Key message
Rice microspore-promoters.
Abstract
Based on microarray data analyzed for developing anthers and pollen grains, we identified nine rice microspore-preferred (RMP) genes, designated RMP1 through RMP9. To extend their biotechnological applicability, we then investigated the activity of RMP promoters originating from monocotyledonous rice in a heterologous system of dicotyledonous Arabidopsis. Expression of GUS was significantly induced in transgenic plants from the microspore to the mature pollen stages and was driven by the RMP1, RMP3, RMP4, RMP5, and RMP9 promoters. We found it interesting that, whereas RMP2 and RMP6 directed GUS expression in microspore at the early unicellular and bicellular stages, RMP7 and RMP8 seemed to be expressed at the late tricellular and mature pollen stages. Moreover, GUS was expressed in seven promoters, RMP3 through RMP9, during the seedling stage, in immature leaves, cotyledons, and roots. To confirm microspore-specific expression, we used complementation analysis with an Arabidopsis male-specific gametophytic mutant, sidecar pollen-2 (scp-2), to verify the activity of three promoters. That mutant shows defects in microspore development prior to pollen mitosis I. These results provide strong evidence that the SIDECAR POLLEN gene, driven by RMP promoters, successfully complements the scp-2 mutation, and they strongly suggest that these promoters can potentially be applied for manipulating the expression of target genes at the microspore stage in various species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In flowering plants, microgametogenesis is defined as the early stages of pollen development, during which unicellular microspores are released from tetrads to produce two daughter cells via pollen mitosis I (PMI) at the bicellular stage (Borg et al. 2009). Microspores obtain nutrients from closely linked tissues the innermost layer of the anther wall, i.e., the tapetum, and undergo further division to generate twin sperm cells after pollen mitosis II (PMII).
Several mutants defective at the microspore stages have been isolated and functionally characterized for genes, such as SIDECAR POLLEN (SCP), GEMINI POLLEN 1 and 3, that are required for microspore polarity at PMI (Chen and McCormick 1996; Park et al. 1998; Twell et al. 2002; Oh et al. 2010, 2016). Also, many genes involved in pollen development and male gametophyte-specific promoters have been identified. Genes such as MSP-1, -2, and -3 are specifically active in either the microspores or developing pollen (Honys et al. 2006). In addition, transcriptome analyses based on various microarray experiments have provided a collection of 13,977 genes, including 11,565 that exhibit male-gametophytic expression in unicellular microspores from Arabidopsis (reviewed by Rutley and Twell 2015).
Although numerous genes have been identified and characterized in rice (Oryza sativa), most are related to the tapetum/anther and late pollen development, such as TDR, Osg6B, RA8, RTS, UDT1, CSA, OsLTP6, OsSCP1–3, RIP1, OSIPA, OSIPK, OsLPS1–9, OsLSP10, and OsLPS11 (Yokoi et al. 1997; Jeon et al. 1999; Jung et al. 2005; Han et al. 2006; Li et al. 2006; Luo et al. 2006; Park et al. 2006; Gupta et al. 2007; Zhang et al. 2010; Swapna et al. 2011; Khurana et al. 2013b; Liu et al. 2013; Oo et al. 2014; Nguyen et al. 2015). However, only a few genes associated with early pollen/microspore development have been described for rice. Transcriptome data are now publicly available for developing pollen and anthers in rice (Suwabe et al. 2008; Fujita et al. 2010; Wei et al. 2010; Aya et al. 2011). These data should be valuable sources for identifying genome-wide candidate genes that are significantly expressed during microspore development. Furthermore, manipulation of rice genes through promoters driving gene expression at this specific stage may give novel insight about various reproductive processes in plants, and might also be useful in biotechnological approaches.
