Abstract
Conifer pollen tubes are an important but underused experimental system in plant biology. They represent a major evolutionary step in male gametophyte development as an intermediate form between the haustorial pollen tubes of cycads and Ginkgo and the structurally reduced and faster growing pollen tubes of flowering plants. Conifer pollen grains are available in large quantities, most can be stored for several years, and they grow very well in culture. The study of pollen tube growth and development furthers our understanding of conifer reproduction and contributes towards our ability to improve on their productivity. This review covers taxonomy and morphology to cell, developmental, and molecular biology. It explores recent advances in research on conifer pollen and pollen tubes in vivo, focusing on pollen wall structure, male gametophyte development within the pollen wall, pollination mechanisms, pollen tube growth and development, and programmed cell death. It also explores recent research in vitro, including the cellular mechanisms underlying pollen tube elongation, in vitro fertilization, genetic transformation and gene expression, and pine pollen tube proteomics. With the ongoing sequencing of the Pinus taeda genome in several labs, we expect the use of conifer pollen tubes as an experimental system to increase in the next decade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of pollen tubes occupies a crucial role in the sexual reproduction of seed plants. In conifers, pollen tubes deliver the male gametes (sperm) into the egg cells, and in the process, interact with the nucellar cells of the ovule and the archegonia of the female gametophyte. The pollen tubes of conifers represent an intermediate form between the haustorial pollen tubes of cycads and Ginkgo and the organizationally simplified and faster growing pollen tubes of flowering plants. Conifer pollen and pollen tubes are characterized by many features that are not found in flowering plants. The differences are not subtle, but represent a major evolutionary divergence in the development of the male gametophytes. Therefore, the study of pollen tube development in conifers not only offers insight into a more primitive form of sexual reproduction, but also expands our understanding of this important stage in the reproductive process of conifers and seed plants in general.
Reports in this area of conifer biology are few, which could be attributed to several factors including the long generation time and size of these conifers. In spite of the limitations, a few laboratories have consistently produced interesting data and thereby contributed to the foundation of our understanding of the mechanisms of pollen tube development in conifers. In the last 10 years, various research efforts have paralleled some of the ongoing research work in flowering plants. In fact, a model system has been established for the conifers, and sequencing of the genome of Pinus taeda is on going in various laboratories (Lev-Yadun and Sederoff 2000). Although we do not anticipate the level of research work in conifers will ever match that of flowering plants, we believe that the use of conifer pollen tubes as an experimental system will increase in the next decade. This review is an attempt to compile and summarize much of the available literature on conifer pollen tubes and is not meant to be a comprehensive analysis of the subject.
Classification and evolution of conifers
Conifers are an ancient group of gymnospermous woody plants that is presently considered to consist of eight families, 68 genera, 629 species and numerous varieties and cultivars. They represent some of our most important lumber species as well as ornamentals throughout the world. The families include the Araucariaceae (three genera), Cephalotaxaceae (one genus), Cupressaceae (28 genera), Phyllocladaceae (one genus), Pinaceae (11 genera), Podocarpaceae (18 genera), Sciadopityaceae (one genus), and Taxaceae (five genera) (Farjon 1998). Some authors still differentiate the Cupressaceae into the Cupressaceae and the Taxodiaceae, this giving nine families, but the present trend is to combine these two.
The first occurrence of recognizable conifers was in the Triassic, about 220 mn years ago, from primitive gymnosperms with seed-bearing cupules (Miller 1975). The cupules were single- or multi-seeded and composed of modified branchlets that became variously fused and arranged to eventually give rise to strobilus-like structures containing gymnospermous integumented ovules that formed naked seeds (Meyen 1984). The earliest extant conifer family recognized in the fossil record is thought to be the Podocarpaceae, a tropical and southern hemisphere family, but within about 30 mn years (at the end of the Triassic and beginning of the Jurassic), fossils of most of the other families are recognizable. With the location of continents quite different from the present and the climate much warmer, rapid evolution occurred, producing a tremendous diversity of conifers bearing vegetative and reproductive structures quite similar to modern conifers. In more recent times, as temperatures cooled, there has been a contraction in the distribution of conifers towards equatorial and tropical regions, followed by expansion in warmer periods towards polar regions and higher elevations. These cycles have repeated several times during the ice ages, resulting in many monotypic families and genera and isolated species (Farjon 1998).
During early conifer evolution, separate and distinctive compound megasporangiate strobili (seed cones) and simple microsporangiate strobili (pollen cones) appeared and these remained as distinct conifer traits. Bisporangiate strobili occasionally form in some modern species but this is considered to be a developmental anomaly, even though they may produce fertile pollen and viable seeds. Evolutionary diversification in conifers resulted in great diversity for such a small group, extending not only to cone morphology, but also male and female gametophyte development, gamete formation, fertilization, cytoplasmic inheritance (Bruns and Owens 2000), embryogenesis, and seed development within the cones (Singh 1978). This is particularly true of the male gametophyte and conifer pollination mechanisms (Owens et al. 1998), most of which are microscopic and less obvious than cone and seed morphology. All conifers are wind pollinated (anemophily) and form pollen tubes (siphonogamy), however, pollen and sperm structures, the method by which pollen enters the ovule, the number of cells within the shed pollen, and the time and method by which pollen tubes form have evolved along several different pathways.
Pollen wall structure
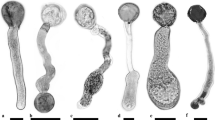
Conifer pollen may be saccate (Fig. 1) or non-saccate (Figs. 2, 3, 4), with smooth (Fig. 2), orbiculate (Fig. 3), or highly sculptured walls (Fig. 4) and contain varying numbers of cells when shed. These features may vary among families and even within families, as is the case within the Pinaceae (Figs. 1, 2, 4), which is the most diverse family in this regard (Owens and Simpson 1986).
Scanning electron micrographs of the four basic types of pollen in conifers
Pinus ponderosa pollen showing sacci and body (corpus)
Pseudotsuga menziesii pollen with indentation caused by normal dehydration before shedding
Chamaecyparis nootkatnesis pollen showing many small orbicules characteristic of the Cupressaceae
Tsuga heterophylla pollen showing spines on the exine
Conifer pollen has a thick multi-layered sporoderm or pollen wall. The outer wall is known as the exine (Fig. 5) and although complex in structure and chemical composition, it appears similar to that of angiosperms (Johri 1984). The exine consists of several layers. The outer exine layer (sexine) consists of a thick ektexine with a sculptured outer tectum supported by rod-like structures (infractectal units) that may fuse, forming ridges and valleys joining to a foot layer below, that in turn joins a thin, laminated inner endexine. In saccate pollen, large spaces form below the infractectal units separating the tectum from the foot layer. The exine is highly resistant to desiccation and decay because of sporopollenin (Kurmann 1989). In some non-saccate conifer pollen, the exine is shed when the pollen is hydrated whereas in other conifers, the pollen tube penetrates through a thin area of the exine, usually between the sacci (Fig. 5). The intine is a thin (Fig. 5), two-layered wall that forms the pollen tube.
