Abstract
Through the process known as programmed cell death (PCD), nucelli of Pinus densiflora serve as the transmitting tissue for growth of the pollen tube. We sought to clarify the processes of degradation of nucellar cell components and their transport to the pollen tube during PCD in response to pollen tube penetration of such nucelli. Stimulated by pollination, synthesis of large amounts of starch grains occurred in cells in a wide region of the nucellus, but as the pollen tube penetrated the nucellus, starch grains were degraded in amyloplasts of nucellar cells. In cells undergoing PCD, electron-dense vacuoles with high membrane contrast appeared, assumed a variety of autophagic structures, expanded, and ultimately collapsed and disappeared. Vesicles and electron-dense amorphous materials were released inside the thickened walls of cells undergoing PCD, and those vesicles and materials reaching the pollen tube after passing through the extracellular matrix were taken into the tube by endocytosis. These results show that in PCD of nucellar cells, intracellular materials are degraded in amyloplasts and vacuoles, and some of the degraded material is supplied to the pollen tube by vesicular transport to support tube growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most seed plants, the pollen tube plays the essential role of conveying the sperm cell to the egg and thereby enabling fertilization. This process is called siphonogamy. In angiosperms, the style serves as the path for the pollen tube. Morphologically, styles are classified into two types: hollow styles, which are common in monocots, and solid styles, which are common in dicots. Hollow styles have a canal extending from the stigma to the base of the style, while solid styles contain specialized cells known as transmitting tissue in their center. Gymnosperms show siphonogamy or zoidogamy (in which motile sperms swim to the egg), depending on the evolutionary path (Poort et al. 1996). At the stage known as the prepollen condition seen in fossils, sperms were released from pollen grain without formation of a pollen tube. In Ginkgo and Cycas, the branched pollen tube that grows into the extracellular matrix (ECM) of the nucellus does not convey sperms; instead, the pollen tube is thought to play a haustrial role. Late in development, the broad end of the tube swells or elongates and eventually ruptures to release the sperms. In many coniferophyta, the pollen grains germinate in the pollen chamber, which is the cavity at the micropylar end of the nucellus, and the pollen tubes grow into the ECM of the nucellus to form multiple branches. A single branch of the pollen tube conveys sperm cells toward the female gametophyte, playing a reproductive role. In most Gnetophyta, the pollen tube is unbranched.
In eukaryotes, specific sets of cells die in various processes of differentiation and growth, and the phenomenon by which the organism itself initiates or controls such cell death is known as programmed cell death (PCD). The form of PCD known as apoptosis occurs in animals. In plants, PCD is seen in a wide variety of processes, including reproduction, senescence, tracheary element formation, and hypersensitive responses (Pennell and Lamb 1997; Wu and Cheung 2000), and several types of PCD mechanism are thought to exist (Reape and McCabe 2008). In many plants, pollen tube intrusion is associated with degeneration of the stylar transmitting tissue of the angiosperm (Cheung 1996; Herrero and Dickinson 1979; Wang et al. 1996; Wu and Cheung 2000) and nucellar tissue of gymnosperm (Choi and Friedman 1991; Owens et al. 1981a, b; Owens and Morris 1990; Owens et al. 1995). We have previously reported that, accompanying pollen tube growth in Pinus densiflora, nucellar cells around the pollen tube undergo PCD with characteristics of apoptosis including chromatin condensation, internucleosomal DNA fragmentation, and cytoplasmic shrinkage. Furthermore, this PCD also displays plant-specific features, including disappearance of starch grains, enlargement and collapse of vacuoles, and expansion of the ECM. In contrast, PCD does not occur in the nucellus before pollination (Hiratsuka et al. 2002).
With the tobacco style, transmitting tissue becomes highly disorganized throughout by the time most pollen tubes reach halfway down the style (Wang et al. 1996). In Solamum americana, DNA fragmentation was detected in the transmitting tissue of the pollinated style, but was absent in that of the unpollinated style (Sin and Chye 2004). Herrero and Dickinson (1979) reported that transmitting tissue cells close to pollen tubes appear necrotic in Petunia hybrida. In the gymnosperm Zamia, several layers of nucellar cells that surround the pollen tube appeared degenerated (Choi and Friedman 1991). In Pseudotsuga menziesii, nucellar cells that came into contact with the tip of the pollen tube degenerated, but many adjacent cells that did not contact the tip showed no degeneration (Owens and Morris 1990). Conversely, Crawford et al. (2007) reported that initiation and progression of PCD in the transmitting tissue does not specifically require pollination. Those findings suggest that several types of PCD may occur in the transmitting and nucellar tissues accompanying pollen tube growth.
In tobacco transmitting tissue, arabinogalactan proteins (AGPs) are secreted in the ECM from the transmitting tissue when pollen tubes extended into styles, but AGPs decreased when pollen tubes passed through the style tissue (Qin et al. 2007). In vitro, transmitting tissue-specific (TTS) protein, which is one of the AGPs distributed to the transmitting tissue of tobacco, promotes pollen tube growth and attracts the pollen tube (Cheung 1996; Wu and Cheung 2000). Wu et al. (1995) observed that TTS proteins are incorporated into the pollen tube wall and are deglycosylated by the pollen tube and suggested that TTS proteins may form a nutrient source for pollen tubes. Those findings suggest that the PCD of the transmitting tissue plays a role in generating the materials necessary for pollen tube growth. PCD is also thought to create space for the elongating pollen tubes (Wu and Cheung 2000). However, the actual mechanisms involved in this process have yet to be sufficiently elucidated.
