seed cone structure homology has caused divergence for decades. The conifer seed cone comprises several bract/seed scale complexes (Florin 1954). Although the seed cones of modern conifers present a consistent Bauplan (Tomlinson 2012), the fossil records demonstrated a very diverse morphology (Taylor et al. 2009). According to the Florin Model (1931) of the conifer evolution, the seed scale of fossil and extant conifers comprises a stalked ovule, sterile appendages, and a rudimentary axis, which may be fused to different degrees.
Currently, there is a different interpretation of the seed scale homology. The traditional view still rests on the pivotal argument of the Florin Model, interpreting the seed scale as a rudimentary lateral shoot that bears multiples appendages and develops at the axil of the bract, homolog to a leaf (Clement-Westerhof 1989; Rothwell et al. 2011). In recent studies based on ontogenetic, paleobotany, and phylogenetic data, a new model of seed scale evolution was proposed (Herting and Stützel 2020, 2022). This new interpretation points out that the seed scale would not be interpreted as a homolog to a leaf for three main reasons: it does not take into account the seed cone ontogeny of extant conifers, it does not explain the seed cone morphology of all extant conifers and because excluded, originally, species from Taxaceae. Therefore, based on ontogenetic studies with Araucaria araucana and comparative development with other conifers, the seed scale should be interpreted as a modified funiculus of the ovule (Herting and Stützel 2020).
Our study demonstrated that in A. angustifolia, the bract arises months earlier in the ovule development. In the early spring, the ovule starts its morphogenesis as an axillary structure of the bract in which a group of meristematic cells differentiates firstly the free portion of the seed scale (interpreted as the funicular region of the ovule). Immediately after the seed scale emergence, the nucellus differentiates. In sequence, these two zones keep growing and differentiating simultaneously.
The seed scale and nucellus development of A. angustifolia, identified by the sequential histological section, repeat the pattern described by A. araucana (Herting and Stützel 2020) by scanning electron microscopy and endorse the idea that both structures arise from the same primordium being the seed scale a component of the ovule once the whole axillary structure is undifferentiated before the nucellus and integument emerges. The formation of the third bulge of cell observed in A. angustifolia during the ovule ontogeny was first described in A. araucana, and its enlargement was linked to a change in ovule orientation from the cone apex to the cone axis (Herting and Stützel 2020).
In Aghatis australis and Wollemia nobilis, the other two genera of Araucariaceae, the ovule presents only one point of attachment to the bract, and the seed scale is not evident (Owens et al. 1995a; Dörken and Rudall 2019). However, it is unclear if the bract and seed scale are completely congenitally fused in those species or if any ontogenetic step has been missed from the seed cone development. In the Podocarpaceae family, also included in Araucariales (Leslie et al. 2018), the ovulate cone bears one or more bracts subtending an axillary epimatium supporting a single adaxial ovule (Tomlinson 1992). According to (Tomlinson 1992), the role of the epimatium is to change the ovule orientation, turning it into an inverted ovule, as in Araucaria (Herting and Stützel 2020). Furthermore, regarding the ovule homology in these two families, ontogenetic data infer that the epimatium of Podocarpaceae and the seed scale of Araucariaceae present the same development pattern. Both structures arise at the axillary portion of the bract, and quickly after its initiation, the nucellus, and the integument emerge. The pattern of development of this structure suggests that seed scale and epimatium are homologous structures (Herting and Stützel 2020; Khan and Hill 2021).
Megasporogenesis/Megagametogenesis
The nucellus of seed plants corresponds to the megasporangium, in which the megaspore mother cell undergoes meiosis to form megaspores (Williams 2009). In conifers, the nucellus could present one or several archesporial cells, which develop subdermally and divide periclinally to form many parietal cells that overlie the sporogenous cells. The sporogenous cells could divide once or more or develop directly as a megaspore mother cell (Biswas and Johri 1997). The general process of megasporogenesis in conifers describes the formation of tetrads or triads. In the latter case, the upper dyad cell does not undergo meiosis II (Pennell 1989; Biswas and Johri 1997). Different tetrad shapes occurred, such as linear, T-shape, tetrahedral, and isobilateral (Fiordi 1987; Pennell 1989), and in all cases, the functional megaspore is the chalazal one. In A. angustifolia, we described a distinct process in which the megaspore mother cells undergo meiosis I and form a coenocytic dyad, and subsequently, meiosis II forms a coenocytic tetrad. After meiosis, three nuclei degenerate, and only one acts as a megaspore to originate a female gametophyte with monosporic origin. The nuclear degeneration process in the megasporogenesis of plants is conducted by programmed cell death, which plays a significant role in forming the female gametophyte (Doronina et al. 2020). In Zea mays, the deaths of three megaspores are accompanied by aggregation of heterochromatin at the nucleus periphery and discharge of the plasmalemma from the cell wall (Russel 1979; Doronina et al. 2020). The causes that determine the fate of these cells have been poorly studied. In Arabidopsis, mutant plants with inactivated ICK/KRPs (interactor/inhibitor of cyclin-dependent kinase (CDK)/Kip-related proteins) genes that are CDK inhibitors, and cell cycle regulators, more than one megaspore mother cell and functional megaspore were developed. Besides, the authors show that a positional signal and more than one ICK protein may occur, selecting the functional megaspore (Cao et al. 2018). In A. angustifolia, right after meiosis II was completed, three pycnotic nuclei showed shrinking, compacting cell nuclei and peripheric position on the tetrad. Conversely, the viable nuclei presented two nucleoli indicating nuclear activity, as described for functional nuclei (Shaw and Brown 2012).