We recently selected eight promoters based on a meta-analysis of expression data from developing anthers and pollen (Nguyen et al. 2016). The activity of these promoters was characterized in rice, a homologous species, and confirmed the early pollen-stage-specific expression, designated as rice microspore-preferred (RMP), by evaluating GUS expression from the uni-nucleated stage to mature pollen (Nguyen et al. 2016). Here, we extend the applicability of these RMP promoters by analyzing their expression patterns in dicotyledonous Arabidopsis plants. We previously reported a similar approach using late pollen-preferred (OsLPS) promoters (Oo et al. 2014). In this study, we tested stage-specific expression patterns of nine RMP promoters (RMP1 through RMP9) by using promoter-GUS reporter gene vectors in a heterologous system, Arabidopsis. To confirm their functionality, we utilized the RMP1, RMP2, and RMP5 promoters to complement sidecar pollen-2 (scp-2) homozygous mutants in Arabidopsis, which exhibit defects in their early pollen development.
Materials and methods
Meta-expression analysis
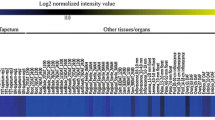
Based on the Affymetrix microarray datasets generated from developing anthers and pollen grains, we selected nine RMP genes (RMP1 through RMP9) out of 410 candidates because they are highly expressed in early stages of pollen development, including the microspores (Nguyen et al. 2016). We also compared the expression patterns of these genes by using laser-captured microdissection (LCM) microarray data (Suwabe et al. 2008) (Fig. 1, Table S1). Orthologs of RMPs in Arabidopsis were searched for using the online programs Rice Gene Annotation tool (Ouyang et al. 2007; http://rice.plantbiology.msu.edu/index.shtml) and PANTHER classification system (Mi et al. 2013; http://pantherdb.org).
Heatmap analysis of RMP genes using laser-captured microdissection (LCM) microarray data for developing anthers, starting from pre-meiosis to mature pollen stages. All LCM data, except RMP4, are presented. Most candidate genes were highly expressed in unicellular (UC) or bicellular (BC) pollen. Blue boxes indicate low level of expression; yellow boxes, high level
Comparative analysis of cis-acting elements (CREs) between promoters of RMP and OsLPS groups
To identify consensus CREs in the promoter sequences of RMP genes, we analyzed three upstream sequences of 1,343 bp for the RMP1 (LOC_Os01g34920, from −18 to −1360), 754 bp for the RMP2 (LOC_Os04g47400, from −97 to −850), and 1,962 bp for the RMP5 (LOC_Os12g44080, from −19 to −1980) (Table S1) that had been analyzed through GUS assays. In addition, promoter sequences of 9 genes of rice late pollen-specific gene (OsLPS) group, which have previously been reported (Oo et al. 2014), were used as a reference group to identify unique CREs conserved in promoters of these RMP genes. Gene group analysis was performed to identify the co-occurrence of transcription factor binding site or cis-acting elements in a group of gene promoter regions. We looked for up to four CREs in only RMPs by using gene group analysis tool from the Plant Promoter Analysis Navigator (PlantPAN, http://plantpan2.itps.ncku.edu.tw/).
Construction of promoter::GUS gene vectors
The promoter regions of RMP genes are located in the 5′ region upstream from the start codon (ATG). These were amplified by two-step Gateway PCR from rice genomic DNA (japonica “Nipponbare”), using specific primers (Table S2) as described by Nguyen et al. (2015). After verification by sequencing, promoter sequences were fused to the GUS reporter gene in the binary Gateway vector pKGWFS7 (http://www.psb.ugent.be/) and were transformed into Agrobacterium tumefaciens strain GV3101.
Transformation and selection of transgenic plants
Wild-type Arabidopsis plants (ecotype Columbia, or Col-0) were grown in a controlled environment chamber (22 °C, 16-h photoperiod). They were transformed with Agrobacterium strain GV3101, harboring RMP-GUS vectors, by the standard floral-dip method (Clough and Bent 1998). Transgenic T1 plants were selected on a solid Murashige and Skoog (MS) medium [4.4 g L−l MS salts and vitamins (Duchefa Biochemie, RV Haarlem, The Netherlands), and 6 g L−l phyto agar (pH 5.8)] that also contained 200 mg L−l cefotaxime (Duchefa Biochemie) and 50 mg L−l kanamycin (Duchefa Biochemie).