Autophagy is a major degradation and recycling system in eukaryotic cell. In plants, autophagy has been shown to play diverse roles during plant development and environmental stress responses. According to van Doorn and Woltering (2005), autophagy in plants may be categorized into three types: micro-autophagy, macro-autophagy, and mega-autophagy. Micro-autophagy involves direct invagination and fission of the vacuolar membrane, macro-autophagy involves the formation of double membrane-bound autophagosomes that enclose the cytoplasm, and mega-autophagy is shown by rupture of the vacuolar membrane. In some angiosperms, cell contents are degraded by autophagy in association with PCD (Vigil 1970; Fukuda 1996; Matile 1997; Inada et al. 1998; Toyooka et al. 2001; Gaffal et al. 2007). In gymnosperms, autophagy has been observed in Picea abies in association with suspensor degradation (Filonova et al. 2000) and in Pinus sylvestris in association with degradation of subordinate embryos in a polyembryonic seed (Filonova et al. 2002). However, little is known about autophagy in transmitting tissue for growth of the pollen tube.
In most gymnosperms, the period from pollination to fertilization is much longer than that in angiosperms (Biswas and Johri 1997). Indeed, the pollen tube in P. densiflora remains in nucellar tissue for a period of a year or more. Substances degraded in PCD of nucellar cells may thus be reused as a source of nutrients during that time. However, details of the processes of degradation of starch grains stored in nucellar cells and other intracellular components accompanying cell death, as well as the role of vacuoles in the degradation process and the manner of transporting degraded substances from nucellar cells to the pollen tube, have yet to be elucidated.
The present study focused on the processes of degradation for nucellar cell components during PCD and their transport to the pollen tube and discussed how the recycling of the dead cell materials contributes to pollen tube growth in the gymnosperm P. densiflora.
Materials and methods
Plant material
Female cones of P. densiflora Siebolt et Zucc. were collected at selected intervals over a 14-month period before and after pollination in Tokyo. Some cones were covered with bags to prevent pollination for 2 weeks.
Embedding in Technovit 7100
Ovules were fixed with 4% (w/v) paraformaldehyde in 50 mM phosphate-buffered saline (pH 7.4) at 4°C overnight, dehydrated in a graded ethanol series, and embedded in Technovit 7100 resin (Heraeus Kulzer, Germany).
Embedding in epoxy resin
Samples were prepared using chemical fixation (CF) and high-pressure freezing fixation (HPF). For CF, ovules were fixed overnight with 2% glutaraldehyde in 0.1 M phosphate buffer (PB), pH 6.8 at 4°C. They were then rinsed in PB, postfixed in 2% buffered osmium tetroxide at 4°C for 1 h, dehydrated in an ethanol series, and embedded in Spurr’s resin (Polysciences, USA) at 70°C for 48 h.
For HPF, ovules were placed in a sample carrier and the carrier was filled with 1-hexadecene. They were frozen in a HPM010 apparatus (BAL-TEC, Liechtenstein) and were stored in liquid nitrogen until freeze substitution. Samples were subjected to substitution in 2% OsO4-acetone for 2–3 days, dehydrated, and embedded in Quetol 812 resin (Nissin EM, Japan).
Measurement of pollen tube length
Serial semi-thin sections (2 μm) of epoxy resin-embedded ovule were mounted on glass slides and stained with toluidine blue. Lengths of pollen tubes were measured using an Olympus DP25 microscope digital camera (Olympus, Japan). Measurements were made on the longest branch of the pollen tube for each ovule, and four pollen tubes were measured for each stage.
Three-dimensional structure of the pollen tube
The serial semi-thin sections (2 μm) stained with toluidine blue were observed with a light microscope. Outlines of the pollen tube and ovule were traced on photograph of each section. Serial drawings were digitized and the 3-D reconstruction was made with TRI computer software (RATOC, Japan).
Terminal deoxy nucleotidyl transferase-mediated dUTP-fluorescein nick-end labeling assay
Technovit-embedded sections were stained using a TaKaRa in situ apoptosis detection kit (Takara Bio, Japan) as described by Hiratsuka et al. (2002).
Reducing sugar localization
Ovules were fixed in a mixture of 4% paraformaldehyde and 1% glutaraldehyde in PB (pH 7.2) at 0°C for 1 h. They were rinsed with PB at 0°C overnight, incubated in Benedict’s solution (Nacalai Tesque, Japan) at 80°C for 5–10 min, and then rinsed in distilled water. The samples were postfixed in 2% osmium tetroxide in PB for 1 h, dehydrated, and embedded in epoxy resin.
Starch staining
For whole mount and starch visualization, ovules were fixed in a 9:1 mixture of ethanol and acetic acid. The samples were rinsed with 90%, 70%, 50%, and 30% ethanol for 20 min, respectively, and were incubated in chloral hydrate/glycerol solution (chloral hydrate 8 g, glycerol 1 ml, distilled water 2 ml) for several days until most tissues had become transparent. They were stained with Lugol solution for starch grains.
The extent of macro-autophagy
The ratio of total autophagosome area to cytoplasmic area was assessed on electron micrographs. Twelve cells in each area were analyzed using Scion Image software (Scion Corporation, USA).
Results
Pollen tube growth and nucellar cell death
The period from pollination to fertilization is approximately 13 months in P. densiflora. In Tokyo, pollination and pollen tube germination occur in May. During that time, the pollen tube intermittently grew approximately 900 μm through nucellar tissue toward the female gametophyte (Fig. 1a). After germination, the pollen tube grew for approximately 3 months forming multiple branches, with the apex of each branch extending about 200 μm. In the region up to about 200 μm from the nucellar apex, most of the region was occupied by the pollen tube. Growth then ceased for approximately 9 months and the tube cell nucleus remained in the base of the branched pollen tube (Fig. 1b). Around May of the following year, the generative cell divided in the pollen grain, and the body cell and stalk cell formed by that division moved into the pollen tube and co-located with the tube cell nucleus, after which they all entered a single branch of the pollen tube, as a reproductive tube. The single branch included a large quantity of starch grains (Fig. 1c). At about this time, archegonia formed in the female gametophyte. The single branch then began to grow again, showing rapid elongation. At the beginning of June, the single branch reached the female gametophyte (Fig. 1d). The body cell divided, producing a bi-nuclear sperm cell (one large, one small), and fertilization occurred. The single branch was thicker than the other branches (Fig. 1e) that did not resume growth (Fig. 1f).
