Abstract
Main conclusion
Extensive histology of pistillate flowers revealed two pollen tube arresting sites (the style-joining and micropyle) within the pistil of Quercus acutissima during the postpollination–prezygotic stage, which reflects a unique female and male gametophyte recognition/selection mechanism.
Abstract
Sexual reproduction is among the most delicate and essential stages in plant life cycles and involves a series of precise interactions between pistils and male gametophytes. Quercus is a woody genus that dominates Northern Hemisphere forests and is notorious for interspecific hybridization, but its sexual reproduction is poorly understood, especially its pollen tube (PT) growth dynamics within pistils. This study used microtome techniques and scanning electron microscopy to observe the postpollination–prezygotic process in the biennially fruiting oak Quercus acutissima. Many pollen grains germinated at anthesis instantly, and PTs penetrated stigmatic surfaces and elongated through the stylar transmitting tissue, then arrested at style-joining for about 12–13 months. Few PTs resumed growth along the compitum in the upper ovarian locule wall in the subsequent April, concurrent with the rapid growth of rudimentary ovules. PTs arrived in the micropyle, and upper septum during megaspore mother cell meiosis, then arrested again for 7–10 days waiting for the embryo sac maturation. Fertilization occurred one week later. Our study shows a clear female dominant crosstalk growth pattern between PT and the ovule. The intermittent PT growth might reflect a unique male gametophyte recognition/selection mechanism to avoid self-pollination and enhance PT competition while increasing interspecific hybridization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual reproduction is one of the most delicate and essential stages in the plant life cycle (Barrett 2013). During this process, a series of complex and precise selection/recognition interactions between the pistil and the male gametophyte plays a critical role in plant evolution (Friedman and Floyd 2001). At anthesis, pollen grains (male gametophytes) fall on the stigma, followed by their rapid germination. Generally, several pollen tubes (PTs) penetrate the stigmatic surface and grow downward through the transmitting tissue in style, but only one PT will reach the embryo sac to accomplish fertilization (van Went and Willemse 1984). In many angiosperms, fertilization generally occurs within 24–48 h (or even less) after pollination (Johri 1984; Gao et al. 1992; Tian and Russell 1997; Williams 2008). Nevertheless, delayed fertilization can be found in many unrelated angiosperm lineages, e.g., in the Fagales (Satake and Kelly 2021), Rosales (Pimienta and Polito 1983), Ericales (Palser et al. 1989), and Saxifragales (Endress and Igersheim 1999). It is unclear where and how PTs remain within the pistil during such delays, and little is known about PT growth dynamics.
The Fagales is a representative order of angiosperms with delayed fertilization (Sogo and Tobe 2006c). The plants of this clade are primarily wind-pollinated, and PT growth in pistils shows different developmental rhythms during the delay period. Previous studies in Casuarinaceae (Sogo et al. 2004a, b), Alnus (Sogo and Tobe 2005), Myrica (Sogo and Tobe 2006c), and Fagus (Sogo and Tobe 2006a) have shown that PT growth ceases upon reaching a particular site in the pistil. These cessation sites are highly diverse, including the style base (i.e., style-joining site), the upper ovarian locule wall, locule, the funicles of the ovules, the micropyle, and at the chalaza or on the nucellar surface (reviewed by Sogo and Tobe 2006a, b). Thus, it seems the PT navigation signal system in the Fagales is highly diverse.
The family Fagaceae, comprising about 1000 species, dominates forests in the temperate, seasonally dry regions of the Northern Hemisphere (Govaerts and Frodin 1998). Delayed fertilization in this family was first recorded over a century ago, and in particular, the pollination-fertilization process in oaks has been documented in detail. Oak pollen grains germinate instantly to penetrate the stigmatic surface to the stylar transmitting tissue once they land on the stigma. PT growth is halted at the point at which styles join for 5–6 (but up to 8) weeks in Q. alba and Q. schottkyana and for 1 year in Q. rubra, Q. velutina, and Q. acutissima (Cecich 1997; Borgardt and Nixon 2003; Deng et al. 2008). The body of literature documenting this phenomenon has provided crucial information on the sexual reproductive process in oaks. However, few studies have directly observed PT growth dynamics in the pistils of oaks, and their results have revealed very different PT growth modes, especially after PTs arrive in the ovarian locules. For example, PTs of Q. suber ceased growth at the style-joining site for 4–5 weeks, entered the ovarian locule, and elongated along the micropyle to reach the embryo sac (Boavida et al. 1999). Two biennially fruiting species, Q. rubra and Q. velutina, showed a similar intermittent PT growth mode within the pistil, but the period of PT arrest at the style-joining was up to 12 months (Cecich 1997). (Shi (2020) reported a very unusual PT growth pattern in Q. acutissima: PT growth ceased at the base of the style for 12 months after pollination, and only a few PTs resumed growth in the following season (at the end of April), thereafter penetrating the upper ovarian locule wall to reach the placenta, proceeding to the chalaza, and growing upward along the integuments to the micropyle to fertilize the embryo sac. This PT growth mode is very different from those observed in other studies.
In other genera of the family Fagaceae, for example, Castanea, the PT growth mode seems to be continuous with no prominent growth cessation in the pistils (Xiong et al. 2019). The genus Fagus is very different, with two PT growth-cessation sites inside the pistils at the integument and micropyle, respectively (Sogo and Tobe 2006a). Therefore, the sexual reproduction process in the Fagaceae might be particularly complex and thus merits further investigation and confirmation.
Moreover, oaks are long-standing models for studying hybridization and species concepts. Darwin (1859) was aware of the complexity of oak species relationships. Thereafter, hybridization in oaks has been reported and extensively investigated. Many population genetic studies have demonstrated that hybridization commonly occurs between species in the same section (Bacilieri et al. 1996) and even between species in different sections, e.g., Quercus ilex and Q. suber (Burgarella et al. 2009). Recent genomic studies have demonstrated that many adaptive alleles could be passed through interspecific gene flow, thereby profoundly impacting the adaptation of multiple species (Kremer and Hipp 2020). However, the physiological background that leads to such common interspecific gene flow remains poorly understood. In this study, we observed PT growth and substantially delayed fertilization processes in the pistils of Q. acutissima, a biennially fruiting species widespread in East Asia. This study was undertaken to provide fine details on the pollination-double fertilization process in Q. acutissima and to increase the present understanding of the biological significance of delayed fertilization and intermittent PT growth in the family Fagaceae and the Fagales more broadly.
Materials and methods
Plant materials
Female inflorescences of Q. acutissima Carruth. were collected from five trees weekly, from early anthesis in March 2017 to August 2018 in Chenshan Botanical Garden (Shanghai, China) and from March 2020 to August 2021 in Kunming Botanical Garden, CAS (Kunming, China). The trees of the two botanical gardens were 12 years old and about 40 years old at the time of sampling, respectively.
The morphology of the collected pistillate flowers was observed using a stereomicroscope (Nikon SMZ72; Nikon, Tokyo, Japan) and photographed to record morphological changes before they were fixed in FAA (50% ethanol:stock formalin:glacial acetic acid, 90:5:5, by vol.).