The meiotic stages have not been observed in any other Araucariaceae species, and in the closed-related family Podocarpaceae, no evidence of coenocytic tetrads was described. Instead, the authors showed the formation of a linear tetrad of megaspores in four species of Podocarpus (Del Fueyo 1999; Wilson and Owens 1999). In conifers, the formation of a coenocytic tetrad was only found in Cupressus sempervirens. However, in this species, the female gametophyte is tetrasporic, given that the four nuclei contribute to female gametophyte formation (El Maâtaoui et al. 1998; El Maâtaoui and Pichot 1999). Regarding other gymnosperms, the classic literature only describes the occurrence of a tetrasporic female gametophyte in two genera of Gnetales, Welwitschia (Biswas and Johri 1997; Friedman 2015) and Gnetum (Carmichael and Friedman 1996). In Welwitschia, the observation of a coenocytic tetrad led the authors to conclude the tetrasporic origin of the female gametophyte, and no degenerative megaspores were described (Friedman 2015). Hence, although these two genera share many sexual reproduction aspects that differ from Ephedra and other gymnosperms, the megasporogenesis process in seed plants is likely much more variable than has been thought.
Our study also identified a thick cell wall with pectic compounds and no signal of callose compound in the megaspore mother cell. This same pattern of the cell wall was observed until the end of the meiosis on the megaspore tetrad. In the same way, the ultrastructure analysis of the megaspore of Ginkgo biloba demonstrated a thick and complex cell wall that presents a conspicuous middle lamella, a primary wall similar to the other ordinary cells, and an inner cell layer resembling the structure of the middle lamella (Stewart and Gifford 1967). The thickening cell wall of the megaspore is a general character of gymnosperms, which allows the cytoplasm to isolate itself to accomplish meiosis. Although the thick structure is constant between species, the cell wall composition may differ. In species that present callose wall deposits, the megaspore mother cells show this material for a short moment, however, this compound disappears quickly after meiosis (Fiordi 1987). Callose isolates the viable megaspore from the degenerative ones, providing its vital activity to initiate female gametophyte development. Since A. angustifolia doesn't present cell walls isolating each megaspore within the tetrad, the ultrastructure analysis of megaspore development could provide the mechanism for functional megaspore selection.
A key event in the seed's evolution was the formation of the megaspores inside the ovule (Williams 2009). In the reproductive development of seed plants, the functional megaspore gives rise to a female gametophyte inside the ovule through multiple mitotic cycles. The conifer female gametophyte must move through several well-defined stages before it reaches maturity: free nuclear stage, cellularization stage, and cellular growth stage (Maheshwari and Singh 1967). Our study described the first steps of the free nucleus and cellular growth stages, including archegonium formation. In A. angustifolia, the first mitotic divisions of the female gametophyte development produced conspicuous nuclei organized dispersedly. Some weeks later, hundreds of nuclei were formed, presenting compacted chromatin and organizing in a thin layer of cytoplasm that revest the inner wall of the coenocytic female gametophyte. This development pattern was also described for many gymnosperms, which showed a large central vacuole that maintains the scanty cytoplasm at the female gametophyte periphery (Konar and Moitra 1980). After the coenocytic phase, the cellularization phase takes place. This phase is marked by alveolar growth in gymnosperms, except in Gnetum and Welwitschia (Maheshwari and Singh 1967). Because the alveoli present a specific development pattern, it is regarded as a fossil fingerprint (Rudall and Bateman 2019).
Except for angiosperms, Gnetum, and Welwitschia, all female gametophytes form archegonia, and according to Sokoloff and Remizowa (2021), this sexual organ presents a commonly developed motif called the “t-shape” pattern. Our data show that the archegonium of A. angustifolia presents the t-shape pattern once the first division of the archegonial initial is horizontal and the primary neck cell (upper cell) divides (vertically). The archegonium is a multicellular and three-dimensional organ that presents a precisely regulated cellular division in each plant group. Detailed studies on the ontogeny of this organ may help to understand the homologies of archegonium and embryo sac and may solve many questions about land plant evolution.