Pollen water content and storage products
Mature pollen of the Pinaceae dehydrates to less than 10% water content before being shed resulting in remarkable aerial buoyancy. This allows the dehydrated pollen to be collected and stored at low temperatures (< −20°C) for several years (Webber and Bonnet-Masimbert 1989). Mature pollen of the Cupressaceae in contrast have a water content of about 30% making it difficult to store for long periods of time. Pollen water content and storage products are poorly understood for most other conifer families. Although storage products vary among families, the main storage product in Pinaceae is starch, with smaller amounts of lipid and proteins, whereas Cupressaceae pollen have large amounts of lipid and little starch (Owens 1993).
Male gametophyte development in relation to pollen tube formation
When conifer pollen is shed, different species may contain different numbers of cells or free nuclei but in none are the male gametes already formed. The terminology for conifer male gametophyte development within the pollen wall and following pollen germination has changed since first proposed by Strasburger in 1879 (Singh 1978). In many cases, the same cells have been called by more than one name, depending on the author and the species being described, and in no case has the terminology been consistent with that used for angiosperms (Jorhi 1984). This has resulted in confusion and misinterpretations by authors in many articles over the years and has made it difficult to compare pollen development among conifers and specifically between conifers and angiosperms. In response to this, a standard terminology that applies equally to all conifers and is consistent with that of angiosperms has been proposed (Fig. 6) (Owens and Bruns 2000). Such codification can only aid in the understanding of molecular models and cellular equivalence for non-flowering seed plants.
In conifers, meiosis occurs in the microsporocytes (pollen mother cells) within the microsporangia to form tetrads of haploid microspores. This entire process may occur before winter dormancy (some Chamaecyparis and Juniperus species in the Cupressaceae), or meiosis may begin before winter dormancy and become arrested at a diffuse diplotene stage, then resume and form microspores after winter dormancy (Larix, Pseudotsuga and Tsuga in the Pinaceae and Thuja in the Cupressaceae), whereas in other species, all stages of meiosis and pollen development occur after winter dormancy (Owens 1993). This phenology may be altered by environmental conditions, such as temperature, that may change the length of the growing season in temperate regions. Phenology is commonly variable and less predictable in tropical conifers. In all cases, meiosis results in the formation of a tetrad of haploid microspores that separate to form four unicellular microspores of equal-size.
Four patterns of cell division are recognized in conifer male gametophyte development (Fig. 6). These are as follows:
-
a.
In the Cupressaceae, the microspore divides by mitosis to form a large tube cell and a smaller generative cell before pollen is shed. Pollen is normally shed at the two-cell stage and the generative cell divides to form two sperms after pollen tube formation (Fig. 6a) (Singh 1978). The sperms are cells with cell walls and contain abundant organelles.
-
b.
In the Taxaceae and Cephalotaxaceae, pollen is normally shed at the one-cell stage. After being shed and entering the ovule, the microspore divides by mitosis to form a large tube cell and a smaller antheridial cell. The antheridial cell then divides to form a sterile cell and a generative cell, all contained within the large tube cell in the developing pollen tube. During pollen tube growth, the generative cell divides to form two sperm nuclei that remain closely associated with the organelles from the generative cell but are not separated by a cell membrane or wall (Fig. 6b) (Anderson and Owens 2000).
-
c.
In the Pinaceae, before the pollen is shed, the microspore divides unequally to form a first small prothallial cell and a large embryonal cell. The embryonal cell then divides unequally to form a second small prothallial cell and a large antheridial initial. Both prothallial cells are sterile and are pushed to one side forming a stack of two lens-shaped prothallial cells. The antheridial initial then divides unequally to form a large tube cell and a small antheridial cell; this is the condition of the four-cell pollen. The antheridial cell then divides equally to form a sterile cell and a generative cell. These are stacked on top of the two prothallial cells and result in the mature five-cell pollen (Fig. 5). Pollen may be shed at the four- or five-cell stage of development. After pollination, pollen germination, and formation of a pollen tube, the generative cell nucleus divides by mitosis to form two sperm nuclei of equal-size that remain enclosed within the generative cell wall and share its abundant organelles (Fig. 6c) (Owens and Bruns 2000). The sperm nuclei are not separated by a cell membrane or wall.
-
d.
The Araucariaceae, a southern hemisphere group, and the Podocarpaceae, a southern hemisphere and tropical group, have male gametophyte development similar to the Pinaceae, except that the first and second primary prothallial cells may undergo further divisions to form many secondary prothallial cells (Fig. 6d). The prothallial cells do not form a distinct cell wall and lose their cell membranes resulting in many free prothallial nuclei in the tube cell cytoplasm. The antheridial initial divides unequally to form a large tube cell and a smaller antheridial cell. All these cells and nuclei are contained within the tube cell. The antheridial cell divides equally to form a sterile cell and a generative cell. The generative cell divides to form two sperm nuclei during pollen tube growth. These sperm nuclei are not separated by a cell wall and remain within the generative cell cytoplasm within the thin generative cell wall until the time of fertilization. In Agathis of the Araucariaceae, the sperm nuclei are equal in size and engulf generative cell cytoplasm and organelles forming large complex sperm nuclei (Owens et al. 1995b). In Podocarpus of the Podocarpaceae, the sperm are unequal in size (Wilson and Owens 1999).
Pollination mechanisms in relation to pollen morphology and pollen tube formation
All conifers are siphonogamous, but the time and method of pollen tube formation and the length of the pollen tube relate to the method by which the pollen enters the ovule. There are five pollination mechanisms known for conifers (Owens et al. 1998). The methods correlate with four features: saccate or non-saccate pollen; erect or inverted ovules; presence or absence of a pollination drop; and length of the pollen tube. Two of these mechanisms involve a pollination drop that forms at the tip of the micropyle through secretions from the ovule.
-
a.
In the Cupressaceae, Taxaceae, Cephalotaxaceae and some Podocarpaceae the ovules are erect or have variable orientation, and are flask-shaped at pollination. The ovule secretes a large pollination drop that is exuded out of the micropyle to form a visible drop on the ovule tip. Pollen is non-saccate (Fig. 3) and upon landing on the drop sinks into the surface of the nucellus within the ovule, probably aided by the evaporation of the drop. On the surface of the nucellus, the pollen germinates and forms a pollen tube that grows into the nucellus and into an archegonium.
-
b.
In some Pinaceae (Pinus,Picea, Cedrus, and some Tsuga species) and some Podocarpaceae (Tomlinson et al. 1991), the ovules are inverted and a pollination drop is exuded out of the micropyle between micropylar arms. Pollen that lands on the micropylar arms is “picked up” by the pollination drop. Other pollen may be scavenged from adjacent cone surfaces by large pollination drops (Runions and Owens 1996a, 1996b). Pollen is saccate and the air-filled sacci cause the pollen to float up into the drop, through the micropyle and to the surface of the nucellus. The pollen germinates on the surface of the nucellus and grows through the nucellus and into an archegonium (Runions et al. 1995). Considerable branching of the pollen tube may occur during this growth (de Win et al. 1996, Owens et al. 2005). In this mechanism, short pollen tubes are formed.
-
c.
Abies species in the Pinaceae have saccate pollen, but the ovule does not appear to secrete a large pollination drop, rather rain or dew accumulate within the cone and form an artificial pollination drop that is functionally similar to the droplets of Pinus and Picea. In Abies amabilis, pollen often germinates in the micropylar canal and the pollen tube grows toward the nucellus as the nucellus grows toward the pollen tube; the two structures meet about midway in this unique mechanism (Chandler and Owens 2004).