Observation of pollen tube growth
Five–ten fixed flowers/inflorescences were randomly selected, and were dissected in a solution of 50% ethanol to collect the pistils, ovary, and ovules. The pistils and ovaries were immersed in a 1.0% sodium hypochlorite solution overnight at room temperature to decolorize them. After being rinsed three times in deionized water, the pistils and ovary were macerated in 1 M sodium hydroxide (NaOH) at 60 ℃ for two hours and then transferred to a 0.1 M K3PO4 buffer solution for half an hour to adjust the pH. The materials then were stained with 0.5% aniline blue in 0.1 N K3PO4 for three hours. The pistils and ovules were mounted in a few drops of staining medium on two glass coverslips on a glass slide and were then covered with another glass coverslip with force to spread the materials (Herr 1971). Pollen-tube growth in the pistils and ovules was observed with a fluorescent microscope (Zeiss LSM410; Zeiss, Oberkochen, Germany) using a UV filter set (Model No. 01) with an excitation filter (365 nm; bandpass 12 nm), dichroic mirror (FT395), and barrier filter (LP397).
A scanning electron microscope (SEM) was used to observe pollen grains on stigmas and pollen tube growth in ovarian locules. The FAA fixed pistils were dissected to expose ovules, then dehydrated with an ethanol series (30, 50, 70, 85, and 95%, followed by three incubation in 100% ethanol), then incubated in fresh 100% ethanol overnight before continuing on to a critical point drying apparatus (Tousimis SAMDRI-795; Tousimis Research Corp., Rockville, MD, USA). The detailed steps followed the protocol of Bray (2000) and Franks (2014). Finally, the pistils were coated with gold and observed with a SEM (FEI Quattro; Thermo Fisher, Waltham, MA, USA).
Observations of ovule and embryo sac development
To locate the specific PT growth-cessation site(s) and assess growth dynamics, the pistil morphological changes (e.g., of ovules and embryo sacs) were examined in microtome sections. 15–20 fixed pistillate flowers were dehydrated by an ethanol series (30, 50, 70, 85, and 95%, followed by two incubations in 100% ethanol with 1–2 h incubation time for each step depending on the size of the sample, then were infiltrated gradually with xylene/100% ethanol series (25, 50, 75%) with 1 h for each step, then followed by two incubations in xylene with 30 min for each time) and embedded in Paraplast® (Leica, Wetzlar, Germany) with a melting point of 57–58 ℃ for subsequent microtome sectioning. Serial Sects. (6 or 15 µm thick) were stained with Heidenhain’s Hematoxylin, Safranin-O, and Fast Green, then mounted in Entellan (Merck, Darmstadt, Germany) and observed with a bright-field microscope (Leica DM3000). To further identify the precise location of the PTs, the same batch of serial Sects. (12 µm thick) were first stained with 0.05% (w/v) Toluidine Blue and then stained with 0.5% aniline blue in 0.1 N K3PO4 for three hours. The slides were mounted with glycerin, then observed with a fluorescent microscope (Zeiss LSM410) using a UV filter set (Model No. 01) with an excitation filter (365 nm; bandpass 12 nm), dichroic mirror (FT395), and barrier filter (LP397), and subsequently observed again with a bright-field using the same microscope. At least 3 pistillate flowers were observed thoroughly to confirm the location of the pollen tube tips within the pistillate flower.
Comparison of the sexual reproduction process in the Fagaceae and the Fagales
The terminology of pistil anatomy follows that of Cecich (1997), Borgardt and Nixon (2003), and Sogo and Tobe (2006a). The comparison of pistil morphology and the sexual development process in the Fagaceae and the Fagales is based on current and previous studies, including those in Quercus alba (Stairs 1964; Mogensen 1965; Cecich 1997), Q. rubra (Hjelmqvist 1953; Cecich 1997), Q. velutina (Cecich 1997); Castanea spp. (Nakamura 1986, 1991, 1992a, b, 1994, 2001, 2003; Zou et al. 2014; Xiong et al. 2019), Fagus japonica (Sogo and Tobe 2006a), Myrica rubra (Sogo and Tobe 2006b, c), Alnus spp. (Sogo and Tobe 2005), Betula pendula (Dahl and Fredrikson 1996), Casuarina equisetifolia (Sogo et al. 2004b), Ticodendron incognitum (Tobe 1991; Sogo and Tobe 2008), and Juglans regia (Langdon 1934; Luza and Polito 1991; Zhang et al. 2021). All the character states were unordered, unpolarized, and unweighted, and missing values were scored as “?”. The character states were thus scored and mapped onto the simplified phylogenetic trees of the Fagales and the Fagaceae obtained by Li et al. (2004) and Oh and Manos (2008) to reconstruct their evolutionary patterns.
Results
We examined the pistillate flower morphology of two batches of samples collected from Shanghai and Kunming, respectively. Except for the phenology of samples from Kunming following a progression 15–20 days (anthesis time on 15 March) earlier than that in Shanghai, the morphological changes and the PT arresting and resuming time duration were identical in the two batches of samples. This study thus focuses on the results based on the samples collected from Shanghai Chenshan Botanical Garden from 2017 to 2018. We summarized the number of the pistils that had a pollen tube tip at a particular time and position after pollination in Table S1.
Development stages of pistillate flowers and PT growth within the pistils
Pollination stage (early to late April) (Figs. 1a, 2a–e)
The development of the pistillate flowers of Quercus acutissima since anthesis to the post-fertilization stage. Scale bar = 2.0 mm. a Pistillate flower of the current year at pollination time on 3 April 2017, showing the recurved style (Sy) and many pollen grains on the stigmatic surface (St). Bracts were marked as *. b Pistillate flower of the current year at a postpollination time on 17 April 2017. The stigmatic surface (St) and upper style (Sy) become dry and brown. c Pistillate flower of the current year on 26 May 2017. The ovary is surrounded by a young couple with dense scale (Se), the styles join is surrounded by perianths (Pe). d Pistillate flower of the current year on 14 June 2017 showing the thickened pedicle (ped) and scales (Sc). e–f Pistillate flowers of the current year on 11 July 2017 (e) and 20 Sept 2017 (f), respectively, showing pistillate flowers stopping growth. The styles join is hard and dry, enclosed by perianth (Pe). g The pistillate flower of the 2nd year on 19th April 2018. The pistillate flower size is almost unchanged compared to that of the previous fall. h Pistillate flower resumed growth on 13 May 2018. The cupule wall and scales (Sc) enlarged. i Pistillate flower of the 2nd year at the fertilization time (14 June 2018), showing the pistil enclosed in cupule with major long scales (Sc). j The longitudinal section of the pistillate flower of h (13 May 2008), showing the ovary (Ov) and the ovules (O). k–m The embryo sac stage of the pistils on 14 June 2018. k The longitudinal section of the pistil. The pistil is enclosed in a cupule with incrassate long scales (Sc). l Magnified section of the pistil in k. Arrows indicate the two ovules attached to the placenta (Pl), three septa (Se) adjacent to the ovarian locule wall. The pistil is enclosed by the palisade layer (PA) and the abscission zone (AZ) at the base. m Top view of the pistil, showing the septum (Se) and six well-developed ovules (O) in the pistil. n The post-fertilization stage on 20 June 2018 showing one fertilized ovule (O), the other aborted ovules (indicated by the arrows), and the septum (Se). ped, pedicle
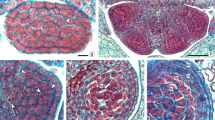