Pollen tube growth in nucellus. a Graph of pollen tube growth. The pollen tube grows intermittently by approximately 900 μm and reaches the female gametophyte (June of the following year). Results represent means ± SD (n = 4). b A 3-D reconstruction from serial transverse sections of an ovule 2 months after pollination. The pollen tube branches profusely. The tube cell nucleus (TN) is located at the base of the branched pollen tube. Blue area, pollen tube (PT); green area, nucellar tissue (NU); pink area, female gametophyte (FG). c Pollen tube 1 year after pollination. TN, body cell (BC) and stalk cell (SC) are moved into one of the branch. The pollen tube includes many starch grains (arrows). d The nucellus 13 months after pollination. A single branch of the pollen tube (star) reaches the FG through the nucellus. Dotted lines show the distance from the nucellar apex. e Cross-section of the nucellus 200 μm from the nucellar apex. One large branch (star) and many other narrow branches (asterisks) are observed. Two to three layers of nucellar cells around the pollen tube degenerate. f Cross-section of the nucellus 260 μm from the nucellar apex. Only the large branch (star) is seen in the nucellus and two to three layers of nucellar cells around the branch degenerate. g A dying cell around the branch. Cell wall thickening, ECM expansion, and nuclear condensation are observed. h Nucellus 1 year after pollination. Nucellar cells around the tubes are labeled by TUNEL (arrows). i Nucellar cells that do not undergo PCD before pollination
After pollination, two to three layers of nucellar cells around the pollen tube degenerated, exhibiting cell wall thickening, ECM expansion, cytoplasmic shrinkage, and nuclear condensation (Fig. 1e–g). In about five layers of the nucellar cells away from the multiple branches of the pollen tube, nuclei were terminal deoxy nucleotidyl transferase-mediated dUTP-fluorescein nick-end labeling (TUNEL)-positive, indicating cell death accompanied by DNA fragmentation (Fig. 1h). PCD did not occur in nucellar cells before pollination (Fig. 1i).
Relationship of nucellar starch grain levels and pollen tube growth
The distribution of starch grains in the nucellus longitudinal section 1 year after pollination was investigated by double staining with toluidine blue and the periodic acid Schiff (PAS) reaction, revealing that the nucellus could be divided into three zones of starch grain distribution. The pollen tube zone (PTZ), located at the apex of the nucellus, was occupied by the branched pollen tube and contained almost no starch grains. The starch grain zone (SGZ) located in the nucellus middle contained numerous starch grains. The female gametophyte zone (FGZ) located near the female gametophyte contained a small amount of starch grains (Fig. 2a). Almost no starch grains appeared anywhere in the nucellus when pollination was artificially blocked (Fig. 2b), and the nucellus degenerated after about 3 months. To investigate changes in the distribution of starch grains in the nucellus (i.e., extent of the SGZ) over time after pollination, nucelli collected at different times were subjected to Lugol staining. The Lugol solution stains the starch grains dark blue or black.
Relationship between nucellar starch grain levels and pollen tube growth. a Toluidine blue and PAS staining of the nucellus longitudinal section 1 year after pollination. The nucellus can be divided into three zones. The PTZ is located at the apex of the nucellus, is occupied by the multi-branched pollen tube, and contains almost no starch grains. The SGZ is located in the middle of the nucellus, containing numerous starch grains. The FGZ located near the female gametophyte contains a small amount of starch grains. b Nucellus artificially blocked from pollination. Almost no starch grains appear anywhere in the nucellus. c–f Whole mount Lugol-stained nucellus indicating the presence of starch grains. c Soon after pollination, abundant starch grains appear in the region up to about 200 μm from the nucellar apex and form SGZ. d Two months after pollination, starch grains are lost from the region transited by the pollen tube and that region changes to PTZ. The pollen grain (PG) and growing pollen tube (PT) are stained with Lugol solution. e Four months after pollination, starch grains that formed up to 200 μm from the nucellar apex have disappeared. f One year after pollination, the synthesis of starch grains resume in all parts of the nucellus except for PTZ. Starch grains also appear in the PG and PT. Bar, 100 μm
In early May, soon after pollination, pollen germinated in the pollen chamber formed at the apex of the nucellus, but the pollen tube had yet to penetrate the nucellar tissue. In the region up to about 200 μm from the nucellar apex, abundant starch grains appeared and formed an SGZ (Fig. 2c). About 2 months later, after pollen tube growth and branching in the nucellus, starch grains were lost from the region transited by the pollen tube. The nucellar apex region changed to the PTZ, and the SGZ shrank from the nucellar apex side and expanded approximately 50 μm on the female gametophyte side. Pollen grains and tubes were also stained with Lugol solution (Fig. 2d). About 4 months after pollination, the pollen tube stopped growing. The part of the SGZ formed up to 200 μm from the nucellar apex immediately after pollination disappeared, and starch grains were not observed in the pollen tube (Fig. 2e). In some cases, during the period when the pollen tube was not growing, synthesis of new starch grains in the FGZ was observed (Fig. 2e). In May of the following year, the pollen tube began to grow again (Fig. 1a), with synthesis of starch grains resuming in all parts of the nucellus except for the PTZ (Fig. 2f). Starch was also synthesized in the pollen grain and tube.