In the archegonium development of A. angustifolia described here, the ventral canal cell was not observed. In the same way, Burlingame (1914) didn’t notice this cell in A. angustifolia, and the ventral canal cell of Aghatis, which also belongs to Araucariaceae, was described as an ephemeral cell (Eames 1913). However, Owens et al. (1995a) identified a persistent ventral canal nucleus in A. australis (Owens et al. 1995a). The occurrence of ventral canal cells in other conifers, such as Podocarpaceae, is usually observed (Maheshwari and Singh 1967), as well as in Pinus and Abies (Sokoloff and Remizowa 2021). Still, Biswas and Johri (1997) point out questions about the regular formation of this cell in Taxus canadensis. Therefore, it is likely that for many conifers, the ventral canal cell presents an ephemeral behavior instead its complete absence, and probably the division of the central cell was missed in our study.
The number and arrangement of the neck cells of gymnosperms vary between plant lineages. In siphonogamous groups, such as conifers and Ephedra, the neck cells' configurations seem even more plastic once these sets of cells play a role in pollen tube reception (Sokoloff and Remizowa 2021). The necks cells of A. angustifolia are organized in one tier of 13 cells, which present a significant amount of starch grains at maturity. A. australis, even though jack cells and the egg cell present starch, this substance wasn´t found in the neck cells' cytoplasm (Owens et al. 1995b). In angiosperms, the pistil offers support and controls pollen tube growth. Therefore, the presence of starch in the neck cells of Araucaria agrees with the idea that the female gametophyte neck of conifers and Ephedra operates like angiosperm pollen-tube transmitting tissue (Sokoloff and Remizowa 2021).
Pollen tube growth in nucellar tissue
In gymnosperms, the usual pollination mechanism occurs when the pollen is carried through the micropyle and then to the nucellus by the pollination drops (Rudall and Bateman 2007). In most of the conifers, the pollen grains germinate inside the ovule (Owens et al. 1995a), where it is hydrated, making the exine burst, allowing the projection of the intine, which will establish the pollen tube (Singh 1978; Owens et al. 1998; Fernando et al. 2010). Differently, in Araucariaceae as well as many Tsuga (Pinaceae) and Saxegothaea (Podocarpaceae) species, the pollen grain germinates extra-ovulary (Singh 1978; Owens et al. 1998; Owens and Bruns 2000; Fernando et al. 2005). In A. angustifolia, pollen grains laid on the ovuliferous scales germinate and grow toward the seed cone axis to reach the micropyle. Over this pathway, the pollen tube doesn’t branch as Konar and Oberoi (1969) suggested for Araucaria and Agathis.
Owens et al. (1995b) showed that the pollen grain of A. australis has a delay of about one to two months between the landing of the pollen on the scales until its gemination, and this time is like our results. The delay in the pollen tube emission through the scale probably occurs due to the hydration of the pollen grains on the scale of the seed cone instead on the nucellus. This pollination mechanism highlights the close relationship between favorable climatic conditions to successful germination.
In Cycadales and Ginkgoales, the pollen tube is exclusively a vegetative structure and does not conduct the sperm cells to the egg cell (Rudall and Bateman 2007). The vegetative role of the pollen tube in these plants is linked with its haustorial behavior, which shows many branches in their pollen tubes while it is developing through the nucellus cells (Johri 1992). In conifers and Gnetales, pollen tubes display a siphonogamic role (Fernando et al. 2005). However, Pinaceae pollen tubes have been shown to branch (Dawkins and Owens 1993; Hiratsuka and Terasaka 2011). In those cases, the multiple branches observed in the pollen tube play a haustorial role, while the main branch, which holds the reproductive cells lineage, is siphonogamic (Hiratsuka and Terasaka 2011). Within Araucariaceae, pollen tube branches have also been described in Aghatis (Owens et al. 1995a, b) and Araucaria (Burlingame 1913, 1915). Indeed, in our study, the pollen tube histologic serial section analyses lead to a misunderstanding of this structure once many tubes border each other in nucellar tissue. Through the three-dimensional reconstruction, we reevaluate the pollen tube growth of A. angustifolia, demonstrating that it doesn't branch at nucellar tissue and evidences its straight trajectory toward the female gametophyte. The pollen tube behavior of A. angustifolia strengthens the idea that this structure has a primarily siphonogamic role. However, because the sperm cells do not form until 12 months after pollen germination, the pollen tube still performs a haustorial role. Therefore, the pollen tube growth in A. angustifoia differs from Agathis, highlighting a new pattern of growth in Araucariaceae. The analysis of pollen tube growth in other conifer species, especially Araucariaceae, may help to understand how this structure evolved in this family and conifers.