-
d.
Pseudotsuga and Larix in the Pinaceae have non-saccate pollen (Fig. 2), inverted ovules and no pollination drop. Pollen that lands on the lobed integument tip is then engulfed into the micropylar canal of the ovule over several days. In the ovules of Pseudotsuga, the pollen sheds its exine and elongates the length of the micropylar canal. Only after the elongated pollen contacts the nucellus does a thin pollen tube form and grow through the nucellus and into an archegonium (Owens et al. 1981). In the ovules of Larix, the micropylar canal becomes filled with an ovular secretion that hydrates the pollen, causing it to swell and shed the exine. These pollen, enclosed only by the intine, float to the nucellar surface, where a pollen tube forms and grows into the nucellus (Owens et al. 1994).
-
e.
In some Tsuga species in the Pinaceae and all species of Araucariaceae, pollen is non-saccate and lands on a cone surface, usually near the ovules. There, the pollen may remain for several weeks before it germinates and forms a long pollen tube that grows to the ovule, through the micropyle and to the nucellar tip where it penetrates the nucellus and grows into an archegonium. In Tsuga heterophylla, the pollen bears spines on the exine (Fig. 4) that become attached to the cobweb-like wax cuticle on the bract of the seed cone. Pollen germinates there and forms a pollen tube several millimeters long that grows to a nearby ovule (Colangeli and Owens 1989). In Agathis, pollen lands on the large exposed nucellus, the ovule or scale surface, or the cone axis. Pollen germinates there and a long pollen tube grows directly to the nucellus, which has grown out of the large micropyle, or in some cases grows through the ovule wall to the nucellus. Once on the nucellar surface or within the nucellus, the pollen tube forms many long branches, some of which contain one of the secondary prothallial nuclei. One of the pollen tubes also contains the generative cell, and this tube grows into one of the several separate archegonia (Owens et al. 1995b).
Pollen tube growth and development in vivo
One of the remarkable features of conifer pollen tubes is their slow germination and growth rate when compared to that of most angiosperms. Germination involves hydration of the pollen followed by either shedding of the exine or formation of a pollen tube that penetrates through the exine. Hydration may occur outside the ovule, as in Larix (Owens et al. 1994), Tsuga heterophylla (Colangeli and Owens 1990b) and most of the Araucariaceae (Owens et al. 1995a) or after the pollen has been taken into the ovule as occurs in most Pinaceae, Cupressaceae and Podocarpaceae (Owens et al. 1998). Pollen of most conifers contains only about 10% water when shed and cytological structure within the pollen is difficult to interpret. Hydration commonly occurs within about 24 h, during which time the cytological structure appears more normal and organelles can be readily recognized (Owens et al. 1994). Hydration causes pollen to swell and the exine to swell or split open. In most conifers, such as in Pinus and Picea, a pollen tube emerges within a few days through a thin portion of the exine, commonly located between the sacci (Fig. 7). In some conifers, such as those in the Cupressaceae, Araucariaceae and Pseudotsuga and Larix of the Pinaceae, the exine splits releasing pollen that is then enclosed only by a thin intine. In the Cupressaceae, pollen is carried into the micropyle and down the micropylar canal by the pollination drop whereas in Larix, pollen is engulfed, then carried down the micropylar canal by a secretion formed within the micropylar canal after the pollen is taken in. A pollen tube forms only after the pollen has been carried close to the nucellus and this may take several weeks after pollination (Owens et al. 1994, Takaso and Owens 1997). In Pseudotsuga there is no large drop flooding the micropylar canal, but rather, the pollen elongates several hundred micrometers down the micropylar canal, then a pollen tube forms when the elongated pollen reaches the nucellus (Takaso et al. 1996).
Several factors that may contribute to the slow rate of pollen tube growth in conifers have been suggested including low moisture content and the lack of polysomes in dry pollen (Dawkins and Owens 1993), the requirement for specific secretions from the micropylar canal, nucellus or female gametophyte that trigger pollen tube development (Takaso and Owens 1994), absence of acidic pectin and callose layers in the pollen tube wall (Derksen et al. 1999), or the requirement for proteins, some of which are only synthesized at the onset of pollen tube elongation (Fernando et al. 2001). In most conifer genera, pollen tube growth is completed during a few weeks in the spring or early summer soon after pollination. In other genera, such as Pinus and some species in the Araucariaceae, pollen may germinate soon after pollination and pollen tubes penetrate the nucellus, but then, the seed cone and pollen tubes become dormant by midsummer and resume growth the following spring.
Plant growth substances have been identified in conifer pollen and pollen tubes and they appear to promote ovule development and prevent cone abortion (Sweet and Lewis 1969, 1971). Some conifers show a low level of pre-fertilization self-incompatibility during pollen germination and pollen tube growth through the nucellus (Runions and Owens 1996b) but most self-incompatibility reactions are post-fertilization, i.e., occur during early embryo development (Owens et al. 2005). Pre-fertilization incompatibility has not been extensively studied in conifers. Conifer pollen tubes grow through the nucellus prior to egg maturity. Ovule secretions initiate pollen tube development 1 week before fertilization in Pseudotsuga (Takaso et al. 1996). The pre-fertilization fluid stimulates pollen tube growth and even distorts pollen tube morphology, which may be related to pre-zygotic selection (Takaso et al. 1996). The time between pollination and fertilization may be as little as a few weeks in most Cupressaceae and Pinaceae, but 1 year in Pinus (Bruns and Owens 2000) and some Araucariaceae (Owens et al. 1995b).
Programmed cell death
Pollen tube growth in vivo is closely correlated with the collapse of nucellar cells that come in contact with the advancing pollen tube (Fig. 8). This is not a general collapse of all nearby nucellar cells but occurs only in those directly contacted by the pollen tube tip. Willemse (1968) reported that in Pinus sylvestris the matrix of the nucellar cell wall is affected by an enzyme produced by ungerminated pollen or germinating pollen tubes. The nucellar cells are also pushed aside by the elongating pollen tube, thus resulting in degeneration. According to Pettitt (1985), proteins are released from pollen walls and tubes of various conifers. These diffusible proteins are believed to be involved in cellular degradation, as an integral part of the pollen tube growth through the nucellus. In Pseudotsuga (Owens and Morris 1990) and Larix occidentalis (Owens et al. 1994), pollen tubes were not branched and electron micrographs of the pollen tube tip (Fig. 8) show that it contains dense cytoplasm with numerous vesicles, lipid bodies and mitochondria just inside the thin intine. It was concluded that the vesicles released substances from the pollen tube tip that caused the collapse of individual nucellar cells. In lodgepole pine, the pollen tube splays out and forms many short branches in the nucellar tip, in the shape of an inverted funnel. Each short branch contacts several cells triggering their collapse and the pollen tube branches fill the spaces left by the collapsed nucellar cells (Owens et al. 2005).