Longitudinal sections of pistils from the pollination time of the current year to the 2nd growing season. a Pistillate flower of the current year on 3 April 2017. A large number of pollen tubes penetrated the transmitting tissue (TT) of the style (Sy). The arrows indicate the pollen tubes (gain) and undeveloped ovary (Ov) at the basal part. St, stigmatic surface; Pe, perianth. b-c Pistillate flower of the current year at the end of the pollination time (23 April 2017). The pollen tubes (indicated by white arrows) elongated to the lower part of the style. Undeveloped ovarian locules (Lo) at the basal of the pistil. d Magnified section of the stigmatic surface of b, showing the outmost water layer and the receptive stigmatic cells (arrows). e Transverse section of the style-joining site (the square of c). f Pistil of the current year on 20 Sept 2017 at the end of the growing season, showing the senesced style (Sy) with several suberized cells (Sc). Callose plugs of the pollen tubes (PT) are concentrated at the style’s base region (illustrated in a square) at the level of the perianths (Pe), the ovarian locule still undeveloped. g–k The longitudinal section of pistil at the 2nd growth season on 19 April. g Section of the whole pistil. The torn area in the upper section where the tree styles join region contains a dark stained hard resin-like substance that is hard to cut, showing the ovarian locules (Lo) enlarged, rudimentary ovule (Ro) formed on the placenta (Pl). h Magnified square region of g. Arrows showing the resin-like substance surrounding the compitum (Co) and paracarpous portion of the ovary (PPO). i Magnified the ovary of g, showing the placenta (Pl) and rudimentary ovules (Ro). j Magnified style join region. Lots of pollen tubes (PT) (indicated by green arrows), and a pollen tube (PT) penetrating the compitum (Co). k Magnified square region of j. Arrows showing the traces of the two pollen tubes. Scale bars = 200 µm (a–i), 100 µm (j), 50 µm (k)
Anthesis occurred in early April and lasted for about 2 weeks. The staminate flowers are grouped into catkins. The staminate anthesis period was 4–5 days before the stigma receptivity of the pistillate flowers. Therefore, there was a 3–7-day overlap of anthesis and stigmatic receptivity for the same tree. Many pollen grains were attached to the stigmatic surface between early and late April with visibly fluorescing apertures during anthesis, indicating that the pollen tube would soon germinate (Fig. 1a). The stigma receptivity lasted for about 10 days, after which its surface turned brown and became necrotic (Fig. 1b). The cupule and the scale of the pistillate flowers were significantly thickened in the middle of May (Fig. 1c–f). The stigma of the species is of the “dry” type, without papillate cells but with receptive cells on its surface (Fig. 2d).
The pollen grains germinated instantly once they landed on the stigmatic surface at anthesis in early–mid-April. We detected a bright fluorescence signal around the pollen tubes when stained with aniline blue, indicating the synthesis of the callose plug (Fig. 2a). Transmitting tissue was observed to extend from the tip of the stigma to the acropetal portion of the ovarian locules located at the style canal surrounded by vascular and cortical tissues (Fig. 2b, e). After PTs penetrated the stigmatic surface, they grew through the cellular transmitting tissue of the style until middle to late May, leaving a trace of callose plugs (Fig. 2a, c, e). At this stage, the styles occupied the majority of the pistil with an undeveloped ovary at the base (Fig. 2c). The rudimentary ovule was undeveloped at this stage.
Pollen tube arrest (late May–April of the following year) (Fig. 1b–f)
At the end of May, the pistillate flowers of the current year stopped growing, and the scales surrounding them became woody and thick. At the same time, the elongation of PTs ceased at the juncture of the three styles (the style-joining site), which is at the level of the visible portion of the perianth (Fig. 2f). Strong fluorescence signals from the callose plugs of the PTs were detected in this region (Fig. 2f). A hard resin-like brown substance was found in the style-joining site of the bleached pistils under the light microscope.
Our study showed that there is no vascular or cortical tissue surrounding the transmitting tissue below the style-joining site. The compitum is a tube-like structure extending from the style-joining site into the paracarpous portion of the ovary (Fig. 3g, h). Before the next growing season, the compitum is invisible, as the cells of the upper region of the ovarian locule and paracarpous portion are tightly appressed to each other without crevices (Fig. 2a–f). Such a morphology lasted for about 12 months, from late May to the next April, during which the size of the pistillate flower, its cupule, and scales did not change substantially.
Longitudinal section of 2nd-year pistils at the micropyle formation stage (4 May 2018–11 May 2018). a,b, e–h 2nd-year pistils on 4 May. c–d 2nd-year pistils on 11 May. a Overall morphology of the pistil, showing prominent enlarged ovary (Ov). Green arrows indicate the callose plugs of pollen tubes. Se, septum; Co, compisum; O, ovule; Pl, placenta. b Magnified style-joining site and upper ovarian locule wall. Green arrows show the pollen tubes still at the styles join region, few PT grew downward to the ovary. c The overall morphology of the pistil on 11 May 2018. The integuments enclosing the nucellus and micropyle (mic) have been formed. Lo, ovarian locule; Se, septum; Co, compisum; O, ovule. d Magnified ovary of c. PTs (indicated by green arrows) have penetrated the compisum and paracarpous portion of the ovary (PPO) to reach the micropyle (mic) of the ovules. e–f Magnified sections of a, observed under the light microscope. e The morphology of the styles join region and adjacent compisum (Co), showing the resin-like substance in the tissue. f The morphology of the upper ovarian locule below the styles join region, showing compisum (Co) and the paracarpous portion of the ovary (PPO). A dark stained region (indicated by *) is the boundary during the pollen tube arrest, the PPO below is mostly composed by fast-growing parenchymal cells. g The morphology of the ovary (Ov). The outer integument (OI) of the ovule (O) has developed but still not enclosed the nucellus (nu). PPO was almost attached to the septum. h Magnified compisum (Co) and PPO region. The green arrow shows a pollen tube. Pe, perianth; St, style. Scale bars = 200 µm
Resumption of growth and fertilization (mid-April of the 2nd year to mid-June of the 2nd year)
During the anthesis time of the following spring, the previous year’s pistillate flower showed no significant appearance variation (Fig. 1g), but the ovarian locule had enlarged (Fig. 2g, j). The rudimentary ovule grew fast at this stage (Fig. 2g, j). In late April, three ovarian locules with two ovule primordia were visible in the 2nd-year pistillate flowers. The volume of the pistillate flower increased quickly afterward (Fig. 1h–i). The scales on the cupule wall significantly elongated and recurved in mid-May (Fig. 1h–k).
We detected many intense and persistent fluorescence signals of callose plugs at the basipetal end of pollen tubes around the style-joining site (Figs. 2f, h, 3a–c). Only very few pollen tubes had resumed growth at the end of April to early May (Fig. 3b), either via the compitum and the paracarpous portion of the ovary to the ovarian locule or by growing along with the basipetal piece of the transmitting tissue without entering the compitum (Figs. 2h, 3c) to the ovarian locule. Callose plugs were only occasionally detected when the pollen tubes entered the compitum or ovarian locule after they resumed growth.