The pollen tube wall chemical composition in gymnosperms markedly differs from angiosperms, and specific pollen tube chemical composition patterns presented in different seed plant lineages may have taxonomic value (Yatomi et al. 2002). The pollen tube of A. angustifolia shows the same chemical composition as the intine of mature pollen grains, as Kuhn et al. (2014) described, and slight differences were observed regards other gymnosperms. In A. angustifolia, the pollen tube wall has a homogeneous distribution of cellulose in all its extensions, as observed in Picea abies (Lazzaro et al. 2003). In a different way, calcofluor labeling in Picea wilsonii (Sheng et al. 2006) and Pinus sylvestris pollen tubes showed a lower density of cellulose microfibers at the tube apex (Derksen et al. 1999). The pollen tube of Cycas revoluta, Ginkgo biloba, and 12 conifers species (representing four families) was stained with ruthenium red (Yatomi et al. 2002). Although the authors indicate that pectin content seems very rare in conifers species analyzed in this study, these data differ from A. angustifolia that do not stain with ruthenium red. The occurrence of callose, stained by aniline blue, in tube wall also varies between conifers. The absence of callose was highlighted in our data and most conifers studied by (Yatomi et al. 2002). Derksen et al. (1999) described the presence of callose in young parts of the pollen tube in Pinus silvestris, and (Chichiriccò et al. 2009) detected this substance in the side of the pollen tube continuous to the intine.
Along its extension, the pollen tube wall composition of Gymnosperms is relatively homogeneous compared to Angiosperms, and this feature seems to be related to the slow growth of its pollen tube (Wallace and Williams 2017). On the other hand, the specific distribution pattern of callose and pectins in the pollen tube wall of Angiosperms is an essential trait to the fast growth of its pollen tube (Chebli et al. 2012).
The Angiosperms pollen tube presents a primary pecto-cellulosic layer and a secondary callosic wall (Heslop-Harrison 1987). The callose wall is distributed on the pollen tube shank conferring resistance to tensile and compression stress (Parre and Geitmann 2005a). In contrast, the pectins present a gradient of apical esterified to distal de-esterified pectins, which provide, from the tip to the shank, an increase in the degree of cell wall rigidity and a decrease of visco-elasticity (Parre and Geitmann 2005b). The fast-growing pollen tube of Angiosperms, which greatly accelerate the fertilization process, is an important innovation in seed plants associated with the origin of the flowering plants (Wallace and Williams 2017).
The histochemical analysis made during the development of the reproductive structures in A. angustifolia demonstrated that synthesizing stored substances in nucellus cells is associated with pollen tube growth. Many studies have already revealed the presence of starch grains in the nucellar cells of gymnosperms. The mechanism of pollen tube intrusion was also associated with pollen tube in stylar transmitting tissue of angiosperm (e.g., Jensen and Fisher 1968). According to (Hiratsuka et al. 2002), the starch synthesis in the nucellus is spurred by pollination in Pinus densiflora; however, the pollen tubes gradually consume the starch grains by means of programmed cell death (PCD).
Conifers present a high diversity regards their pollination mechanism, and such range reflects in different traits of pollen morphology, seed cone morphology, and ovule structure (Owens et al. 1998). In some lineages, the pollen germination occurs right after its arrival in the ovule, while in others, the germination is postponed for weeks or months (Williams 2009). The period between pollen germination and fertilization also presents variation, which could extend for weeks or even months (Williams 2009). In A. angustifolia, fertilization occurred 14 months after pollination, emphasizing the long-time reproductive cycle of this species.
Using several bioimaging techniques, we presented ontogenetic events of seed cone development in A. angustifolia. We reported updates for the reproductive biology knowledge of this species from the ovule formation until pre-fertilization. Our observations show that the seed scale is born in the same primordium of the ovule, agreeing that this structure is part of the ovule itself and should be interpreted as the modified funiculus of the ovule. Along the ovule ontogeny, the formation of a coenocytic tetrad and the formation of the female gametophyte with monosporic origin was demonstrated. This process highlights a distinct pattern of female gametophyte origin that was never noticed in conifers. Besides, the three-dimensional reconstruction of the ovule revealed the presence of many pollen tubes growing in the nucellus. They border each other but have only one axis that extends straight toward the female gametophyte, performing a primarily siphonogamic role. The pollen tube growth in A. angustifolia differs from Agathis, highlighting a new growth pattern in Araucariaceae.
Author contribution statement
SAK designed the study. SAK and FMN wrote the manuscript. SAK and TS acquired the light microscopy images. SAK acquired the scanning electron microscopy images. FMN performed the microcomputed tomography analysis. TS and JEAM contributed to the writing and revision of the manuscript. All authors read and approved the manuscript.
Data availability
All data generated and analyzed during this study are included in this article.