Histochemical GUS assay
Expression of GUS was examined in both reproductive and vegetative organs of Arabidopsis as described by Nguyen et al. (2015). Samples were submerged in GUS buffer [0.1 M sodium phosphate (pH 7.0), 1 mM EDTA (pH 8.0), 0.1% Triton X-100, and 0.5 mM K3Fe (CN)6] that was supplemented with 1 mM 5 bromo-4-chloro-indoxyl β-d-glucuronic acid (X-GlcA; Duchefa Biochemie). After vacuum infiltration for 10 min, the samples were incubated at 37 °C for 48 h. Stained tissues were subsequently cleared in 70% ethanol and viewed under a stereomicroscope (Zeiss Stemi 2000-C; Carl-Zeiss, Oberkochen, Germany). Images were captured using a ProgC3 camera (Jenoptik, Jena, Germany) under 0.65× magnification.
DAPI staining
Samples were stained with DAPI (4′,6-diamidino-2-phenylindole) as described by Nguyen et al. (2015). Anthers of various sizes were dissected to release microspores/pollen at four stages—unicellular (UC), bicellular (BC), tricellular (TC), and mature pollen (MP)—into the DAPI staining solution (GUS buffer containing 0.4 µg mL−l DAPI) as described by Park et al. (1998). Images of the pollen were viewed by light and UV epi-illumination, using a Nikon ECLIPSE 80i microscope (Nikon, Melville, NY, USA), and were captured with a Prog ResMFcool camera (Jenoptik) at 40× magnification.
Genetic complementation analysis
To examine functionally whether the RMP promoters can direct gene expression in Arabidopsis microspores, we performed a complementation analysis using Arabidopsis scp-2 homozygous mutants, which are developmentally defective at the microspore stage (Oh et al. 2010). For genetic complementation, vectors were constructed by introducing each promoter into a pBluescript (pBS) vector, using AscI and NotI restriction enzymes. The full-length SCP coding region and a double HA fragment from the proSCP-SCP:dHA construct (Oh et al. 2010) were ligated into pBS-proRMP between the NotI and PacI sites to create proRMP-SCP:dHA. After verification by enzyme digestion and sequencing, the proRMP-SCP:dHA fragments generated from double digestion by AscI and PacI were ligated into binary vector pER8, which contains a hygromycin resistance gene as a selection marker (Fig. S1a). To generate complementing lines, we transformed scp-2 homozygotes with Agrobacterium harboring the proRMP-SCP::dHA construct. Transgenic T1 seedlings were selected on an MS medium containing 20 mg L−l hygromycin (Bio Basic Inc., Markham, ON, Canada) and transferred to soil. The generated plants were examined for the T-DNA insertion and genotypes of the scp-2 mutant allele (Fig. S2) via PCR-based analysis using the specific primers listed in Table S2. The pollen phenotypes of those transgenic scp-2 homozygotes were observed with the Nikon ECLIPSE 80i microscope. In addition, T-DNA genotypes of individual T2 complementing lines were evaluated based on frequencies of hygromycin-resistant (hygR) plants. Briefly, T2 seeds were grown on an MS medium containing 20 mg L−l hygromycin. Resistant seedlings were scored 10 d after germination.
Results
Heatmap analysis of selected RMP expression using LCM microarray data
Using genome-wide microarray data of developing rice anthers and pollen samples, we recently identified 410 RMP genes showing rice microspore-preferred expression patterns (Nguyen et al. 2016). From these, we selected eight genes and used promoter-GUS reporter gene vectors to test expression in the homologous system, rice (Nguyen et al. 2016). For the current study, we added one gene, RMP9 (LOC_Os03g26430.1), which encodes a putative aldose 1-epimerase-like protein and exhibited UC and BC microspore-preferred expression (Fig. 1; Table S1). Furthermore, we investigated accurate expression patterns via LCM microarray data during microspore/pollen development, from pre-meiosis to the mature pollen stage (Suwabe et al. 2008). As we had predicted, all of the RMP genes except RMP4 (which had no probe in the Agilent 44 K array) were most highly expressed in the early stages of pollen development, including the microspores (Fig. 1), However, their expression in the tapetum was much lower at corresponding stages, which strongly suggested that these genes have microspore-preferred patterns. Of these, RMP1, RMP2, RMP3, RMP7, and RMP9 were expressed preferentially at both stages of UC microspore and BC pollen. Interestingly, RMP5 was UC-preferred, while RMP8 was BC-preferred expression. But RMP6 showed weak expression at UC compared to other stages. We also determined that eight of those nine genes have putative orthologs in Arabidopsis (Table S1). Of these, At1g31740, At2g76160, and At3g47800 demonstrated high amino acid identity with RMP1 (51%), RMP2 (65%), and RMP9 (53%), respectively. No ortholog for RMP6 was identified in Arabidopsis (Table S1).