In the ovary, the development of primordia in the outer and inner integuments of the ovule occurred in early May (Fig. 3c). The two integuments then grew rapidly to form the micropyle in a week (in the middle of May), followed by the meiosis of the megaspore mother cell (Fig. 3c). The septa of each ovarian locule were incompletely developed at the top of the locules and very close to the paracarpous portion of the ovary (PPO). The placenta is mostly sessile, in which the vascular bundle runs from the placenta into the ovules. Callose visualized by green fluorescence was detectable along the spiral vessels, which are the sieve tubes of the phloem (Fig. 3h). Meanwhile, we found callose deposition around the chalaza of some ovules after megaspore mother cell meiosis (Fig. 3b, h), and those ovules were likely aborted later.
Pollen tubes elongated along the compitum and the upper septum without branching after resuming growth. The earliest time pollen tubes arriving in the micropyle was at the megaspore mother cell tetrad stage in mid-May (Fig. 3c, d), but fertilization did not happen instantly; instead, it occurred 7–14 days later (4 June 2018) when the eight-nucleate embryo sac had matured (Fig. 4g). More pollen tubes entered the ovarian locule during this interval, producing many short blind branches again around the upper septa and micropylar region (Figs. 3c, d, 4b–f, 4j, k). The free-nucleate endosperm was detected in the embryo sac in the pistils collected on 20 June 2018 (Fig. 4i), indicating that fertilization occurred after 13 June. In this family, usually, only one ovule can develop into a seed; the remaining five are subsequently aborted (Fig. 4l). However, the exact dates during which the PT resumed growth and penetrated the ovarian locule could not be determined based on weekly collections, as the development of the pistillate flowers is not synchronous.
Pistils at embryo sac stage to the post-fertilization stage (4 June–3 July 2018). a–f Longitudinal sections of pistils on 4 June 2018. The full view of the pistil under the light (a) and fluorescence microscope (b). a The arrows mark the micropyles of the three ovules (O) and the abscission zone (AZ) at the basal part. b The green arrows indicate the pollen tubes, the white arrows the micropyles (mic). c Magnified ovary of the same sample on 4th June, showing pollen tubes (indicated by green arrows) at the hanging lobes of the paracarpous portion of the ovary (PPO) and around micropyles (mic). d Magnified compitum–PPO region and the adjacent septa (Se). Pollen tubes (indicated by green arrows) elongated along the lobes of PPO to reach the septa (Se) and micropyle (mic) (marked by *). e Longitudinal section of ovules. A pollen tube (green arrow) penetrated the micropyle (mic). f Magnified micropyle (mic, labeled by *) region; green arrows indicate pollen tubes. g–l Ovules at the fertilization stage (g, h, j, k) (13 June 2018) and post-fertilization stage (i, l) (3 July 2018). g A mature ovule at the 8-nucleate embryo sac stage (13 June), showing a pollen tube in micropyle (mic), an egg cell (egg) at the top of the embryo sac with two polar nuclei (pn) at the central zone. h Magnified placenta and basal ovule show the spiral vessels (VB) in the placenta and ovule bundles. The light blue arrows show the sieve tube plates of phloem. i Ovule at free nuclear endosperm stage after fertilization (20 June, 2018), showing the embryo (Em) and the free nuclear endosperm (fn). The inner integument began to disintegrate. j–k Scanning electron microscopy (SEM) pictures of ovules at the fertilization stage. Green arrows mark the pollen tubes in the micropyle (mic) of the ovules (O). l The transverse section of the ovary on 3 July 2018 showing 5 aborted ovules (indicated by *), a normally fertilized ovule (O) with cellular endosperm (end) at the heart shape embryo stage. Se, septum. Scale bars = 200 µm (a–e, h–l), 100 µm (f)
Evolutionary trends of the sexual reproduction process of the Fagaceae and the Fagales
Nine sexual reproduction traits were analyzed across the Fagales. The PT arresting sites within the pistil and PT pathway to enter the embryo sac are highly divergent in the Fagales, e.g., the diversified intermittent PT growth ceasing sites and the very different pathways (porogamy, pseudoporogamy, and chalazogamy). Among these, PT arresting patterns in the pistil, the duration from pollination to fertilization (30 days–13 months), and the rhythm of pistils and PTs are highly diverse in the family Fagaceae. The detailed comparison among the taxa and scored selected character states for ancestral state reconstruction are summarized in Table 1.
Four characters related to the sexual reproduction process, (1) differentiation of the ovary at pollination time, (2) PT growth pattern in style, (3) formation of the micropyle when the PT is in the ovary, and (4) PT pathway to the embryo sac, were selected for ancestral state reconstruction. The characteristic status was scored, and the evolutionary pattern was traced over the phylogenetic tree. The analysis revealed that an undifferentiated ovary at lepollination was derived several times independently in different lineages, showing a paraphyletic pattern (Fig. S1a). The state of PTs being arrested at the style-joining site was unresolved in the ancestral lineage of the Fagales. This character shows high diversity in the family Fagaceae, from lacking such an arrest to slow growth to prominent arresting at the style-joining site (Fig. S1b). A well-formed micropyle when PT(s) enter the ovary is plesiomorphic. Myrica (with an unformed micropyle) and Juglans (with a micropyle either formed or unformed) represented a specialized clade (Fig. S1c) relative to other plants in the Fagales. The ancestral state of PT pathways to the embryo sac (including chalazogamy, porogamy, and pseudoporogamy) was observed to be unresolved in the Juglans–Myrica clade. Chalazogamy in the clade comprised of {[Betulaceae (incl. Betula and Alnus) + Ticodendronaceae] + Casuarinaceae} could be either apomorphic or plesiomorphic. Porogamy was plesiomorphic within the Fagaceae (Fig. S1d).
Discussion
Pollen tube growth mode in Quercus acutissima
The pollen germination and PT growth in the pistil involves a series of complicated cell–cell interactions; some facilitate PT navigation and fertilization, while others prohibit PT access to the female gametophyte (Lord and Russell 2002; Cheung et al. 2010). The genus Quercus has one prominent PT growth-cessation site at the style-joining site (Cecich 1997; Boavida et al. 1999) and an inconspicuous short-term growth-cessation site around the micropyle–upper septum region. The female sporogenous cell was undeveloped in the ovary during pollination in Q. acutissima. It requires about an additional 12–13 months before ovules form. The resumption of PT growth in the pistil is synchronous with the key development events of the ovule, for example, the formation of the rudimentary ovule and embryo sac maturation. Such a growth pattern indicates that the female sporophytic tissue and gametophyte collaboratively regulate PT growth in the pistils.
The transmitting tissue is a specialized column of cells producing extracellular matrix material essential for PT elongation and guidance (Cheung et al. 1995; Erbar 2003; Crawford and Yanofsky 2008). The PTs navigate en route through transmitting tissue to the ovule. Many PTs in Q. acutissima converged into the transmitting tissue at anthesis, reaching the style-joining site in about 2–4 weeks. Two to four PTs can resume growth from the style-joining site in the next growing season. Therefore, the style-joining site in the pistil acts as a powerful filter to block the growth of most PTs. There is an anatomical difference between the transmitting tissue above the pollen arresting site and the region below it in the upper ovarian locule wall. The stylar transmitting tissue was surrounded by vascular bundles and cortical tissue, but not the transmitting tissue below the perianth. This result is partly supported by the speculation of Sogo and Tobe (2006b) that intermittent PT growth might result in the nutritional needs of PTs inside the pistil for their long-term survival during the postpollination and fertilization stage.