It is well-documented that the nucellar cells surrounding pollen and pollen tubes degenerate, and recently, it has been shown that these cells undergo programmed cell death (PCD). The work of Hiratsuka et al. (2002) on Pinus densiflora clearly showed that PCD is initiated by the pollen or pollen tubes, from which the cell death signal diffuses into the surrounding nucellar cells. The dying nucellar cells exhibit features typical of PCD including chromatin condensation, cytoplasmic shrinkage, cytoplasmic and nuclear blebbing, and internucleosomal cleavage. Furthermore, vacuoles collapse, and the release of vesicles and amorphorous material occurs in the nucellar cells located nearest the pollen tubes. It has been interpreted that the cellular contents of the dying nuclear cells are utilized by the developing pollen tubes. The death of the nucellar cells in the immediate vicinity of the pollen tube tip facilitate the passage of the growing pollen tube.
Pollen tube growth and development in vitro
In culture, pollen germination occurs after 1 or 2 days in Thuja plicata (Colangeli and Owens 1990a), Tsuga heterophylla (Colangeli and Owens 1990b), Picea glauca (Dawkins and Owens 1993), Larix occidentalis (Owens et al. 1994), Pseudotsuga menziesii (Webber and Painter 1996; Fernando et al. 1997), Taxus brevifolia (Anderson and Owens 2000), Chamaecyparis nootkatensis (Anderson et al. 2002), Abies amabilis (Chandler and Owens 2004), and Pinus contorta (Owens et al. 2005). Although pollen germination in conifers is slow, they are generally easy to germinate in vitro and a very high germination percentage has been reported in various groups especially in Pinus. Fernando and Owens (2001) reported 95–100% germination rates in Pinus aristata, P. monticola, and P. strobus. In contrast, only about 57% germination rate was obtained for P. sylvestris (Haggman et al. 1997). Pollen cone collection and pollen extraction at the right stage of pollen development, and the storage of the extracted pollen at the appropriate moisture level and temperature significantly improve germination rates (Fernando and Owens 2001). Using pollen extracted from mature surface sterilized pollen cones, pollen tube elongation in vitro was monitored for up to 30 days and exponential growth occurred in various species of Pinus (Fernando and Owens 2001). In P. sylvestris, growth of pollen tubes was never observed beyond 10 day-old cultures (de Win et al. 1996). This could be attributed to microbial contamination brought about by the use of non-sterile pollen.
Pseudotsuga has an unusual pattern of pollen germination that occurs in a two-step process, the first involving elongation of the pollen and the next step is the formation of pollen tubes. Since pollen tubes in Pseudotsuga and Larix are difficult to induce in vitro, the process of pollen tube formation in these conifers is also poorly understood (Owens and Morris 1990), although various experiments have been done to induce pollen tubes from these conifers. Said et al. (1991) used homogenate of ovules at the time of secretion and this successfully induced pollen tubes in Larix. Ovule secretions have also been collected and used to induce pollen tubes in Pseudotsuga (Takaso et al. 1996). Relatively higher rates of pollen tube formation, albeit at the 10–30% level, were obtained using optimized pollen germination media supplemented with flavonols (Dumont-BeBoux and von Aderkas 1996) or mineral salts (Fernando et al. 1997).
Cellular mechanisms underlying pollen tube elongation
The slow growth rate of conifer pollen tubes compared to flowering plants is manifested in the differences in organelle positioning, organelle motility, and cytoskeletal function within the elongating pollen tube tip. These differences have led to the development of conifer pollen tubes as a model for studying an alternative form of polarized cell growth (Fig. 16). Since the generative cells initially remain within the body of the pollen as pollen tubes elongate (Singh 1978), callose plugs cannot form to separate the elongating tip from the older part of the pollen tube. Thus, the entire pollen tube, from the tip back to the pollen, remains as one elongated cell from germination to fertilization.
Cellular mechanism of pollen tube elongation in conifers
Elongating Picea abies pollen tubes have plastids throughout the tube and a clear zone at the tip
A longitudinal array of microtubules extends from the pollen grain throughout the tube
A longitudinal array of microfilaments also extends from the pollen grain throughout the tube
There is a clear zone at the tube tip
There is a radial network of microtubules at the tube tip
There is also a network of microfilaments at the tube tip
a Localization of Ca2+ using Fura-2-dextran shows a two-fold tip focused Ca2+ gradient. b This differential interference contrast microscopy image of the clear zone at pollen tube tip is coincident with the tip focused Ca2+ gradient
This model shows that the microtubules and microfilaments coordinate to drive the fountain-streaming pattern. This moves ER into the tube tip where the Golgi generates secretory vesicles that flow towards higher Ca2+ to fuse with the plasma membrane, scale bars throughout are 25 μm
There are two distinct zones in elongating conifer pollen tubes both in vivo (Dawkins and Owens 1993, Runions and Owens 1999) and in vitro (Terasaka and Niitsu 1994, de Win et al.1996, Lazzaro 1996, Derksen et al. 1999, Anderhag et al. 2000, Justus et al. 2004). One begins in the pollen and extends towards the pollen tube tip (Fig. 9), containing an axial array of microtubules (Fig. 10) and microfilaments (Fig. 11) that position the generative cell, tube nucleus, abundant amyloplasts, vacuoles, and other organelles (Terasaka and Niitsu 1994, de Win et al. 1996, Lazzaro 1996, 1998, 1999). The second zone is at the elongating pollen tube tip, which does not contain the inverted cone of secretory vesicles common to flowering plants. Instead, a clear zone lacking amyloplasts but enriched in mitochondria and endomembrane system components extends 20–30 μm back from the tip (Fig. 12). There is a demarcation running perpendicular to the pollen tube axis between this clear zone and the amyloplasts in the rest of the pollen tube (de Win et al. 1996, Lazzaro 1996).
The cytoskeleton in the pollen tube tip
Organelles do not typically stream in a reverse fountain pattern in conifer pollen tube tips. Instead the dominant pattern in Pinus sylvestris and Picea abies pollen tubes is a regular fountain (Fig. 16), with organelles moving towards the tip in the center of the tube and away from the tip along the cell cortex (de Win et al. 1996, Justus et al. 2004). Videos of this streaming pattern can be seen at http://www.cofc.edu/∼lazzaro. This pattern coincides with microtubule (Fig. 13) and microfilament (Fig. 14) organization (Lazzaro 1996, 1999, Anderhag et al. 2000) and microtubules control the positioning of organelles into and within the tip and influence the direction of streaming by mediating microfilament organization (Justus et al. 2004). Microfilament disruption by cytochalasin or latrunculin reduces organelle motility to Brownian motion (Justus et al. 2004). Disruption of microfilaments and inhibition of myosin will both completely inhibit germination and profoundly inhibit pollen tube elongation in Pinus densiflora (Terasaka and Niitsu 1994) and Picea abies (Anderhag et al. 2000). Numerous short branches consistently form following microfilament disruption in Picea abies (Anderhag et al. 2000). This is unique to conifer pollen tubes since branching is not a common response to microfilament disruption in angiosperms.
Microtubule disruption stops growth, alters organelle motility within the tip, and alters the organization of actin microfilaments. In particular, microtubule disruption by propyzamide and oryzalin cause the accumulation of membrane tubules or vacuoles in the tip that reverse the direction and stream in a reverse fountain as microfilaments reorganize into pronounced bundles in the tip cytoplasm (Justus et al. 2004). Microtubule disruption can also cause bifurcation in Picea abies pollen tubes (Anderhag et al. 2000). The coordinated behavior of microtubules and microfilaments in tip extension is unique to conifer pollen tubes compared to angiosperms (Anderhag et al. 2000, Justus et al. 2004), but has interesting functional parallels to tip growth in protonema cells of ferns (Kadota and Wada 1992, Kadota et al. 1999) and mosses (Doonan et al. 1988, Schwuchow et al. 1990, Schwuchow and Sack 1994, Meske et al. 1996).