Evaluation of the activity of RMP promoters in heterologous plants
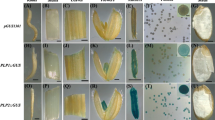
To examine the activity of these promoters in Arabidopsis, a heterologous dicot, we conducted Agrobacterium-mediated transformation with Arabidopsis. In situ GUS expression driven by RMP promoters was examined in both reproductive and vegetative tissues from 50 independent transgenic plants per construct. Distinct expression patterns were observed, including those for the RMP2 and RMP6 promoters, which directed GUS expression in young buds but not in mature flowers (Fig. 2a). To determine the earliest appearance of GUS signals, we examined expression patterns in microspores/pollen grains that were released from dissected anthers at four stages: UC, BC, TC, and MP, and found signals only at the UC and BC stages for RMP2 and RMP6. In contrast, RMP7 and RMP8 exhibited GUS expression in mature flowers at the late pollen stages, TC and MP. The other five promoters showed expression at all stages, from UC through MP (Fig. 2b). We also investigated the activities of these promoters in vegetative tissues from 10-day-old T2 seedlings (20 lines per construct). Although GUS signals were not found in RMP1 and RMP2 seedlings, slight expression was detected in the cotyledon regions of RMP7 and RMP8 and the apical regions of RMP3 seedlings (Fig. 2c). Unexpectedly, we observed a high level of GUS signals in seedlings driven by the other promoters. For example, signals were detected in emerging leaves of RMP4 seedlings, but those signals were much higher in the leaves and shoots of transgenics driven by the RMP5 promoter. Expression under the control of the RMP6 and RMP9 promoters was similar among seedlings for each promoter and was strongest in root tissues (Fig. 2c).
GUS expression driven by RMP promoters during pollen development stages and at seedling stage in Arabidopsis: a in inflorescences. b DAPI- and GUS-stained images of unicellular microspores (UC), bicellular (BC), tricellular (TC), and mature (MP) pollen grains. c GUS expression at seedling stage. Images of inflorescences and seedlings were taken under ×0.65 magnification. Pollen grains were taken under ×40 magnification lens
RMP promoters are effective in genetic complementation analysis for functional verification through a heterologous system
To verify the activity of promoters at the microspore stage, we selected three candidate promoters—RMP1, RMP2, and RMP5—for genetic complementation of gametophytic mutant scp-2. All three showed UC/BC microspore-preferred expression. Whereas ~86% of pollen from non-transformed scp-2 homozygotes had the expected phenotype of aberrant grains, frequencies were reduced for the transgenic scp-2 homozygotes (Fig. S3). Specifically, 45 transformed T1 plants containing the RMP1 promoter showed frequencies of aberrant pollen ranging from 36.9 to 73.8%, including 12 lines with strongly reduced frequencies of less than 50%. Moreover, frequencies for 29 T1 transgenics of RMP2 ranged from 35.3 to 95.9%, which was less than the 80.2 to 89.6% calculated for their corresponding non-transformed plants (Fig. S3). Finally, the 20 T1 transgenic lines of RMP5 showed frequencies of 44.2 to 92.3%.