We also found that the histological staining of the transmitting tissue in the style canal is much darker than that in the lower part of the upper ovarian locule wall (Fig. 2b, g), indicating the two parts may have different functions. Remarkably, we detected a “darkened belt” that occurred below the PT arrest area (Fig. 3f, g) at the time of early April in the 2nd-year pistil. This feature then disappeared, followed by the fast growth of the compitum, and pollen tubes began to elongate basipetally. Simultaneously, the compitum became a narrow crevice along the style base to the upper ovarian locule to allow the PT to penetrate the ovarian locule wall. These significant histological differences (Fig. 2a) might block subsequent PT growth until the initial ovule development in the subsequent growing season.
It took about 1–2 weeks for PTs of Q. acutissima to penetrate the compitum and upper ovarian locule wall after they had resumed growth. In other angiosperms, only 2 days are needed for PTs to grow through a 2–4 cm long style, e.g., in tobacco (Nicotiana tabacum) (Tian and Russell 1997) and Torenia fournieri (Higashiyama et al. 1997). Therefore, the PT elongation speed in the compitum–upper ovarian locule wall of Q. acutissima was still obstructed. Thus, the transmitting tissue in the style and ovarian locule wall are functionally different. The stylar transmitting tissue provides universal guidance to attract PT elongation and enrich PTs at the style-joining site. The lower transmitting tissue in the upper ovarian locule wall serves as a place for PT competition, recognition, and selection. However, the pistil’s physical and/or physiological constraint to PT growth merits further investigation to characterize their interactions.
Based on our observation, PTs arrived in the ovarian locule just about during megaspore mother cell meiosis in the middle to late May, not before. The PTs were branched and had a zigzag form around the micropyle-upper septum region, indicating PTs lost their growth orientation and temporarily ceased growth. This period took about another 7–14 days until the embryo sac had matured, and then, one PT finally fertilized the embryo sac. Thus, the PT growth mode in Q. acutissima can be divided into four stages. (1) The pollen germinates and grows through the stylar transmitting tissue to reach the style-joining site, where their growth is arrested. (2) Megasporogenesis begins to develop in the following spring (late April). After stimulation, a few (2–4) PTs resumed growth to penetrate the upper ovarian locule wall slowly, entering the ovary during megaspore mother cell meiosis in middle to late May. (3) Then, PTs ceased growth around the upper septum and micropyle for a short period (about 7–14 days). (4) When the embryo sac had matured, one PT fertilized the embryo sac in early June. The PT–pistil growth process observed in this study agrees with those surveyed in Q. alba, Q. rubra, and Q. velutina by Cecich (1997), except for the duration of arrested PT growth at the style-joining site in oak species. However, the exact days that PTs were arrested in the micropyle–upper septum is hard to define by the week sampling. Based on our observation of sections from different pistils, a functional megaspore needs about a week to develop into a mature eight-nucleate embryo sac. The short-term arrest of PT growth around the micropyle region before fertilization was confirmed.
Nevertheless, Shi (2020) has reported an unusual PT growth scenario wherein PTs entered the placenta from the upper ovarian locule wall, penetrating the funiculus and chalaza and then growing along the integuments to reach the micropyle and fertilize the embryo sac. We repeated the experiments on the same batch of materials collected from Chenshan Botanical Garden from 2017 to 2018 that Shi (2020) had investigated. Though aniline blue preferentially stains callose, it also stains other tissue. After locating the vascular bundle by carefully observing the sections under both fluorescence and bright-field microscopes, the fluorescent signals reported on the placenta and funiculus by Shi (2020) were actually observed to correspond to the phloem, as the sieve plates of sieve tubes contained callose that was stained by aniline blue.
Evolutionary trends of pollen tube growth in the pistil in the family Fagaceae and the order Fagales
Delayed fertilization and the intermittent PT growth modes have been generally reported in the Fagales. The growth cessation sites of PTs in the pistils can be in the style-joining site, the upper ovarian locule wall, the funiculus, the chalaza, and near the micropyle (reviewed by Sogo and Tobe 2008).
A prominent long-term arrest of PT growth at the style-joining site is a shared trait for the genus Quercus. The PT growth mode in other Fagaceae genera is not the same as that in oaks. In the genus Castanea, PT growth in the stylar transmitting tissue shows no obvious growth cessation, but its elongation is slow, taking approximately 9–12 days for PTs to grow to half of the style length and an additional 18–20 days to penetrate the ovule in Castanea mollissima (Nakamura 1994; Xiong et al. 2019). The genus Fagus has two PT growth cessation sites (the funicle and the micropyle), but both are inside the ovary (Sogo and Tobe 2006a), congruent with an early derived specialized systematic status of Fagus relative to the rest of the genera in the family Fagaceae. The female sporogenous cells in the ovary seem crucial for the continuous growth of pollen tubes in the pistil, as the ovule integument meristem and nucellus were differentiated in Castanea and Fagus at anthesis, but no sporogenous cell in the ovary was observed at pollination in Quercus. Without guidance signals from the immature ovary, the oak PTs were arrested at the style-joining site. Compared to the prolonged growth of the PTs in the transmitting tissue of the stylar canal in Castanea, the prominent intermittent growth of PTs at the style-joining site in the genus Quercus might reflect an extreme scenario during which the PTs with higher potential for development in the female tissue were selected. Further investigation of the PT growth dynamics within the pistil in the genera Lithocarpus and Castanopsis are essential to elucidate the evolutionary pattern of sexual reproduction in the family Fagaceae.
A similar PT growth cessation pattern at the style-joining site was found in other members of the Fagales, e.g., Ticodendron, Casuarina, and Alnus, but the duration was much shorter (Sogo and Tobe 2008). According to the ancestral state reconstruction, PT growth cessation at the style-joining site seems to have been derived several times independently in the Fagales, suggesting this trait is adaptive. However, its roles in sexual reproduction and PT–female tissue interactions merit further investigation.
Nevertheless, the PT pathway and PT progression to the embryo sac in the ovule in the order Fagales are very diversified (Table 1). The PT arrest site in the Fagales is usually rich in vascular bundles, e.g., the style-joining site, funiculus, and chalaza. This phenomenon partly supports the speculation by Sogo and Tobe (2006b) on the nutritional needs of PTs. Based on an examination of the available published literature on the order Fagales, we noticed the vascular bundle in the upper septum and/or placenta is generally well developed, and this area is adjacent to the upper ovarian locule wall in the chalazogamous lineages (e.g., Myrica, Ticodendron, Casuarina, Alnus, etc.). However, the family Fagaceae seems specified without vascular bundles in the upper septum and adjacent upper ovarian locule wall, which might serve as a filter to increase control of the female sporophyte over the male gametophyte. Further histochemical analyses to locate the spatial-tempo distribution of nutrients could clarify the function of the PT growth arrest sites in the Fagales.