Approximately 2 weeks after pollination, nucellar cells were examined by electron microscopy using HPF. Amyloplasts in the SGZ contained large starch grains (Fig. 3a), but starch grains in amyloplasts in the PTZ had shrunk or disappeared (Fig. 3b, c). Amyloplasts observed in the PTZ included those surrounded by the endoplasmic reticulum (ER) (Fig. 3d), those enclosed in multilayered elongate vacuoles (Fig. 3e), and those that had been taken into vacuoles (autophagic vacuoles) (Fig. 3f). Some amyloplasts in the FGZ contained small starch grains, while others contained almost no starch grains.
Electron micrographs of nucellus at 2 weeks after pollination (HPF method). a In the nucellar cell of the SGZ, amyloplasts (arrows) contain large starch grains. b–f Nucellar cells in PTZ. b Amyloplasts containing small starch grains are observed near the pollen tube (PT). c Starch grains (S) in amyloplasts become smaller. d Amyloplast surrounded by ER. e Amyloplast enclosed in multilayer vacuoles. f Amyloplast taken into a vacuole (V)
Using Benedict's reagent, reducing sugars were detected in the PTZ cells around the pollen tube (Fig. 4a), particularly in the periphery of starch grains in amyloplasts (Fig. 4b) and in the vacuoles (Fig. 4c). Reducing sugars were not detected in nucellar cells more than about five layers away from the tube (Fig. 4d).
Reducing sugar localization in a nucellar cell in PTZ. a Reducing sugars are detected in amyloplasts and vacuoles (arrows). b, c Magnified view of nucellar cells close to the pollen tube. Reducing sugars (arrows) distribute to the periphery of starch grains (S) in amyloplasts (b) and throughout the entire vacuoles (c). d Reducing sugars are not detected in nucellar cell more than about five layers away from the tube tip
Morphological changes of vacuole accompanying cell death
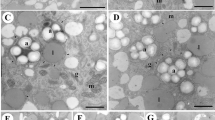
Autophagic vacuoles containing various vesicles and degenerated organelles were observed in the PTZ cells (Figs. 3f and 5g, o). To clarify morphological changes of vacuoles during cell death, the vacuoles of each part of the nucellus were observed in detail. When ovule sections obtained approximately 1 year after pollination were stained with toluidine blue, vacuoles in FGZ cells were barely stained at all (Fig. 5a), while vacuoles in PTZ cells showed dark staining (Fig. 5b). Vacuoles in the SGZ were small and whether any staining was actually present could not be determined under light microscopy. Under electron microscopy, vacuoles in FGZ cells displayed low vacuolar membrane contrast and low internal electron density. The vacuoles did not contain any organelles. These vacuoles were referred to as low-density vacuoles (LVs) (Fig. 5c). On the FGZ side of the SGZ, TUNEL-positive cells were not observed and LVs were present. On the PTZ side, TUNEL-positive cells were observed at low frequency. In addition, LVs, vacuoles with high vacuolar membrane contrast and high internal electron density and showing autophagy (high-density vacuoles—HVs), and intermediate-type vacuoles with features between those of LVs and HVs were observed (Fig. 5d). Intermediate-type vacuoles included vacuoles displaying high vacuolar membrane contrast and low internal electron density and showing autophagy (Fig. 5d) and vacuoles displaying low vacuolar membrane contrast and low internal electron density and showing autophagy. Vacuoles resulting from fusion of HVs and LVs were also identified (Fig. 5d). HVs with a structure in which amyloplasts were enclosed in multiple layers (Figs. 3e and 5d) and those with a structure of folded layers (Fig. 5e) were observed. Large amounts of ER and vesicles were present in cells, and some vesicles were distributed around LVs (Fig. 5f). In the PTZ, most vacuoles were HVs containing numerous vesicles of various size and other organelles (Fig. 5g).
Morphological changes of vacuoles accompanying nucellar cell death. a, b Nucellar cells approximately 1 year after pollination stained with touidine blue. a Vacuoles in FGZ cells are barely stained at all. b Vacuoles in PTZ cells show dark staining. c–o Electron micrographs of vacuoles in nucellar cells (HPF method). c Vacuoles in the FGZ cell. The vacuoles display low membrane contrast and low internal electron density. The vacuoles do not contain any organelles (LV). d–f Vacuoles on the PTZ side of the SGZ. d In addition to LVs, vacuoles with high membrane contrast and high internal electron density showing autophagy (HV) and intermediate-type vacuoles with features between those of LVs and HVs are observed (arrow). Some vacuoles resulting from fusion of HVs and LVs are also identified (arrowheads). e HVs with a structure of folded layers. f LVs fusing with some vesicles (arrows). g HV in PTZ containing various sized vesicles and degenerated organelle (arrow). h–l Autophagosomes in the PTZ and SGZ. Lipid bodies (h, i) and cytoplasm containing some organelles and/or vesicles (j–l) surrounded by a double membrane structure (autophagosome) were observed. h A cup-shaped autophagosome. Ribosomes attached to the membranes (arrows). m Small vacuoles enlarge by vacuolar fusion (arrows). n Engulfment of cytoplasm and organelles by micro-autophagy (arrow). o Collapse of the membrane of an enlarged vacuole (arrowheads) and micro-autophagy (arrow). Many vesicles and degenerated organelle (double arrow) are observed in the vacuole
In cells undergoing PCD (PTZ, SGZ), lipid bodies (Fig. 5h, i) and cytoplasm containing some organelles and/or vesicles (Fig. 5j–l) surrounded by a double membrane structure (autophagosome) were observed, whereas no autophagosomes were seen in the FGZ (Fig. 6). In some cases, cup-shaped autophagosome membranes were attached to ribosomes (Fig. 5h). We also observed a double membrane continuous with amyloplasts (Fig. 5i). Small vacuoles enlarged by vacuolar fusion (Fig. 5m) and engulfment of cytoplasm by micro-autophagy (Fig. 5n, o). As cell death progressed, membranes of enlarged vacuoles collapsed (Fig. 5o), with the vacuoles themselves ultimately breaking down and disappearing.