Pollen tube wall synthesis
The cell wall in Pinus sylvestris pollen tubes contains esterifed pectins and callose (Derksen et al. 1999), but the distribution of these molecules differs from angiosperm pollen tubes. Callose occurs only at the tips of young pollen tubes and pectins show a banded pattern. Callose eventually disappears from pine pollen tubes whereas acidic pectin is completely absent. The cellulose microfibrils in pine pollen tubes are oriented obliquely and densely along the lateral walls, as compared to the pollen tube tip (Derksen et al. 1999). However, the cellulose at the pollen tube tip is still more concentrated in Pinus sylvestris and Picea abies compared to angiosperms (Derksen et al. 1999, Lazzaro et al. 2003).
The radial array of cortical microtubules at the tip of Picea abies pollen tubes (Fig. 13) maintains tip integrity and is coordinated with cellulose synthesis. The specific inhibition of cellulose microfibril deposition by isoxaben leads to the disorganization of these microtubules (Lazzaro et al. 2003). Pollen tubes exposed to isoxaben are significantly shorter with a decrease in cellulose throughout the walls. Isoxaben also significantly increases the frequency of swelling in tip with no effect on pollen tube width outside the swollen tip. The decrease in cellulose is more pronounced in pollen tubes with swollen tips where microtubules have coincidentally reorganized into a random pattern from the radial array normally found beneath the plasma membrane (Lazzaro et al. 2003). The dominant paradigm for cellulose deposition in plant cells is that cortical microtubules direct the deposition of parallel cellulose microfibrils by guiding cellulose synthase complexes within the plasma membrane (Carpita and Gibeaut 1993). An extension of the paradigm includes bidirectional communication across the plasma membrane (Fisher and Cyr 1998) and the findings in Picea abies support this principle. In the elongating cells where cellulose synthesis occurs at the tip, the disruption of this synthesis leads to the disorganization of cortical microtubules.
Ca2+ dynamics and elongation
Ca2+ is a major factor in the control of conifer pollen tube growth. While there are similarities with the Ca2+ status in angiosperm pollen tubes, there are also important differences. External Ca2+ enhances germination and is required for the elongation of conifer pollen tubes (Fig. 16). Pollen tube growth is also inhibited when pollen is germinated in the presence of lanthanides or verapamil, which block calcium uptake. However, no other changes in morphology are induced (Lazzaro et al. 2005). There is a two-fold tip focused gradient of Ca2+ in the cytoplasm (Fig. 15a) that ranges from 450 nM at the plasma membrane to 225 nM at the base of the clear zone (Fig. 15b). Although this gradient is much less than those seen in angiosperms (Holdaway-Clarke and Hepler 2003), it still fluctuates as the pollen tube elongates (videos available at http://www.cofc.edu/∼lazzaro). This gradient is perturbed by a Ca2+ shuttle buffer and depleted by caffeine and external applications of Ca2+ channel blockers. When the Ca2+ gradient diminishes and the basal level in the cytoplasm is lowered below 150 nM, a transient tip-focused surge in cytoplasmic Ca2+ occurs, restoring the gradient and triggering the accumulation of a large vesicle population at the plasma membrane. During recovery from treatment with Ca2+ channel blockers, organelle motility within the tip switches direction from a regular fountain to a reverse fountain pattern. The rapid elevation of cytoplasmic Ca2+ triggered by the initial drop of cytoplasmic calcium below 150 nM and the Ca2+ influenced reversal of motility have not been observed in the pollen tubes of flowering plants (Lazzaro et al. 2005).
In vitro fertilization
In vitro fertilization (IVF) involves the isolation of the male and female reproductive structures and their co-culture to facilitate gametophytic interaction leading to the fusion of gametes. It is a novel breeding technology that could bypass crossing barriers to produce hybrids that otherwise cannot be formed in nature. The first attempt of IVF in conifers was done using Pseudotsuga menziesii (Fernando et al. 1998). When isolated female gametophytes were introduced to growing pollen tubes under in vitro conditions, the pollen tubes penetrated the archegonia as well as the prothalial cells of the female gametophytes (Fernando et al. 1998). Using a culture medium supplemented with lactose and polyethylene glycol, the longevity of pollen tubes and eggs was improved and IVF was eventually achieved in a conifer (Fernando et al. 1998). This was done through co-culture of pollen tubes and isolated female gametophytes resulting in the release of sperm into the egg cytoplasm and fusion of gametes (Fernando et al. 1998). Formation of four-nucleate proembryos was accomplished, but the culture medium was unable to sustain their further development. It appears that the culture requirements for the female gametophytes differ from those of the developing sporophytes.
The IVF approach has been extended to studies such as in vitro crosses involving various genera and species of conifers. Pollen tubes of Larix occidentalis, Picea sitchensis, and Pinus monticola were co-cultured with isolated female gametophytes from Pseudotsuga menziesii, Larix x eurolepis, and Pinus monticola (Dumont-BeBoux et al. 1998). Using co-cultured pollen tubes and isolated female gametophytes, intra- and inter-specific crosses between Pinus aristata, P. monticola, and P. strobus were done (Fernando and Owens 2001). In both studies, penetration of the pollen tubes into the archegonia and release of the male gametes into the egg cytoplasm were observed.
Transformation and gene expression
Pollen or pollen tube transformation is a straightforward approach to analyze the expression of genes. This is useful in woody species since it does not require the regeneration of transgenic plants. Therefore, this approach has been applied to conifers to determine the activities of promoters using uid A as the reporter gene. Hay et al. (1994) examined the effects of four different promoters on the pollen of five conifers (Pinus contorta, P. banksiana, Picea mariana, Tsuga heterophylla and Chamaecyparis nootkatensis). Assays of β-glucuronidase expression in the pollen and pollen tubes of these species showed that the ABA-inducible Em and α-tubulin promoters gave the highest and lowest levels of transient expression, respectively. The CaMV 35S and rice actin promoters showed intermediate expression levels. Martinussen et al. (1995) analyzed the activities of two promoters that have been previously shown to be either preferentially expressed in the mature male gametophyte (LAT52) or highly expressed in both the sporophyte and male gametophyte (Act1). They showed that in growing pollen tubes of Picea abies and Pinus pinaster, the activity of the Act1 promoter was significantly higher than that of the LAT52 promoter. As compared to the CaMV 35S and ABA-inducible EM promoters, Haggman et al. (1997) obtained higher transient expression in Pinus sylvestris pollen using the polyubiquitin (UbB1) promoter from sunflower. In Picea abies pollen, they obtained higher transient expression using the UbB1 promoter. The activities of the CaMV 35S promoter have also been demonstrated in the pollen tubes of several other conifers, but primarily in studies relating to optimization of microparticle bombardment parameters (Tian et al. 1997, Fernando et al. 2000).