After a PT arrives in the ovule, the ultimate step to fertilization is regulated by numerous signals emanating from the embryo sac of the ovule. Recent studies on plant sexual reproduction in Arabidopsis have revealed that the synergid cell (Higashiyama et al. 2001) and the central cell (Chen et al. 2007) secrete PT attractant molecules essential for PT guidance to enter the embryo sac. Among these molecules, non-species-specific cysteine-rich peptides XIUQIU attracted PTs from Arabidopsis thaliana and A. lyrata in a similar manner. In contrast, LUREs [especially, Arabidopsis thaliana LURE1 peptides (AtLURE1s)] function in species-referential and species-specific manners to outcompete heterospecific PTs to promote reproductive isolation in A. thaliana (Zhong et al. 2019). These results well illustrate the cooperation of these attractants to contribute to maintaining reproductive isolation while allowing heterospecific fertilization at a low frequency (Zhong et al. 2019). At the embryo sac formation stage, we found short-term PT arrest around the micropyle region in Q. acutissima. Likewise, these PTs might wait for guidance signals emanating from the mature embryo sac. The PT pathway to the embryo sac is porogamous or chalazogamous in the Fagales, similar to that in Arabidopsis, and PTs enter the embryo sac from the synergid cell side, indicating PT-embryo sac crosstalk signal system in the angiosperm might be conserved. Moreover, only one embryo sac can be developed into a mature seed among the six ovules present. Thus, this process may also be considered competition among the six ovules, with selection mediated by the PT. The PT arrest around the micropyle region might be a final opportunity for male–female gametophyte recognition and selection before double fertilization. After several iterations of the PT arresting process, the PT number was ultimately reduced to one PT that is able to fertilize one embryo sac. The substantially prolonged postpollination–prezygotic period plays a meaningful role in providing time for selection and competition among male and female gametophytes in oaks.
Ecological adaptative significance of delayed fertilization in the family Fagaceae and the order Fagales
Ancestral state reconstruction has shown that delayed fertilization might be a synapomorphy of the Fagales (Sogo and Tobe 2006b), but this trait seems to have been derived independently several times in several angiosperm lineages (Sogo and Tobe 2006d), suggesting it may be an adaptation. It has been proposed that delayed fertilization promotes PT competition by providing a “fair start” for PTs to the ovules, thus enhancing PT competition (Willson and Burley 1983; Dahl and Fredrikson 1996; Sogo and Tobe 2006a, 2008).
It is worth noting that the stigma receptivity period in the Fagales generally lasts for 4–7 (or up to 10) days (Cecich 1997; Boavida et al. 1999; Nakamura 2001; Gomez-Casero et al. 2004; Sogo et al. 2004a), which is much longer than that observed in non-wind-pollinated plant lineages (usually several hours to 2–3 days, or even less) (Dafni 1992). The pollen grain number is not a restrictive factor for fertilization in typical wind-pollinated plants, as a vast number of pollen grains can land on the stigmatic surface, resulting in random pollination. Our previous observations of Q. schottkyana (Deng et al. 2008), Q. variabilis, and Q. acutissima have shown that the flowering time of staminate flowers is 3–4 days in advance of the stigma receptivity period, but there is still a short overlapping period (3–4 days) of pollination and stigma receptivity of the same tree (unpublished data). Without postpollination barrier(s), geitonogamous self-pollination should be common in oaks. However, oaks are almost entirely outcrossing and show a high degree of self-incompatibility according to mating system analysis using molecular markers (Bacilieri et al. 1996; Pakkad et al. 2008; Oyama et al. 2017). During our three years of field observation on a single tree of Q. acutissima in a forest in Lin-an county, Zhejiang province, in which only one tree occurred within an approximately 10 ha memorial park, we detected many PTs germinated on the stigmatic surface at pollination time and were arrested at the style-joining site, but no acorns were ever found in the following year (Shi 2020). Similarly, in Q. ilex, more self-PTs reached the style, resulting in a higher pistillate flower abortion ratio (Yacine and Bouras 1997). Artificial crossing experiments in Q. grisea and Q. gambelii had shown that self-pollinated flowers aborted at the same rate as conspecific and heterospecific flowers before fertilization but were nearly eliminated after fertilization (Williams et al. 2001). All these findings indicate that pollen-stigma recognition is not the crucial step leading to self-incompatibility in oaks. A series of postpollination and prezygotic selection mechanisms (very likely to be late-acting self-incompatibility), thus, must act within the genus to promote outcrossing. Intermittent PT growth in the pistils presents a unique mechanism in oaks and other wind-pollinated trees to reinforce male gametophyte selection and competition to avoid geitonogamous self-fertilization.
Oaks are notorious for hybridization between closely related species. Recent genomic analysis has shown that many adaptative alleles can be exchanged among oak species (even distant lineages) and thus impact the species’ fitness and adaptive evolution. This, in turn, might be related to the remarkably fast divergence of the genus, which has made oaks one of the most successful dominant woody lineages (Kremer and Hipp 2020). This common interspecific gene flow is undoubtedly profoundly rooted in the oak mating system but is not fully understood. A critical step forward in understanding this mystery in oaks is elucidating how the female sporophytic tissue and gametophyte select PTs with different genotypes. Considering the high frequency of interspecific gene flow among oaks (even among very distinct species), the interaction between PTs and the PT guidance system within the pistil must be insufficiently specific to block genetically distant PTs from fertilizing the embryo sac, and this merits further investigation.
In summary, intermittent PT growth in the pistil might serve as a double-edged sword in oaks that, on the one hand, acts as a unique mechanism to avoid geitonogamous self-fertilization and favor outcrossing and, on the other hand, might increase the probability of heterospecific PTs fertilizing the embryo sac resulting in high interspecific gene flow. This hypothesis needs critical experimental verification by comparing pollen tube behavior and development mode within the pistils among geitonogamous self-, outcross-, and interspecific cross-pollination treatments.
Author contribution statement
MD and QL: conceived designed research, and wrote the manuscript. MD, KY, WS, and CS: conducted experiments. MD, QL and KY: revised the draft. All authors read and approved the manuscript.