References
Adelar Mantovani L, Morellato PC, dos Reis MS (2004) Fenologia reprodutiva e produção de sementes em Araucaria angustifolia (Bert.) O. Kuntze. Rev Bras Bot. https://doi.org/10.1590/S0100-84042004000400017
Baillie J, Hilton-Taylor C, Stuart S (2004) A global species assessment IUCN. IUCN Publications Services, Cambridge
Biswas C, Johri BM (1997) The gymnosperms. Springer, Berlin Heidelberg
Breygina M, Klimenko E, Schekaleva O (2021) Pollen germination and pollen tube growth in gymnosperms. Plants 10:1301. https://doi.org/10.3390/plants10071301
Burlingame LL (1913) The morphology of Araucaria brasiliensis. I. The staminate cone and male gametophyte. Bot Gaz 55:97–114. https://doi.org/10.1086/331002
Burlingame LL (1914) The morphology of Araucaria brasiliensis. II. The ovulate cone and female gametophyte. Bot Gaz 57:490–508. https://doi.org/10.1086/331344
Burlingame LL (1915) The morphology of Araucaria brasiliensis. III. Fertilization, the embryo, and the seed. Bot Gaz 59:1–39. https://doi.org/10.1086/331466
Cao L, Wang S, Venglat P et al (2018) Arabidopsis ICK/KRP cyclin-dependent kinase inhibitors function to ensure the formation of one megaspore mother cell and one functional megaspore per ovule. PLOS Genet. https://doi.org/10.1371/journal.pgen.1007230
Carmichael JS, Friedman WE (1996) Double fertilization in Gnetum gnemon (Gnetaceae): its bearing on the evolution of sexual reproduction within the Gnetales and the anthophyte clade. Am J Bot 83:767–780. https://doi.org/10.1002/j.1537-2197.1996.tb12766.x
Chebli Y, Kaneda M, Zerzour R, Geitmann A (2012) The cell wall of the Arabidopsis pollen tube—spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol 160:1940–1955. https://doi.org/10.1104/pp.112.199729
Chichiriccò G, Spanò L, Torraca G, Tartarini A (2009) Hydration, sporoderm breaking and germination of Cupressus arizonica pollen. Plant Biol 11:359–368. https://doi.org/10.1111/j.1438-8677.2008.00134.x
Clement-Westerhof J (1989) Morphology and phylogeny of Paleozoic conifers. Columbia University Press, New York
Dawkins MD, Owens JN (1993) In vitro and in vivo pollen hydration, germination, and pollen-tube growth in white spruce, Picea glauca (Moench) voss. Int J Plant Sci 154:506–521. https://doi.org/10.1086/297134
Del Fueyo GM (1999) Cone and ovule development in the Podocarpus species from Argentina. Phytomorphology 49:49–60
Derksen J, Li Y, Knuiman B, Geurts H (1999) The wall of Pinus sylvestris L. pollen tubes. Protoplasma 208:26–36. https://doi.org/10.1007/BF01279072
Dettmann ME, Clifford HT (2005) Biogeography of araucariaceae. Australia and New Zealand forest histories: Araucarian forests. Australian forest history society Inc. Occas Publ 2:1–9
Dörken VM, Rudall PJ (2019) Structure and abnormalities in cones of the Wollemi pine (Wollemia nobilis). Kew Bull 74:3. https://doi.org/10.1007/s12225-018-9789-7
Doronina TV, Sheval EV, Lazareva EM (2020) Programmed cell death during formation of the embryo sac and seed. Russ J Dev Biol 51:135–147. https://doi.org/10.1134/S1062360420030029
Eames AJ (1913) The morphology of Agathis australis. Ann Bot 27:1–38
El Maâtaoui M, Pichot C (1999) Nuclear and cell fusion cause polyploidy in the megagametophyte of common cypress, Cupressus sempervirens L. Planta 208:345–351. https://doi.org/10.1007/s004250050568
El Maâtaoui M, Pichot C, Alzubi H, Grimaud N (1998) Cytological basis for a tetraspory in Cupressus sempervirens L. megagametogenesis and its implications in genetic studies. Theor Appl Genet 96:776–779. https://doi.org/10.1007/s001220050801
Escapa IH, Catalano SA (2013) Phylogenetic analysis of Araucariaceae: integrating molecules, morphology, and fossils. Int J Plant Sci 174:1153–1170. https://doi.org/10.1086/672369
Farjon A (2010) A handbook of the world’s conifers(vol 1). Brill. https://doi.org/10.1163/9789047430629
Farjon A (2017) A handbook of the world’s conifers (2 vols.). Brill. https://doi.org/10.1163/9789004324510
Fernando DD, Lazzaro MD, Owens JN (2005) Growth and development of conifer pollen tubes. Sex Plant Reprod 18:149–162. https://doi.org/10.1007/s00497-005-0008-y
Fernando DD, Quinn CR, Brenner ED, Owens JN (2010) Male gametophyte development and evolution in extant gymnosperms. Int J Plant Dev Biol 4:47–63