Quantification of autophagosome formation in PTZ, SGZ, and FGZ under electron microscopy. Area indices represent percentage values of the autophagosome area compared to the cytoplasm area. Autophagosome area index is higher in the PTZ than in the SGZ. No autophagosomes are seen in FGZ. Results represent mean ± SE (n = 12)
Release and movement of substances from dead cells to pollen tubes
In the PTZ, cells around the pollen tube underwent PCD, and the degree of PCD was dependent on the distance from the pollen tube. Cells in contact with the pollen tube were crushed and became vestigial. Cell wall thickening and ECM expansion were marked in dying nucellar cells (Fig. 7a). Vesicles of many different sizes and electron-dense amorphous material were released not only from the regions of contact with the pollen tube but also from the whole perimeter of dying nucellar cells and passed through the cell membrane to the thickened cell wall. With reference to the cell membrane, vesicles with a single layer membrane were observed on the cytoplasmic side (Fig. 7b, arrow 1), and vesicles with a double layer membrane (Fig. 7b, arrow 2) and vesicles releasing electron-dense contents due to collapse of the membrane were observed on the cell wall side (Fig. 7b, arrow 3). Electron density of the material released from vesicles resembled that of amorphous material released with the vesicles (Fig. 7b). Depending on the cell, budded vesicles from tubular organelles (Fig. 7c) and beaded vesicles (Fig. 7d) were released at the sites of cell membrane collapse, in the vicinity of the cytoplasm and cell wall border. The pollen tube was in contact with dying nucellar cells via the ECM, and protrusions with diameters of approximately 5 μm in the tip or side of the tube (Fig. 7e) penetrated nucellar cells forming convex–concave junctions, ensuring secure adhesion of the tube to the nucellar cells (Fig. 7f). In these convex–concave junctions, ECM between the pollen tube and nucellar cells was thin. Vesicles and amorphous material passed through the ECM and pollen tube wall (Fig. 7g). When vesicles reached the membrane of the tube cell, the membrane invaginated and vesicles were taken up by endocytosis (Fig. 7h, i).
Release and movement of vesicles and substances from dying cells to pollen tube in PTZ. A HPF method was used to observe the ultrastructure of nucellus and pollen tube. a Cells in contact with the pollen tube are crushed and become vestigial (arrow). b Vesicles with reference to the cell membrane of the dying cell. Vesicles can be seen showing a single layer membrane on the cytoplasmic side (arrow 1), a double layer membrane (arrow 2), and releasing electron-dense contents (asterisk) due to collapse of the membrane (arrow 3) on the cell wall side. c Budded vesicles from tubular organelles (arrows). d Beaded vesicles (arrows) released at sites of the cell membrane collapse (arrowhead). e A light microscopic image of protrusion (arrow) formed on the pollen tube in vitro. f Electron microscopic image of protrusions (arrows) penetrating nucellar cells (NC) forming convex–concave junctions in vivo. g Vesicles (arrows) and amorphous material (asterisk) released from the cytoplasm of dead NC to the tube wall. h When vesicles reach the membrane of the tube, the membrane invaginates (arrows) and takes up vesicles by endocytosis (arrowheads). i Release of vesicle from dying nucellar cell (arrowhead) and endocytosis of vesicles by pollen tube (arrow) are also observed in areas other than the convex–concave junction. N nucleus of nucellus cell, ECM extracellular matrix, PTW pollen tube wall, NCW nucellar cell wall
Discussion
Intermittent growth and functional differentiation of the pollen tube
The period from pollination to fertilization is approximately 13 months in P. densiflora, but the pollen tube actually only grows for about 4 months. During the other 9 months, the tube does not grow. Pollen tube growth also ceases temporarily in Pinus contorta and Agathis australis, which have 2-year life cycles resembling that of P. densiflora (Owens et al. 1981a, b, 1995). The timing and duration of the cessation of pollen tube growth differ in each of those three species, but the period from resumption of pollen tube growth to fertilization is short in all of them. The trigger for cessation of pollen tube growth is unknown, but resumption is synchronous with formation of archegonia in P. densiflora. Since resumption of pollen tube growth in P. contorta is suppressed until immediately before maturation of the female gametophyte, Owens et al. (1981b) suggested that a factor regulating coordinated development of the pollen tube and the female gametophyte may exist. In the angiosperms Alnus firma, Alnus sieboldiana, and Fagus japonica, the pollen tube grows intermittently in coordination with the development of the embryo sac during the period from pollination to fertilization (Sogo and Tobe 2005, 2006).
Two types of functional differentiation were seen in pollen tubes of P. densiflora. The first type was a haustorial function associated with multiple branching of the pollen tube. Due to such branching, the pollen tube occupied most of the region up to about 200 μm from the nucellar apex. The branched tube functions mainly as a haustorial tube. This interpretation was supported by the observations that (1) many nucellar cells around the tube had died, (2) small protrusions from the branched tube extended into the dying cells forming convex–concave junctions that would be useful in performing haustorial functions, and (3) dying cell contents were transported to the pollen tube by vesicles. The small protrusions resembled the outgrowths seen in Zamia although of which pollen tube did not branch (Choi and Friedman 1991). Pollen tubes of Ginkgo and Cycas, representing gymnosperms with primitive sperm fertilization, grow in the nucellus while branching (Friedman 1987). These pollen tubes do not convey sperm cells and are considered to play haustorial roles (Johri 1992). The Pinus haustorial tube thus resembles pollen tubes from zoidogamous gymnosperms. The second type was a reproductive function by the single branch that conveyed sperm cells. That branch, as a reproductive tube, was thicker and grew more rapidly than the other branches. About 1 year after pollination, the tube nucleus, body cell, and stalk cell were co-located into one branch and the branch then resumed growth. The nucleus and/or those cells thus appear to induce differentiation of the pollen tube. In Ephedra, which is regarded as being close to angiosperms, the pollen tube does not branch and functions to convey sperm cells. The Pinus reproductive tube is thought to be equivalent to the pollen tube of Ephedra or angiosperms.