Data availability statement
The data generated and/or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- PT:
-
Pollen tube
References
Bacilieri R, Ducousso A, Petit RJ, Kremer A (1996) Mating system and asymmetric hybridization in a mixed stand of European oaks. Evolution 50(2):900–908. https://doi.org/10.2307/2410861
Barrett SCH (2013) The evolution of plant reproductive systems: how often are transitions irreversible? Proc R Soc Lond B Biol Sci 280(1765):20130913. https://doi.org/10.1098/rspb.2013.0913
Boavida LC, Varela MC, Feijó JA (1999) Sexual reproduction in the cork oak (Quercus suber L.). I. The progamic phase. Sex Plant Reprod 11(6):347–353. https://doi.org/10.1007/s004970050162
Borgardt SJ, Nixon KC (2003) A comparative flower and fruit anatomical study of Quercus acutissima, a biennial-fruiting oak from the Cerris group (Fagaceae). Am J Bot 90(11):1567–1584. https://doi.org/10.3732/ajb.90.11.1567
Bray D (2000) Critical point drying of biological specimens for scanning electron microscopy. In: Williams JR, Clifford AA (eds) Supercritical fluid methods and protocols. Methods Biotechnol 13: 235–243. https://doi.org/10.1385/1-59259-030-6:235
Burgarella C, Lorenzo Z, Jabbour-Zahab R, Lumaret R, Guichoux E, Petit RJ, Soto Á, Gil L (2009) Detection of hybrids in nature: application to oaks (Quercus suber and Q. ilex). Heredity 102(5):442–452. https://doi.org/10.1038/hdy.2009.8
Cecich RA (1997) Pollen tube growth in Quercus. Forest Sci 43(1):140–146. https://doi.org/10.1093/forestscience/43.1.140
Chen YH, Li HJ, Shi DQ, Yuan L, Liu J, Sreenivasan R, Baskar R, Grossniklaus U, Yang WC (2007) The central cell plays a critical role in pollen tube guidance in Arabidopsis. Plant Cell 19(11):3563–3577. https://doi.org/10.1105/tpc.107.053967
Cheung AY, Wang H, Wu H-m (1995) A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82(3):383–393. https://doi.org/10.1016/0092-8674(95)90427-1
Cheung AY, Boavida LC, Aggarwal M, Wu H-M, Feijó JA (2010) The pollen tube journey in the pistil and imaging the in vivo process by two-photon microscopy. J Exp Bot 61(7):1907–1915. https://doi.org/10.1093/jxb/erq062
Crawford BCW, Yanofsky MF (2008) The formation and function of the female reproductive tract in flowering plants. Curr Biol 18(20):R972–R978. https://doi.org/10.1016/j.cub.2008.08.010
Dafni A (1992) Pollination ecology : a practical approach. IRL Press at Oxford University Press, Oxford
Dahl AE, Fredrikson M (1996) The timetable for development of maternal tissues sets the stage for male genomic selection in Betula pendula (Betulaceae). Am J Bot 83(7):895–902. https://doi.org/10.2307/2446267
Darwin C (1859) On the origins of species by means of natural selection. John Murray, London, UK
Deng M, Zhou ZK, Chen YQ, Sun WB (2008) Systematic significance of the development and anatomy of flowers and fruit of Quercus schottkyana (subgenus Cyclobalanopsis: fagaceae). Int J Plant Sci 169(9):1261–1277. https://doi.org/10.1086/591976
Endress PK, Igersheim A (1999) Gynoecium diversity and systematics of the basal eudicots. Bot J Linn Soc 130(4):305–393. https://doi.org/10.1111/j.1095-8339.1999.tb00528.x
Erbar C (2003) Pollen tube transmitting tissue: place of competition of male gametophytes. Int J Plant Sci 164(S5):S265–S277. https://doi.org/10.1086/377061
Franks RG (2014) Scanning electron microscopy analysis of floral development. In: Riechmann JL, Wellmer F (eds) Flower development: Methods and protocols. Springer, New York, pp 263–273
Friedman WE, Floyd SK (2001) Perspective: The origin of flowering plants and their reproductive biology- a tale of two phylogenies. Evolution 55(2):217–231. https://doi.org/10.1111/j.0014-3820.2001.tb01288.x
Gao X, Francis D, Ormrod JC, Bennett MD (1992) An electron microscopic study of double fertilization in allohexaploid wheat Triticum aestivum L. Ann Bot 70(6):561–568. https://doi.org/10.1093/oxfordjournals.aob.a088517
Gomez-Casero MT, Hidalgo PJ, Garcia-Mozo H, Dominguez E, Galan C (2004) Pollen biology in four mediterranean Quercus species. Grana 43(1):22–30. https://doi.org/10.1080/00173130410018957
Govaerts R, Frodin DG (1998) World checklist and bibliography of Fagales (Betulaceae, Corylaceae, Fagaceae and Ticodendraceae). Kew Publishing, London
Herr JM (1971) A new clearing-squash technique for the study of ovule development in angiosperms. Am J Bot 58(8):785–790. https://doi.org/10.2307/2441475
Higashiyama T, Kuroiwa H, Kawano S, Kuroiwa T (1997) Kinetics of double fertilization in Torenia fournieri based on direct observations of the naked embryo sac. Planta 203(1):101–110. https://doi.org/10.1007/s00050170
Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S-y, Kuroiwa H, Kuroiwa T (2001) Pollen tube attraction by the synergid cell. Science 293(5534):1480–1483. https://doi.org/10.1126/science.1062429
Hjelmqvist H (1953) The embryo sac development of Quercus robur L. Phytomorphology 3:377–384
Johri BM (1984) Embryology of angiosperms. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-69302-1
Kremer A, Hipp AL (2020) Oaks: an evolutionary success story. New Phytol 226(4):987–1011. https://doi.org/10.1111/nph.16274
Langdon LM (1934) Embryogeny of Carya and Juglans, a comparative study. Bot Gaz 96 (1):93–117. https://www.jstor.org/stable/2471560
Li RQ, Chen ZD, Lu AM, Soltis DE, Soltis PS, Manos PS (2004) Phylogenetic relationships in Fagales based on DNA sequences from three genomes. Int J Plant Sci 165(2):311–324. https://doi.org/10.1086/381920
Lord EM, Russell SD (2002) The mechanisms of pollination and fertilization in plants. Annu Rev Cell Dev Biol 18(1):81–105. https://doi.org/10.1146/annurev.cellbio.18.012502.083438
Luza JG, Polito VS (1991) Porogamy and chalazogamy in walnut (Juglans regia L.). Bot Gaz 152(1):100–106. https://doi.org/10.1086/337868
Mogensen HL (1965) A contribution to the anatomical development of the acorn in Quercus L. Iowa State Coll J Sci 40:221–255
Nakamura M (1986) Development of ovules in the ovary of Japanese chestnut. J Japan Soc Hort Sci 55(3):251–257. https://doi.org/10.2503/jjshs.55.251
Nakamura M (1991) Bur abscission in Japanese chestnut (Castanea crenata Sieb. et Zucc.) as related to degeneration of ovules. J Japan Soc Hort Sci 60(1):47–53. https://doi.org/10.2503/jjshs.60.47
Nakamura M (1992a) Elongation of styles and optimum pollination time in Japanese chestnut (Castanea crenata Sieb. et Zucc.). J Japan Soc Hort Sci 61(2):265–271. https://doi.org/10.2503/jjshs.61.265
Nakamura M (1992b) Structure of stigma and germination of pollen in Japanese chestnut (Castanea crenata Sieb. et Zucc.). J Japan Soc Hort Sci 61(2):295–302. https://doi.org/10.2503/jjshs.61.295
Nakamura M (1994) Elongation of pollen tubes and degeneration of ovules in Japanese chestnut (Castanea crenata Sieb. et Zucc.). J Japan Soc Hort Sci 63(2):277–282. https://doi.org/10.2503/jjshs.63.277
Nakamura M (2001) Pollen tube growth and fertilization in Japanese chestnut (Castanea crenata Sieb. et Zucc.). J Japan Soc Hort Sci 70(5):561–566. https://doi.org/10.2503/jjshs.70.561