The use of haploid pollen as carriers of foreign DNA allows rapid production of transgenic plants. Pollen is a natural vector for gene transfer because it is involved in the normal process of sexual reproduction. Therefore, the production of transformed conifer pollen has paved the way for the development of a genetic transformation system that is independent of a tissue culture. In Pinus sylvestris, transformed pollen has been used to pollinate female cones (Haggman et al. 1997, Aronen et al. 1998) and transgenic seeds have been recovered (Aronen et al. 2003). The co-culture of transformed pollen tubes and isolated female gametophytes shows great promise in generating transformed hybrids.
Biochemical regulation of pollen germination and tube growth
It has been demonstrated in Pinus ponderosa that the generative and tube nuclei actively synthesize RNAs at an early stage of pollen germination (Young and Stanley 1963). A similar result was reported by Frankis (1990) using P. taeda. By blocking RNA synthesis with actinomycin D, Frankis demonstrated inhibition of pollen tube elongation in P. taeda. This was manifested after 12 h and became more prominent after 2 days. According to Frankis, the retarded pollen tube growth is due to the lack of RNAs which are essential for normal pollen tube growth. Frankis also reported that proteins in P. taeda are synthesized at different times during pollen germination and early tube growth. RNA and protein synthesis in germinating pollen have also been described for P. monticola and eight other species of conifers (Fernando et al. 2001). In all nine conifers, arresting transcription using actinomycin D did not prevent pollen germination, however, pollen tube elongation was slow. On the other hand, blocking protein synthesis through the use of cycloheximide prevented pollen germination. Blocking protein synthesis in pollen that has already germinated also prevented further tube elongation. This means that the mature ungerminated pollen of conifers does not contain the proteins necessary for germination and tube growth (Fernando et al. 2001) yet. In a similar experiment using P. bungeana, Hao et al. (2005) arrived at the same conclusion. Thus, the timing of protein synthesis in conifers (Fernando et al. 2001, Hao et al. 2005) differs from that in most flowering plants, where the mature ungerminated pollen already contains all proteins required for germination and early tube growth (Mascarenhas 1993). Blocking transcription in conifer pollen appears to have the same effect as in most flowering plants, i.e., the RNAs necessary for pollen germination are already in the pollen during dispersal (Mascarenhas 1993).
Protein profiles of pollen and pollen tubes
One- and two-dimensional gel electrophoreses have visualized the protein complement of pine pollen and pollen tubes. Studies comparing the protein profiles of ungerminated and germinated pollen show that a large fraction of the proteome of the mature ungerminated pollen is similar to the proteome of the germinated pollen, as in the case of P. taeda (Frankis 1990), P. monticola (Fernando et al. 2001) and P. bungeana (Hao et al. 2005). Based on quantitative variations (presence and absence of protein spots), the pollen and pollen tubes of P. strobus share about 94% similarity (Fernando 2005). This shows that the majority of the proteins in the pollen tube are already present in the pollen, when pollen is dispersed from the cones. The high correlation of two-dimensional protein profiles between these two developmental stages also suggests that the information obtained through the analysis of pollen would be helpful in understanding what is going on in the pollen tubes.
In P. pinaster, 702 protein spots were consistently resolved from pollen after two-dimensional gel electrophoresis (2-DE). A comparison with the protein profiles of needles and vegetative buds showed that approximately 10% were pollen specific (Bahrman and Petit 1995). The two-dimensional protein profile of P. pinaster pollen is currently available on the Internet (http://www.pierroton.inra.fr/genetics/2D/pollenb.jpg). When the two-dimensional protein profiles of P. pinaster and P. strobus are compared, the lack of correlation between spots is apparent. This is also the case when the latter is compared with the protein profile of P. taeda pollen, where both samples have undergone similar procedures of protein extraction and electrophoresis. This suggests that annotated proteome maps from one species cannot be used to identify proteins from pollen of another species, even if they belong to the same genus. This is equally true in flowering plants (Rose et al. 2004).
Proteome of pine pollen tubes
Proteomic analysis has allowed the identification of proteins from plants whose genomes have not necessarily been sequenced. This approach has been successfully applied to characterize the development of pollen tubes in P. strobus. In this species, the protein profiles of pollen and pollen tubes were compared following 2-DE. The differentially expressed proteins in the pollen tubes were subjected to Matrix-Assisted Laser/Desorption Ionization Time-of-Flight Mass Spectrometry and identified through database search (Fernando 2005). This approach showed that 12% of the differentially-expressed proteins in the pollen tubes matched with hypothetical proteins, whereas 33% had no match in the NCBInr and Swiss-Prot databases. For most of the differentially expressed proteins (55%), a putative function was assigned based on similarity of sequences with previously characterized proteins from other plant species. The identified differentially expressed proteins from the pollen tubes of P. strobus were grouped into five categories based on their functions, including metabolism, stress/defense response, gene regulation, signal transduction, and cell wall formation (Fernando 2005). Many of these proteins have already been described from pollen tubes of various flowering plants, but there are also many proteins that have not yet been reported from pollen tubes of any species, e.g., phenylcoumaran benzylic ether reductase, ascorbate peroxidase, f-box family protein, enhancer-of-zeste, gag-pol polyprotein, At3g18730, and many others. So far, no similar work has been done on any flowering plant. Therefore, this study has expanded our knowledge of the proteins that are expressed in the male gametophytes of seed plants. It also contributes to our understanding of the changes in protein expression associated with pollen tube development and provides insights into the molecular programs that separate the development of the pollen tubes from those of the pollen. Functional analysis, probably through pollen tube transformation vis-á-vis post-transcriptional gene silencing (Moutinho et al. 2001, Waterhouse and Helliwell 2003, Tang et al. 2004) will provide a better understanding of the behavior of pollen tubes and the mechanisms that regulate this critical stage of sexual reproduction in seed plants.
Summary
Conifer pollen tubes are characterized by several distinctive features. These include slow rate and extended period of growth, extremely delayed sperm formation, no cytokinesis following sperm formation, a pollen tube wall made up primarily of cellulose, and distinct cytoskeletal control and organelle zonation. The pollen tube is an ideal experimental system, especially in studies involving genetic transformation, gene expression, cellular dynamics, and polarized growth. In spite of the work that has been done, there are several topics in conifer biology that have not yet been fully described, including the formation and induction of the male gametes, the mechanism behind the effect of pollen tubes to induce ovule and seed cone development, and incompatibility mechanisms in intraspecific and interspecific crosses. There are also many research areas being examined with flowering plant pollen and pollen tubes that have not yet been studied in conifers, such as the mechanism of pollen tube guidance and transcriptome analysis. It appears that progress in this field has been slow but contributions from various laboratories throughout the world are coming out at a regular rate. The field of conifer biology will benefit from the sequencing of the pine genome and therefore, we anticipate that in the next decade, molecular tools for conifers will advance to the stage where conifer reproductive biology and particularly, pollen tube biology, will be more conducive to experimental dissection.