Role of starch grains in pollen tube growth
The present study showed that pollination triggers starch grain synthesis in nucelli of P. densiflora. In the region up to about 200 μm from the nucellar apex, starch grains were successively lost from the area transited by the pollen tube. In P. contorta (Owens et al. 1981a), P. menziesii (Owens and Morris 1990), and A. australis (Owens et al. 1995), starch grains are abundant in the tip of the nucellus and degrade when the pollen tube grows. In Nicotiana tabacum (Bell and Hicks 1976), P. hybrida (Herrero and Dickinson 1979), Persea americana (Sedgley 1979), and other angiosperms, starch is abundant in the stigma and stylar transmitting tissue, which form the path of the pollen tube, and starch disappears as the pollen tube grows in P. hybrida and P. americana. These results suggest that starch grains in nucelli of P. densiflora are synthesized and stored as a nutrient source for pollen tube growth. During the period when pollen tube growth had ceased, new synthesis of starch grains did not occur in the nucellus. Starch grains disappeared from the pollen tube, and transport of nutrients from nucellar cells to the pollen tube seemed to have almost stopped. In some cases, new starch grain synthesis did occur in the FGZ at that time, but those starch grains were thought to play a part in the development of the female gametophyte, which began before the resumption of pollen tube growth. When the pollen tube began to grow toward the female gametophyte again, numerous starch grains appeared throughout the nucellus except in the PTZ. These starch grains seem likely to be used as an energy source and as raw materials for the pollen tube, which suddenly resumes growth, and to be involved in development of the female gametophyte.
To date, starch has been reported to be degraded by α-amylase in vacuoles of Vigna mungo cotyledons (Toyooka et al. 2001) and in the chloroplasts of Oryza sativa leaves (Asatsuma et al. 2005). However, the intracellular location of catabolic enzymes is not well understood, and information on the degradation mechanisms for starch is insufficient. In the present study, starch grains stored in the nucelli of P. densiflora are degraded in amyloplasts of cells undergoing PCD. Enzymes carrying out this degradation were thought to be synthesized in large amounts of ER around amyloplasts. Reducing sugars that were thought to represent a degradation product of starch were detected in amyloplasts and vacuoles of cells undergoing PCD in a experiment using Benedict's reagent, an indicator that detects reducing sugars. Starch degradation was thought to take place mainly in amyloplasts, and degradation products, i.e., reducing sugars, are subsequently stored in vacuoles. Reducing sugars in vacuoles are ultimately released from the nucellar cell by vacuolar collapse and may be made available to the pollen tube.
Role of vacuoles in PCD
Vacuolar morphology and functions change as the plant grows or cells differentiate (Marty 1999). The role of vacuoles in plant PCD is attracting attention, since plants do not have macrophages. Autophagy is known as one of the mechanisms for the degradation of cellular contents in order to recycle nutrients or break down damaged or toxic material (Bassham 2007). We have previously reported that in P. densiflora nucellar cells undergoing PCD, vacuoles expand and then ultimately collapse (Hiratsuka et al. 2002). The present study showed that several types of vacuoles with different morphologies are present in three zones of the nucellus: the FGZ, SGZ, and PTZ. The FGZ, which is composed of living cells, contains LVs. Cells on the FGZ side of the SGZ, which contains abundant starch grains, did not undergo PCD, and small LVs were observed in those cells. On the PTZ side of the SGZ, cells undergoing PCD were present at low frequency, and several types of vacuoles were observed. Those types were LVs, HVs, and vacuoles with features between those of LVs and HVs. In the PTZ nucellar cells undergoing PCD, most vacuoles were HVs containing numerous vesicles and organelles.
Toluidine blue, which stains polyvalent acidic substances, favorably stained HVs but did not stain LVs. Benedict's reagent showed that reducing sugars were stored in HVs of PTZ. These results suggest that substances stored in HVs differ from those stored in LVs. As the membranes of LVs show low contrast while the membranes of HVs show high contrast, differences in membrane components such as channel proteins and transporter proteins are expected. Differences in toluidine blue staining and internal electron density were considered to probably result from HVs and LVs taking up different materials due to differences in membrane components. Whether HVs are formed de novo or result from changes in LVs is unknown. However, vesicles have been observed around LVs. In addition, there are intermediate-type vacuoles with features between those of LVs and HVs. Thus, LVs may change into HVs by the uptake of vesicles containing different proteins as cells undergo PCD.