Fiordi AC (1987) Megasporogenesis in gymnosperms: aspects of the cytoplasm and the cell walls. Atti Della Societá Toscana Di Scienze Naturali, Memorie, Serie B 94:163–179
Florin R (1931) Untersuchungen zur stammesgeschichte der coniferales und cordaitales, 1st edn. Almquist & Wiksells Boktryckeri, Stockholm
Florin R (1954) The Female reproductive organs of conifers and taxads. Biol Rev 29:367–389. https://doi.org/10.1111/j.1469-185X.1954.tb01515.x
Friedman WE (2015) Development and evolution of the female gametophyte and fertilization process in Welwitschia mirabilis (Welwitschiaceae). Am J Bot 102:312–324. https://doi.org/10.3732/ajb.1400472
Gabriel BL (1982) Biological electron microscopy. Wiley, Hoboken
Gerrienne P, Meyer-Berthaud B, Fairon-Demaret M et al (2004) Runcaria, a middle devonian seed plant precursor. Science 306:856–858. https://doi.org/10.1126/science.1102491
Gerrits PO, Smid L (1983) A new, less toxic polymerization system for the embedding of soft tissues in glycol methacrylate and subsequent preparing of serial sections. J Microsc 132:81–85. https://doi.org/10.1111/j.1365-2818.1983.tb04711.x
Gersterberger P, Leins P (1978) Rasterelektronenmikroskopische untersuchungen an blütenknospen von physalis philadelphica (Solanaceae) anwendung einer neuen präparationsmethode. Ber Dtsch Bot Ges 91:381–387
Gleiser G, Speziale KL, Lambertucci SA et al (2019) Uncoupled evolution of male and female cone sizes in an ancient conifer lineage. Int J Plant Sci 180:72–80. https://doi.org/10.1086/700580
Goeten D, Rogge-Renner GD, Schmidt ÉC et al (2020) Updating embryonic ontogenesis in Araucaria angustifolia: from Burlingame (1915) to the present. Protoplasma 257:931–948. https://doi.org/10.1007/s00709-020-01481-5
Haines R, Prakash N, Nikles D (1984) Pollination in Araucaria Juss. Aust J Bot 32:583. https://doi.org/10.1071/BT9840583
Herting J, Stützel T (2020) Morphogenesis of the seed cone of Araucaria araucana (Molina) K. Koch and the evolution of the coniferous seed scale. Flora 273:151719. https://doi.org/10.1016/j.flora.2020.151719
Herting J, Stützel T (2022) Evolution of the coniferous seed scale. Ann Bot 129:753–760. https://doi.org/10.1093/aob/mcab154
Heslop-Harrison J (1987) Pollen germination and pollen tube growth. In: Bourne GH, Jeon KW, Friedlander M (eds) International review of cytology, vol 107. Elsevier, Netherlands, pp 1–78
Hiratsuka R, Terasaka O (2011) Pollen tube reuses intracellular components of nucellar cells undergoing programmed cell death in Pinus densiflora. Protoplasma 248:339–351. https://doi.org/10.1007/s00709-010-0176-y
Hiratsuka R, Yamada Y, Terasaka O (2002) Programmed cell death of Pinus nucellus in response to pollen tube penetration. J Plant Res 115:141–148. https://doi.org/10.1007/s102650200019
Hughes J, McCully ME (1975) The use of an optical brightener in the study of plant structure. Stain Technol 50:319–329. https://doi.org/10.3109/10520297509117082
IBAMA (1992) Lista Oficial das Espécies da Flora Brasileira Ameaçadas de Extinção: Portarian°, 06-N, de 15 de janeiro de 1992. Diário Oficial (da República Federativa do Brasil)
Jensen WA, Fisher DB (1968) Cotton embryogenesis: the tissues of the stigma and style and their relation to the pollen tube. Planta 84:97–121. https://doi.org/10.1007/BF00398389
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company, London
Johri BM (1992) Haustorial role of pollen tubes. Ann Bot 70:471–475. https://doi.org/10.1093/oxfordjournals.aob.a088504
Khan R, Hill RS (2021) Morpho-anatomical affinities and evolutionary relationships of three paleoendemic podocarp genera based on seed cone traits. Ann Bot 128:887–902. https://doi.org/10.1093/aob/mcab113
Koch Z, Corrêa MC (2002) Araucária: a floresta do Brasil meridional. Olhar Brasileiro, Curitiba
Konar RN, Moitra A (1980) Ultrastructure, cyto- and histochemistry of female gametophyte of gymnosperms. Gamete Res 3:67–97. https://doi.org/10.1002/mrd.1120030108
Konar RN, Oberoi YP (1969) Recent work on reproductive structures of living conifers and taxads—a review. Bot Rev 35:89–116. https://doi.org/10.1007/BF02858911
Kuhn SA, de Mariath JE, A, (2014) Reproductive biology of the “Brazilian pine” (Araucaria angustifolia–Araucariaceae): development of microspores and microgametophytes. Flora Morphol Distrib Funct Ecol Plants 209:290–298. https://doi.org/10.1016/j.flora.2014.02.009