Nakamura M (2003) Fertilization and in vitro growth of ovules in Japanese chestnut (Castanea crenata Sieb. et Zucc.). J Japan Soc Hort Sci 72(6):482–487. https://doi.org/10.2503/jjshs.72.482
Oh SH, Manos PS (2008) Molecular phylogenetics and cupule evolution in Fagaceae as inferred from nuclear CRABS CLAW sequences. Taxon 57(2):434–451. https://doi.org/10.2307/25066014
Oyama K, Herrera-Arroyo ML, Rocha-Ramírez V, Benítez-Malvido J, Ruiz-Sánchez E, González-Rodríguez A (2017) Gene flow interruption in a recently human-modified landscape: The value of isolated trees for the maintenance of genetic diversity in a Mexican endemic red oak. For Ecol Manage 390:27–35. https://doi.org/10.1016/j.foreco.2017.01.018
Pakkad G, Ueno S, Yoshimaru H (2008) Gene flow pattern and mating system in a small population of Quercus semiserrata Roxb. (Fagaceae). For Ecol Manage 255(11):3819–3826. https://doi.org/10.1016/j.foreco.2008.03.017
Palser BF, Rouse JL, Williams EG (1989) Coordinated timetables for megagametophyte development and pollen tube growth in Rhododendron nuttallii from anthesis to early postfertilization. Am J Bot 76(8):1167–1202. https://doi.org/10.2307/2444828
Pimienta E, Polito VS (1983) Embryo sac development in almond [Prunus dulcis (Mill.) D. A. Webb] as affected by cross-, self- and mon-pollination. Ann Bot 51(4):469–479. https://doi.org/10.1093/oxfordjournals.aob.a086492
Satake A, Kelly D (2021) Delayed fertilization facilitates flowering time diversity in Fagaceae. Phil Trans R Soc B 376(1839):20210115. https://doi.org/10.1098/rstb.2021.0115
Shi CC (2020) Observation of delayed fertilization of Quercus acutissima (Fagaceae) and its evolutionary significance. Dissertation, Shanghai Normal University, Shanghai, China
Sogo A, Tobe H (2005) Intermittent pollen-tube growth in pistils of alders (Alnus). Proc Natl Acad Sci USA 102(24):8770–8775. https://doi.org/10.1073/pnas.0503081102
Sogo A, Tobe H (2006a) Delayed fertilization and pollen-tube growth in pistils of Fagus japonica (Fagaceae). Am J Bot 93(12):1748–1756. https://doi.org/10.3732/ajb.93.12.1748
Sogo A, Tobe H (2006b) The evolution of fertilization modes independent of the micropyle in Fagales and “pseudoporogamy.” Plant Sys Evol 259(1):73–80. https://doi.org/10.1007/s00606-006-0409-x
Sogo A, Tobe H (2006c) Mode of pollen-tube growth in pistils of Myrica rubra (Myricaceae): A comparison with related families. Ann Bot 97(1):71–77. https://doi.org/10.1093/aob/mcj015
Sogo A, Tobe H (2006d) Mode of pollen tube growth in pistils of Eucommia ulmoides (Eucommiaceae, Garryales). Int J Plant Sci 167(5):933–941. https://doi.org/10.1086/505756
Sogo A, Tobe H (2008) Mode of pollen tube growth in pistils of Ticodendron incognitum (Ticodendraceae, Fagales) and the evolution of chalazogamy. Bot J Linn Soc 157(4):621–631. https://doi.org/10.1111/j.1095-8339.2008.00807.x
Sogo A, Jaffre T, Tobe H (2004a) Pollen-tube growth and fertilization mode in Gymnostoma (Casuarinaceae): their characteristics and evolution. J Plant Res 117(3):249–251. https://doi.org/10.1007/s10265-004-0148-4
Sogo A, Noguchi J, Jaffre T, Tobe H (2004b) Pollen-tube growth pattern and chalazogamy in Casuarina equisetifolia (Casuarinaceae). J Plant Res 117(1):37–46. https://doi.org/10.1007/s10265-003-0129-z
Stairs GR (1964) Microsporogenesis and embryogenesis in Quercus. Bot Gazette 125(2):115–121
Tian HQ, Russell SD (1997) Calcium distribution in fertilized and unfertilized ovules and embryo sacs of Nicotiana tabacum L. Planta 202(1):93–105. https://doi.org/10.1007/s004250050107
Tobe H (1991) Reproductive morphology, anatomy, and relationships of Ticodendron. Ann Mo Bot Gard 78(1):135–142. https://doi.org/10.2307/2399598
Williams JH (2008) Novelties of the flowering plant pollen tube underlie diversification of a key life history stage. Proc Natl Acad Sci USA 105(32):11259–11263. https://doi.org/10.1073/pnas.0800036105
Williams JH, Boecklen WJ, Howard DJ (2001) Reproductive processes in two oaks (Quercus) contact zones with different levels of hybridization. Heredity 87(6):680–690. https://doi.org/10.1046/j.1365-2540.2001.00968.x
Willson MF, Burley N (1983) Mate choice in plants: tactics, mechanisms, and consequences. Princeton University Press, Princeton, New Jersey, pp 46–115
Xiong H, Zou F, Guo S, Yuan D, Niu G (2019) Self-sterility may be due to prezygotic late-acting self-incompatibility and early-acting inbreeding depression in Chinese chestnut. J Am Soc Hortic Sci 144(3):172–181. https://doi.org/10.21273/jashs04634-18
Yacine A, Bouras F (1997) Self- and cross-pollination effects on pollen tube growth and seed set in holm oak Quercus ilex L (Fagaceae). Ann for Sci 54(5):447–462. https://doi.org/10.1051/forest:19970503
Zhang L, Guo C, Lu X, Sun X, Liu C, Zhou Q, Deng J (2021) Flower development of heterodichogamous Juglans mandshurica (Juglandaceae). Front Plant Sci 12:541163. https://doi.org/10.3389/fpls.2021.541163
Zhong S, Liu M, Wang Z, Huang Q, Hou S, Xu YC, Ge Z, Song Z, Huang J, Qiu X, Shi Y, Xiao J, Liu P, Guo YL, Dong J, Dresselhaus T, Gu H, Qu LJ (2019) Cysteine-rich peptides promote interspecific genetic isolation in Arabidopsis. Science 364(6443):851. https://doi.org/10.1126/science.aau9564
Zou F, Guo SJ, Xie P, Xiong H, Lv WJ, Li GH (2014) Megasporogenesis and development of female gametophyte in chinese chestnut (Castanea mollissima) cultivar 'Yanshanzaofeng'. Int J Agric Biol 16(5):1001–1005. http://www.fspublishers.org/published_papers/93953_..pdf
Acknowledgements
We are thankful to Dr. Zhuo Feng (Institute of Palaeontology, Yunnan Key Laboratory for Palaeobiology, Yunnan University) for kindly providing the microscopy research platform; Xishuangbanna Station of Tropical Rainforest Ecosystem Studies, and Baima Snow Mountain complex ecosystem vertical transect Field Observation and research Station for their help on the field work; Jin-Jin Hu, Si-Si Zheng, Ying Li, Duoqing Lin, Xing Ning, and Fu Gao for their help on sampling the flower materials. This study was funded by the National Scientific Foundation of China (NSFC) 31972858, the Fund of Yunnan Key Laboratory for Integrative Conservation of Plant Species with Extremely Small Populations (PSESP2021F01), and the Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences (Y4ZK111B01).
Funding
The National Scientific Foundation of China (NSFC), 31972858, Min Deng, Fund of Yunnan Key Laboratory for Integrative Conservation of Plant Species with Extremely Small Populations, PSESP2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this study was conducted without any commercial relationships that could lead to any potential conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deng, M., Yao, K., Shi, C. et al. Development of Quercus acutissima (Fagaceae) pollen tubes inside pistils during the sexual reproduction process. Planta 256, 16 (2022). https://doi.org/10.1007/s00425-022-03937-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-022-03937-9