In cells undergoing PCD, intracellular components are thought to be rapidly degraded in vacuoles as a result of induction of autophagic vacuoles that contain more enzyme groups involved in degradation than do living cells. The present results revealed three autophagic processes: micro-autophagy, macro-autophagy, and mega-autophagy. Most autophagosomes are about 1 μm in diameter, similar to the size of animal autophagosomes. In some cases, autophagosome membranes attach to ribosomes, suggesting that these membranes are derived from ER. In other cases, a double membrane is continuous with amyloplasts. Autophagosomes were frequently observed in PTZ cells, but were not seen in the FGZ. This result suggested that PCD induces autophagosome formation. To date, several types of membrane structures are thought to form plant autophagosomes, as cytoplasm engulfed by proplastids was observed in PCD of P. abies embryo cells (Filonova et al. 2000), and microbodies surrounded by ER have been reported in the germination process of Ricinus communis seeds (Vigil 1970). The autophagosome membrane has also been suggested to derive from the ER in yeast and rat liver (Bernales et al. 2006; Dunn 1990) and a subdomain of the ER forms a cradle for autophagosome formation in mammalian culture cells (Hayashi-Nishino et al. 2009). The origin of the autophagosome membrane remains controversial. On the other hand, ER-derived compartment, ER bodies were reported in Arabidopsis (Hayashi et al. 2001), but their structure and size are different from the autophagosomes found in Pinus nucellus. In this study, structures consisting of amyloplasts wrapped in multiple layers of vacuole were observed in the SGZ, but relationships between these structures and autophagy are unknown.
In the final stage of PCD in plants, cell contents are degraded as a result of the collapse of vacuoles (Fukuda 1996; Matile 1997). Likewise in P. densiflora nucelli, vacuoles expand by micro- and macro-autophagy and fusion and then ultimately collapse (mega-autophagy), leading to degradation of the entire cell. Degraded cell contents are then released into the ECM, which is the path of the pollen tube, and are thus supplied to the pollen tube.
Transport of degraded cell contents to the pollen tube
To date, studies have shown that pollen tubes of N. tabacum and N. sylvestris grow while taking up material by endocytosis (Moscatelli et al. 2007; Lisboa et al. 2008). In fungi, vesicular transport to deliver substances across their cell walls was reported (Casadevall et al. 2009). In gymnosperms, the pollen tubes of Ginkgo, Cycas, and Zamia are thought to play haustorial roles, but no evidence suggests uptake of material from their peripheral cells by endocytosis.
In the present study, vesicles that had budded from tubular organelles, amorphous material, and vesicles of many different sizes formed by degradation of vacuoles were released from the cytoplasm of cells undergoing PCD. After release, some vesicles collapsed. As the electron density of the material released from vesicles resembled that of amorphous material, some of the amorphous material had probably represented vesicular content. The identity of the tubular organelles from which vesicular budding occurred could not be specified, as those organelles showed diverse sizes and orientations, and did not display attached ribosomes.
After release from the cytoplasm, vesicles and amorphous material might have passed through the thickened cell wall and expanded ECM to reach the pollen tube. Since movement of vesicles in cell walls and ECM was not seen in locations where PCD did not occur, movement of vesicles and material was thought to probably be facilitated by structural changes in cell walls and ECM, changes in micropore size, and other such mechanisms in association with PCD. The vesicles that reached the pollen tube were taken up by the tube via endocytosis of individual vesicles occurring mainly at the tip of the pollen tube and at convex–concave junctions. These results show that the multiple branches of pollen tubes that grow up to about 200 μm play a haustorial role in P. densiflora.
Involvement of clathrin-coated vesicles in endocytosis in tobacco pollen tubes was reported (Lisboa et al. 2008), but no structures showing a clathrin coating were observed in the present study. The identity of materials in vesicles transported to the pollen tube remains unclear. The analysis of vesicular transport in fungi revealed that vesicles contain proteins, lipids, and polysaccharides (Casadevall et al. 2009). Those materials can be candidates for vesicular cargo in Pinus.
The present study showed that in nucelli of P. densiflora, materials are supplied from nucellar cells undergoing PCD to the pollen tube, and vesicular transport accompanied by endocytosis is one mechanism by which that supply occurs.
References
Asatsuma S, Sawada C, Itoh K, Okito M, Kitajima A, Mitsui T (2005) Involvement of α-amylase I-1 in starch degradation in rice chloroplasts. Plant Cell Physiol 46:858–869. doi:10.1093/pcp/pci091
Bassham DC (2007) Plant autophagy—more than a starvation response. Curr Opin Plant Biol 10:587–593. doi:10.1016/j.pbi.2007.06.006
Bell J, Hicks G (1976) Transmitting tissue in the pistil of tobacco: light and electron microscopic observations. Planta 131:187–200
Bernales S, McDonald KL, Walter P (2006) Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4:e423. doi:10.1371/journal.pbio.0040423
Biswas C, Johri BM (1997) The gymnosperms. Springer, Berlin
Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML (2009) Vesicular transport across the fungal cell wall. Trends Microbiol 17:158–162. doi:10.1016/j.tim.2008.12.005
Cheung AY (1996) Pollen–pistil interactions during pollen-tube growth. Trends Plant Sci 1:45–51
Choi JS, Friedman WE (1991) Development of the pollen tube of Zamia furfuracea (Zamiaceae) and its evolutionary implications. Am J Bot 78:544–560