Lazzaro MD, Donohue JM, Soodavar FM (2003) Disruption of cellulose synthesis by isoxaben causes tip swelling and disorganizes cortical microtubules in elongating conifer pollen tubes. Protoplasma 220:201–207. https://doi.org/10.1007/s00709-002-0042-7
Leslie AB (2011) Shifting functional roles and the evolution of conifer pollen-producing and seed-producing cones. Paleobiology 37:587–602. https://doi.org/10.1666/10049.1
Leslie AB, Beaulieu J, Holman G et al (2018) An overview of extant conifer evolution from the perspective of the fossil record. Am J Bot 105:1531–1544. https://doi.org/10.1002/ajb2.1143
Lillie RD (1965) Histopathologic technic and practical histochemistry. McGraw-Hill Book Company, New York
Maheshwari P, Singh H (1967) The female gametophyte of gymnosperms. Biol Rev 42:88–129. https://doi.org/10.1111/j.1469-185X.1967.tb01341.x
Martin FW (1959) Staining and observing pollen tubes in the style by means of fluorescence. Stain Technol 34:125–128. https://doi.org/10.3109/10520295909114663
McDowell EM, Trump BF (1976) Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med 100:405–414
Miller CN (1999) Implications of fossil conifers for the phylogenetic relationships of living families. Bot Rev 65:239–277. https://doi.org/10.1007/BF02857631
Nogueira FM, Kuhn SA, Palombini FL et al (2017) Tank-inflorescence in Nidularium innocentii (Bromeliaceae): three-dimensional model and development. Bot J Linn Soc 185:413–424. https://doi.org/10.1093/botlinnean/box059
Nogueira FM, Palombini FL, Kuhn SA et al (2019) Heat transfer in the tank-inflorescence of Nidularium innocentii (Bromeliaceae): experimental and finite element analysis based on X-ray microtomography. Micron 124:102714. https://doi.org/10.1016/j.micron.2019.102714
O’Brien TP, McCully ME (1981) The study of plant structure principles and selected methods
Owens JN, Bruns D (2000) Western white pine (Pinus monticola Dougl.) reproduction: I Gametophyte development. Sex Plant Reprod 13:61–74. https://doi.org/10.1007/s004970000042
Owens JN, Catalano GL, Morris SJ, Aitken-Christie J (1995a) The reproductive biology of kauri (Agathis australis). I. Pollination and prefertilization development. Int J Plant Sci 156:257–269. https://doi.org/10.1086/297248
Owens JN, Catalano GL, Morris SJ, Aitken-Christie J (1995b) The reproductive biology of kauri (Agathis australis). II. Male gametes, fertilization, and cytoplasmic inheritance. Int J Plant Sci 156:404–416. https://doi.org/10.1086/297262
Owens JN, Takaso T, Runions CJ (1998) Pollination in conifers. Trends Plant Sci 3:479–485. https://doi.org/10.1016/S1360-1385(98)01337-5
Palombini FL, Nogueira FM, Kindlein W et al (2020) Biomimetic systems and design in the 3D characterization of the complex vascular system of bamboo node based on X-ray microtomography and finite element analysis. J Mater Res 35:842–854. https://doi.org/10.1557/jmr.2019.117
Panti C, Pujana RR, Zamaloa MC, Romero EJ (2012) Araucariaceae macrofossil record from South America and Antarctica. Alcheringa Australas J Palaeontol 36:1–22. https://doi.org/10.1080/03115518.2011.564562
Parre E, Geitmann A (2005a) More than a leak sealant. The mechanical properties of callose in pollen tubes. Plant Physiol 137:274–286. https://doi.org/10.1104/pp.104.050773
Parre E, Geitmann A (2005b) Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 220:582–592. https://doi.org/10.1007/s00425-004-1368-5
Pennell RI (1989) Sporogenesis in conifers. In: Callow JA (ed) Advances in botanical research. Academic Press, Cambridge, pp 179–196
Ran J-H, Shen T-T, Wang M-M, Wang X-Q (2018) Phylogenomics resolves the deep phylogeny of seed plants and indicates partial convergent or homoplastic evolution between Gnetales and angiosperms. Proc R Soc B Biol Sci 285:20181012. https://doi.org/10.1098/rspb.2018.1012
Rothwell GW, Stockey RA, Mapes G, Hilton J (2011) Structure and relationships of the Jurassic conifer seed cone Hughmillerites juddii gen. et comb. nov.: implications for the origin and evolution of Cupressaceae. Rev Palaeobot Palynol 164:45–59. https://doi.org/10.1016/j.revpalbo.2010.11.004
Rudall PJ, Bateman RM (2007) Developmental bases for key innovations in the seed-plant microgametophyte. Trends Plant Sci 12:317–326. https://doi.org/10.1016/j.tplants.2007.06.004