References
Anderhag P, Hepler PK, Lazzaro MD (2000) Microtubules and microfilaments are both responsible for pollen tube elongation in the conifer Picea abies (Norway spruce). Protoplasma 214:141–157
Anderson ED, Owens JN (2000) Microsporogenesis, pollination, pollen germination and male gametophyte development in Taxus brevifolia. Ann Bot 86:1033–1044
Anderson ED, Owens JN, Colangeli AM, Russell JH (2002) Challenges facing yellow cypress (Chamaecyparis nootkatensis) seed orchards: low filled seed production, pollen-cone abortion, self-pollination, and accelerated embryo development. Can J For Res 32:1411–1419
Aronen TS, Nikkanen TO, Haggman HM (1998) Compatibility of different pollination techniques with microprojectile bombardment of Norway spruce and Scots pine pollen. Can J For Res 28:79–86
Aronen TS, Nikkanen TO, Haggman HM (2003) The production of transgenic Scots pine (Pinus sylvestris L.) via the application of transformed pollen in controlled crossings. Trans Res 12:375–378
Bahrman N, Petit RJ (1995) Genetic polymorphism in maritime pine (Pinus pinaster Ait.) assessed by two-dimensional gel electrophoresis of needle, bud and pollen proteins. J Mol Evol 41:231–237
Bruns D, Owens JN (2000) Western white pine (Pinus monticola) reproduction: II. Fertilization and cytoplasmic inheritance. Sex Plant Reprod 13:75–84
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3:1–30
Chandler LM, Owens JN (2004) The pollination mechanism in Abies amabilis. Can J For Res 34:1–10
Colangeli AM, Owens JN (1989) Post-dormancy seed-cone development and the pollination mechanism in western hemlock (Tsuga heterophylla). Can J For Res 19:44–53
Colangeli AM, Owens JN (1990a) The relationship between time of pollination, pollination efficiency, and cone size in western red cedar (Thuja plicata). Can J Bot 68:439–443
Colangeli AM, Owens JN (1990b) Cone and seed development in wind-pollinated western hemlock (Tsuga heterophylla) clone bank. Can J For Res 20:1432–1437
Dawkins MD, Owens JN (1993) In vitro and in vivo pollen hydration, germination, and pollen tube growth in white spruce, Picea glauca (Moench) voss. Int J Plant Sci 164:506–521
Derksen J, Li Y, Knuiman B, Geurts H (1999) The wall of Pinus sylvestris L. pollen tubes. Protoplasma 208:26–36
de Win AHN, Knuiman B, Pierson ES, Geurts H, Kengen HMP, Derkson J (1996) Development and cellular organization of Pinus sylvestris pollen tubes. Sex Plant Reprod 9:93–101
Doonan JH, Cove DJ, Lloyd CW (1988) Microtubules and microfilaments in tip growth: evidence that microtubules impose polarity on protonemal growth in Physcomitrella patens. J Cell Sci 89:533–540
Dumont-BeBoux N, von Aderkas P (1996) In vitro pollen tube growth in Douglas fir. Can J For Res 27:674–678
Dumont-BeBoux N, Weber M, Ma Y, von Aderkas P (1998) Intergeneric pollen-megagametophyte relationships of conifers in vitro. Theor Appl Genet 97:881–887
Farjon A (1998) World checklist and bibliography of conifers. The Royal Botanical Gardens, Kew, UK, p 297
Fernando DD (2005) Characterization of pollen tube development in Pinus strobus (Eastern white pine) through proteomic analysis of differentially expressed proteins. Proteomics 56:2619–2628
Fernando DD, Owens JN, von Aderkas P, Takaso T (1997) In vitro pollen tube growth and penetration of female gametophyte in Douglas fir (Pseudotsuga menziesii). Sex Plant Reprod 10:209–216
Fernando DD, Owens JN (2001) Development of an in vitro technology to confer white pine blister rust resistance. In: Sniezko, R.A. et al. eds. 2004. Breeding and genetic resources of five-needle pines: growth, adaptability and pest resistance; 2001 July 23–27 Medford, OR, USA. IUFRO Working Party 2.02.15. Proceedings RMRS-P-32. Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station. pp 163–168
Fernando DD, Owens JN, Misra S (2000) Transient gene expression in pine pollen tubes following particle bombardment. Plant Cell Rep 19:224–228
Fernando DD, Owens JN, von Aderkas P (1998) In vitro fertilization from co-cultured pollen tubes and female gametophytes of Douglas fir (Pseudotsuga menziesii). Theor Appl Genet 96:1057–1063
Fernando DD, Owens JN, Yu X (2001) RNA and protein synthesis during in vitro pollen germination and tube elongation in Pinus monticola and other conifers. Sex Plant Reprod 13:259–264
Fisher DD, Cyr RJ (1998) Extending the microtubule/microfibril paradigm: cellulose synthesis is required for normal cortical microtubule alignment in elongating cells. Plant Physiol 116:1043–1051
Frankis RC (1990) RNA and protein synthesis in germinating pine pollen. J Exp Bot 41:1469–1473
Haggman HM, Aronen TS, Nikkanen TO (1997) Gene transfer by particle bombardment to Norway spruce and Scots pine pollen. Can J For Res 27:928–935
Hao H, Li Y, Hu Y, and Lin J (2005) inhibition of RNA and protein synthesis in pollen tube development of Pinus bungeana by actinomycin D and cycloheximide. New Phytol 165:721–730
Hay I, Lachance D, von Aderakas P, Charest PJ (1994) Transient chimeric gene expression in pollen of five conifer species following microparticle bombardment. Can J For Res 24:2417–2423
Hiratsuka R, Yamada Y, Terasaka O (2002) Programmed cell death of Pinus nucellus in response to pollen tube penetration. J Plant Res 115:141–148
Holdaway-Clarke TL, Hepler PK (2003) Control of pollen tube growth: role of ion gradients and fluxes. New Phytol 159:539–563
Jorhi BM (1984) Embryology of Angiosperms. Springer, Berlin, Heidelberg, New York
Justus CD, Anderhag P, Goins JL, Lazzaro MD (2004) Microtubules and microfilaments coordinate to direct a fountain-streaming pattern in elongating conifer pollen tube tips. Planta 219:103–109
Kadota A, Wada M (1992) Reorganization of the cortical cytoskeleton in tip growing fern protonemal cells during phytochrome mediated phototropism and blue light induced apical swelling. Protoplasma 166:35–41
Kadota A, Yoshizaki N, Wada M (1999) Cytoskeletal changes during resumption of tip growth in non-growing protonema cells of the fern Adiantum capillus-veneris L. Protoplasma 207:195–202
Kurmann MH (1989) Pollen wall formation in Abies concolor and a discussion on wall layer homologies. Can J Bot 67:2489–2504
Lazzaro MD (1996) The actin microfilament network within elongating pollen tubes of the gymnosperm Picea abies (Norway spruce). Protoplasma 194:186–194
Lazzaro MD (1998) The spermatogenous body cell of the conifer Picea abies (Norway spruce) contains actin microfilaments. Protoplasma 201:194–201
Lazzaro MD (1999) Microtubule organization in germinated pollen of the conifer Picea abies (Norway spruce, Pinaceae). Amer J Bot 86:759–766
Lazzaro MD, Donohue JM, Soodavar FM (2003) Disruption of cellulose synthesis by isoxaben causes tip swelling and disorganizes cortical microtubules in elongating conifer pollen tubes. Protoplasma 220:201–207