Crawford BCW, Ditta G, Yanofsky MF (2007) The NTT gene is required for transmitting-tract development in carpels of Arabidopsis thaliana. Curr Biol 17:1101–1108. doi:10.1016/j.cub.2007.05.079
Dunn WA Jr (1990) Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J Cell Biol 110:1923–1933
Filonova LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivotovsky B, von Arnold S (2000) Two waves of programmed cell death occur during formation and development of somatic embryos in the gymnosperm, Norway spruce. J Cell Sci 113:4399–4411
Filonova LH, von Arnold S, Daniel G, Bozhkov PV (2002) Programmed cell death eliminates all but one embryo in a polyembryonic plant seed. Cell Death Differ 9:1057–1062
Friedman WE (1987) Growth and development of the male gametophyte of Ginkgo biloba within the ovule (in vivo). Am J Bot 74:1797–1815
Fukuda H (1996) Xylogenesis: initiation, progression, and cell death. Annu Rev Plant Physiol Plant Mol Biol 47:299–325
Gaffal KP, Friedrichs GJ, El-Gammal S (2007) Ultrastructural evidence for a dual function of the phloem and programmed cell death in the floral nectary of Digitalis purpurea. Ann Bot 99:593–607. doi:10.1093/aob/mcm002
Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, Yamamoto A (2009) A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol 11:1433–1437. doi:10.1038/ncb1991
Hayashi Y, Yamada K, Shimada T, Matsushima R, Nishizawa NK, Nishimura M, Hara-Nishimura I (2001) A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol 42:894–899
Herrero M, Dickinson HG (1979) Pollen–pistil incompatibility in Petunia hybrida: changes in the pistil following compatible and incompatible intraspecific crosses. J Cell Sci 36:1–18
Hiratsuka R, Yamada Y, Terasaka O (2002) Programmed cell death of Pinus nucellus in response to pollen tube penetration. J Plant Res 115:141–148
Inada N, Sakai A, Kuroiwa H, Kuroiwa T (1998) Three-dimensional analysis of the senescence program in rice (Oryza sativa L.) coleoptiles. Planta 206:585–597
Johri BM (1992) Haustorial role of pollen tubes. Ann Bot 70:471–475
Lisboa S, Scherer GEF, Quader H (2008) Localized endocytosis in tobacco pollen tubes: visualisation and dynamics of membrane retrieval by a fluorescent phospholipid. Plant Cell Rep 27:21–28. doi:10.1007/s00299-007-0437-1
Marty F (1999) Plant vacuoles. Plant Cell 11:587–599
Matile P (1997) The vacuole and cell senescence. Adv Bot Res 25:87–112
Moscatelli A, Ciampolini F, Rodighiero S, Onelli E, Cresti M, Santo N, Idilli A (2007) Distinct endocytic pathways identified in tobacco pollen tubes using charged nanogold. J Cell Sci 120:3804–3819. doi:10.1242/jcs.012138
Owens JN, Simpson SJ, Molder M (1981a) Sexual reproduction of Pinus contorta. I. Pollen development, the pollination mechanism, and early ovule development. Can J Bot 59:1828–1843
Owens JN, Simpson SJ, Molder M (1981b) Sexual reproduction of Pinus contorta. II. Postdormancy ovule, embryo, and seed development. Can J Bot 60:2071–2083
Owens JN, Morris SJ (1990) Cytological basis for cytoplasmic inheritance in Pseudotsuga menziesii. I. Pollen tube and archegonial development. Am J Bot 77:433–445
Owens JN, Catalano GL, Morris SJ, Aitken-Christie J (1995) The reproductive biology of Kauri (Agathis australis). I. Pollination and prefertilization development. Int J Plant Sci 156:257–269
Pennell RI, Lamb C (1997) Programmed cell death in plants. Plant Cell 9:1157–1168
Poort RJ, Visscher H, Dilcher DL (1996) Zoidogamy in fossil gymnosperms: the centenary of concept, with special reference to prepollen of late paleozoic conifers. Proc Natl Acad Sci USA 93:11713–11717
Qin Y, Chen D, Zhao J (2007) Localization of arabinogalactan proteins in anther, pollen, and pollen tube of Nicotiana tubacum L. Protoplasma 231:43–53. doi:10.1007/s00709-007-0245-z
Reape TJ, McCabe PF (2008) Apoptotic-like programmed cell death in plants. New Phytol 180:13–26. doi:10.1111/j.1469-8137.2008.02549.x
Sedgley M (1979) Structural changes in the pollinated and unpollinated avocado stigma and style. J Cell Sci 38:49–60
Sin SF, Chye ML (2004) Expression of proteinase inhibitor II proteins during floral development in Solanum americanum. Planta 219:1010–1022. doi:10.1007/s00425-004-1306-6
Sogo A, Tobe H (2005) Intermittent pollen-tube growth in pistils of alders (Alnus). Proc Natl Acad Sci USA 102:8770–8775
Sogo A, Tobe H (2006) Delayed fertilization and pollen-tube growth in pistils of Fagus japonica (Fagaceae). Am J Bot 93:1748–1756
Toyooka K, Okamoto T, Minamikawa T (2001) Cotyledon cells of Vigna mungo seedlings use at least two distinct autophagic machineries for degradation of starch granules and cellular components. J Cell Biol 154:973–982
Vigil EL (1970) Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol 46:435–454
van Doorn WG, Woltering EJ (2005) Many ways to exit? Cell death categories in plants. Trends Plant Sci 10:117–122. doi:10.1016/j.tplants.2005.01.006
Wang H, Wu Hm, Cheung AY (1996) Pollination induces mRNA poly (A) tail-shortening and cell deterioration in flower transmitting tissue. Plant J 9:715–727
Wu Hm, Wang H, Cheung AY (1995) A pollen tube growth stimulatory glycoprotein is deglycosylated by pollen tubes and displays a glycosylation gradient in the flower. Cell 82:395–403
Wu Hm, Cheung AY (2000) Programmed cell death in plant reproduction. Plant Mol Biol 44:267–281
Acknowledgments
We wish to thank Laboratory of Electron Microscopy, Japan Women’s University for the use of the HPM010 apparatus. This work was partially supported by a grant-in-aid for Scientific Research from the Japan Society for the Promotion of Science (19657023).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Benedikt Kost
Rights and permissions
About this article
Cite this article
Hiratsuka, R., Terasaka, O. Pollen tube reuses intracellular components of nucellar cells undergoing programmed cell death in Pinus densiflora . Protoplasma 248, 339–351 (2011). https://doi.org/10.1007/s00709-010-0176-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0176-y