Rudall PJ, Bateman RM (2019) Coenocytic growth phases in land plant development: a paleo-evo-devo perspective. Int J Plant Sci 180:607–622. https://doi.org/10.1086/702758
Russel SD (1979) Fine structure of megagametophyte development in Zea mays. Can J Bot 57:1093–1110
Sass J (1951) Botanical microtechnique. The Iowa State College, Iowa
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Shaw P, Brown J (2012) Nucleoli: composition, function, and dynamics. Plant Physiol 158:44–51. https://doi.org/10.1104/pp.111.188052
Sheng X, Hu Z, Lü H et al (2006) Roles of the ubiquitin/proteasome pathway in pollen tube growth with emphasis on MG132-induced alterations in ultrastructure, cytoskeleton, and cell wall components. Plant Physiol 141:1578–1590. https://doi.org/10.1104/pp.106.081703
Singh H (1978) Embryology of gymnosperms: Gebrüder Borntraeger
Sokoloff DD, Remizowa MV (2021) Diversity, development and evolution of archegonia in land plants. Bot J Linn Soc 195:380–419. https://doi.org/10.1093/botlinnean/boaa077
Stewart KD, Gifford EM (1967) Ultrastructure of the developing megaspore mother cell of Ginkgo biloba. Am J Bot 54:375–383. https://doi.org/10.1002/j.1537-2197.1967.tb06932.x
Taylor EL, Taylor TN, Krings M (2009) Paleobotany: the biology and evolution of fossil plants. Academic Press, Cambridge
Tomlinson PB (1992) Aspects of cone morphology and development in Podocarpaceae (Coniferales). Int J Plant Sci 153:572–588. https://doi.org/10.1086/297081
Tomlinson PB (2012) Rescuing robert brown—the origins of angio-ovuly in seed cones of conifers. Bot Rev 78:310–334. https://doi.org/10.1007/s12229-012-9104-5
Wallace S, Williams JH (2017) Evolutionary origins of pectin methylesterase genes associated with novel aspects of angiosperm pollen tube walls. Biochem Biophys Res Commun 487:509–516. https://doi.org/10.1016/j.bbrc.2017.04.027
Williams CG (2009) Conifer reproductive biology. Springer, Netherlands, Dordrecht
Wilson VR, Owens JN (1999) The reproductive biology of totara (Podocarpus totara) (Podocarpaceae). Ann Bot 83:401–411. https://doi.org/10.1006/anbo.1998.0836
Yatomi R, Nakamura S, Nakamura N (2002) Immunochemical and cytochemical detection of wall components of germinated pollen of gymnosperms. Grana 41:21–28. https://doi.org/10.1080/00173130260045468
Acknowledgements
`The authors are grateful to the Laboratório de Anatomia Vegetal at the Universidade Federal do Rio Grande do Sul (LAVeg, UFRGS) and the Laboratório de Física Nuclear Aplicada at Universidade Estadual de Londrina (UEL) for technical support. The authors thank the Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), which sponsored grants to FMN (Number 151156/2022-0), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)-Finance Code 001. The authors also thank the anonymous reviewers for contributing to the improvement of this work.
Funding
The authors thank the Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), which sponsored grants to FMN (Number 151156/2022–0), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)–Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest within this article.
Ethical approval
Not applicable.
Consent to participate
All authors have seen and agreed with the content of this article. All authors agree with the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Glossary
- Archegonium
-
The reproductive structure of the multicellular female gametophyte that produces the egg cell.
- Seed cone
-
The compound reproductive structure of conifers in which each unit produces one or more ovules.
- Seed cone unit
-
Composed of a bract and all axillary structures (Herting and Stutzel 2022).
- Seed Scale
-
A single or multiple structures axillary to a bract and all axillary structures (Herting and Stutzel 2022).
- Siphonogamy
-
Oriented growth of the pollen tube to the female gametophyte. Sperm cells are not motile in lineages with this type of growth.
- Coenocyte
-
Cell with several nuclei without division of the cytoplasm.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuhn, S.A., Nogueira, F.M., Schürer, T. et al. Reproductive biology of the “Brazilian pine” (Araucaria angustifolia-Araucariaceae): the pollen tube growth and the seed cone development. Plant Reprod 37, 1–13 (2024). https://doi.org/10.1007/s00497-023-00473-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-023-00473-8