Lazzaro MD, Cardenas L, Bhatt AP, Justus CD, Phillips MS, Holdaway-Clarke TL, Hepler PK (2005) Calcium gradients in conifer pollen tubes: dynamic properties differ from those seen in angiosperms. J Exp Bot (DOI: 10.1093/jxb/eri256)
Lev-Yadun S, Sederoff R (2000) Pinus taeda as a model system for studying plant evolution, wood formation, and perennial growth. J Plant Growth Regul 19:290–305
Martinussen I, Bate N, Weterings K, Junttila O, Twell D (1995) Analysis of gene regulation in growing pollen tubes of angiosperm and gymnosperm species using microprojectile bombardment. Physiol Plant 93:445–450
Mascarenhas JP (1993) Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 5:1303–1314
Meske V, Ruppert V, Hartmann E (1996) Structural basis for the red light induced repolarization of tip growth in caulonema cells of Ceratodon purpureus. Protoplasma 192:189–198
Meyen SV (1984) Basic features of gymnosperm systematics and phylogeny as evidenced by the fossil record. Bot Rev 50:1–115
Miller CN (1975) Mesozoic conifers. Bot Rev 43:217–280
Moutinho A, Camacho L, Haley A, Salome Pais M, Trewavas A, Malho R (2001) Antisense perturbation of protein function in living pollen tubes. Sex Plant Reprod 14:101–104
Owens JN, Simpson SJ, Molder M (1981) The pollination mechanism and the optimal time of pollination in Douglas-fir (Pseudotsuga menziesii). Can J For Res 11:36–50
Owens JN, Simpson S (1986) Pollen from conifers native to British Columbia. Can J For Res 16:955–967
Owens JN, Morris SJ (1990) Cytological basis for cytoplasmic inheritance in Pseudotsuga menziesii. I. Pollen tube and archegonial development. Amer J Bot 77:433–445
Owens JN (1993) Chapter 1. Pollination Biology. In: Pollen management Handbook. Vol. II. ISDA, For Ser Agric Handbook, 698:1–13
Owens JN, Morris S, Catalano G (1994) How the pollination mechanism and prezygotic and postzygotic events affect seed production in Larix occidentalis. Can J For Res 24:917–927
Owens JN, Catalano G, Morris SJ, Aitken-Christie J (1995a) The reproductive biology of Kauri (Agathis australis). I. Pollination and prefertilization development. Int J Plant Sci 156:257–269
Owens JN, Catalano G, Morris SJ, Aitken-Christie J (1995b) The reproductive biology of Kauri (Agathis australis). II. Male gametes, fertilization and cytoplasmic inheritance. Int J Plant Sci 156:404–416
Owens JN, Takaso T, Runions CJ (1998) Pollination in conifers. Trends Plant Sci 3:479–485
Owens JN, Bruns D (2000) Western white pine (Pinus monticola) reproduction: I. Gametophyte development. Sex Plant Reprod 13:75–84
Owens JN, Bennett J, L’Hirondelle S (2005) Pollination and cone morphology affect cone and seed production in lodgepole pine seed orchards. Can J For Res 35:383–400
Pettitt JM (1985) Pollen tube development and characteristics of the protein emissions in conifers. Ann Bot 56:379–397
Rose JKC, Bashir S, Giovannoni JJ, Jahn MM, Saravanan RS (2004) Tackling the plant proteome: practical approaches, hurdles and experimental tools. Plant J 39:715–733
Runions JC, Catalano GL, Owens JN (1995) Pollination mechanism of seed orchard interior spruce. Can J For Res 25:1434–1444
Runions CJ, Owens JN (1996a) Pollen scavenging and rain involvement in the pollination mechanism of interior spruce. Can J Bot 74:115–124
Runions CJ and Owens JN (1996b) Evidence of pre-zygotic self-incompatibility in a conifer. In: Owens SJ, Rudall PJ (eds) Reproductive Biology. Royal Botanic Gardens, Kew, pp 255–264
Runions CJ, Owens JN, (1999) Sexual reproduction of interior spruce (Pinaceae). II. Fertilization to early embryo formation. Int J Plant Sci 160:641–652
Said C, Villar M, Zandonella P (1991) Ovule receptivity and pollen viability in Japanese larch (Larix leptolepis Gord.) Silvae Genet 40:1–6
Schwuchow J, Sack FD, Hartmann E (1990) Microtubule distribution in gravitropic protonemata of the moss Ceratodon. Protoplasma 159:60–69
Schwuchow J, Sack FD (1994) Microtubules restrict plastid sedimentation in protonemata of the moss Ceratodon. Cell Motil Cytoskel 29:366–374
Singh H (1978) Embryology of Gymnosperms. (Handbuch der Pflanzenanatomie). Gebruder Borntraeger, Berlin Heidelberg New York, p 302
Sweet GB, Lewis PN (1969) A diffusible auxin from Pinus radiata pollen and it’s possible role in stimulating ovule development. Planta 89:380–384
Sweet GB, Lewis PN (1971) Plant growth substances in the pollen of Pinus radiata at different levels of germination. NZ J Bot 9:146–156
Takaso T, Owens JN (1994) Effects of ovular secretions on pollen in Pseudotsuga menziesii (Pinaceae). Am J Bot 81:504–513
Takaso T, Owens JN (1997) Pollen movement in the micropylar canal of Larix and its simulation. J Plant Res 110:259–264
Takaso T, Von Aderkas P, Owens JN (1996) Prefertilization events in ovules of Pseudotsuga: ovular secretion and its influence on pollen tubes. Can J Bot 74:1214–1219
Tang W, Samuels V, Whitley N, Bloom N, Delagarza T, Newton RJ (2004) Post-transcriptional gene silencing induced by short interfering RNAs in cultured transgenic plant cells. Genomics, Proteomics and Bioinformatics 2:97–108
Terasaka O, Niitsu T (1994) Differential roles of microtubule and actin-myosin cytoskeleton in the growth of Pinus pollen tubes. Sex Plant Reprod 7:264–272
Tian L, Seguin A, Charest PJ (1997) Expression of the green flourescent protein gene in conifer tissues. Plant Cell Rep 16:267–271
Tomlinson PB, Braggins JE, Rattanbury JA (1991) Pollination drop in relation to cone morphology in Podocarpaceae: a novel reproductive mechanism. Am J Bot 78:1289–1303
Waterhouse PM, Helliwell CA (2003) Exploring plant genomics by RNA-induced gene silencing. Nature Reviews: Genetics 4:29–38
Webber JE, Bonnet-Masembert M (1989) Influence of moisture content of forest tree pollen on its response to different viability tests. Ann Sci For 46:605–635
Webber JE, Painter RA (1996) Douglas fir pollen management. Sec Ed Res. Br. B.C. Min. For., Victoria, B.C. Pap. 02/1996. p 91
Willemse MTM (1968) Development of the micro- and macro-gametophytes of Pinus sylvestris L. Acta Bot Neerl 17:330–331
Wilson V, Owens JN (1999) The reproductive biology of totara (Podocarpus totara) (Podocarpaceae). Ann Bot 83:401–411
Young LCT, Stanley RG (1963) Incorporation of tritiated nucleosides thymidine, uridine and cytidine in nuclei of germinating pine pollen. Nucleus 6:83–90
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernando, D.D., Lazzaro, M.D. & Owens, J.N. Growth and development of conifer pollen tubes. Sex Plant Reprod 18, 149–162 (2005). https://doi.org/10.1007/s00497-005-0008-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-005-0008-y