For analyzing the T2 generation, we selected two T1 plants that showed strong reductions in the occurrence of mutant pollen in each complementing line. In all, 20 hygR T2 progenies for each promoter construct were evaluated by calculated the frequency of the scp phenotype. Most T2 plants showed much stronger reductions in aberrant pollen grains (average of 27.0–33.0%; Fig. 3a). Specifically, frequencies of scp pollen ranged from 15.4 to 56.9% for the RMP1 promoter, 19.5 to 49.1% for RMP2, and 16.9 to 47.5% for RMP5. This strongly contrasted with the 80.2 to 89.6% aberrant pollen produced by non-transgenic scp-2 back-crossed plants. Frequencies of hygR were also analyzed in T3 seedlings to determine the genotype or the copy number of T-DNA. Those frequencies ranged from 76.3 to 100.0%, suggesting that at least one was a homozygote among the 20 T2 plants harboring the RMP1 promoter. The others were likely to be heterozygous for T-DNA, with at least one copy (Fig. 3a, Table S3). Eight of the 20 T2 plants containing the RMP2 promoter seemed to be homozygous for T-DNA, exhibiting a lower frequency of scp-2 pollen. Moreover, among 12 plants presenting hygromycin segregation, only one showed an obvious 3:1 ratio, indicating a single copy of T-DNA as a heterozygote. The other 11 displayed higher frequencies of resistant seedlings, demonstrating that they carried more than one T-DNA copy (Fig. 3a, Table S4). For the RMP5 promoter, three homozygous plants were observed on hygromycin plates while the other 17 plants showed various frequencies, suggesting that all were heterozygous for T-DNA. Among these 17 plants, five carried a single copy of T-DNA (based on frequencies), with approximately 75% having a 3:1 segregation ratio. The remaining plants were expected to contain more than one copy in their genomes because they had higher hygR frequencies (Fig. 3a, Table S5). As predicted, most homozygous and heterozygous T2 plants that had high frequencies of hygR T3 seedlings and were thought to have multiple copies of T-DNA, showed strong reductions in the occurrence of scp-2 mutant pollen when compared with heterozygotes carrying single copies (Fig. 3a).
a Percentages of aberrant pollen grains from non-transformed scp-2 homozygotes (scp-2) and transformed scp-2 homozygotes harboring proRMP-SCP:dHA were calculated at mature pollen stage in T2 generation. **Homozygous plants; *heterozygous plants carrying either single (s*) or multiple (m*) T-DNA copies. b Silique production in complementing lines (proRMP-SCP) compared with scp-2 hm mutant background (scp-2) and wild-type plants
The high frequencies of aberrant pollen in scp-2 homozygous plants led to reduced fertility and, ultimately, shorter siliques. By comparison, most of the T2 complementing lines exhibited strong decreases in mutant pollen frequencies and highly restored fertility, and they produced siliques with lengths relatively similar to those of normal wild-type plants (Fig. 3b, Fig. S1b). These results provided solid evidence that expression levels of SCP driven by RMP promoters are biologically relevant, enough to complement the scp-2 mutation.
Analysis of cis-acting regulatory elements for the promoters of three RMP genes confirmed by the GUS reporter system
To identify the CREs associated with microspore-preferred expression, we examined three promoters of RMP1, RMP2, and RMP5 used for complementation analysis and nine late pollen-specific genes (OsLPS) which have previously been reported (Oo et al. 2014) as a control. The promoter sequences were analyzed using the PlantPAN 2.0 (Chang et al. 2008; Chow et al. 2016). As a result, we found four CREs identified only in the promoters of three RMP genes but not in the OsLPS genes, including CGCGBOXAT, RHERPATEXPA7, PALINDROMICCBOXGM, and CCA1ATLHCB1. The functions and sequences of these CREs are summarized in Fig. 4 and Table S6. Of these, CGCGBOXAT is related to Ca2+/calmodulin (Yang and Poovaiah 2002); RHERPATEXPA7, root hair distribution patterns (Kim et al. 2006); PALINDROMICCBOXGM, soybean apical hypocotyl (Cheong et al. 1998); and CCA1ATLHCB1, regulation by phytochrome (Wang et al. 1997). These CREs are not related to previously identified motifs involved in pollen expression, suggesting the potential as novel CREs for early pollen development. Thus, we propose that they will be useful in future efforts to manipulate useful agronomic traits in rice microspore.
Conserved CREs analysis in the promoter sequences of three RMP genes. a Diagram of distribution of four conserved CREs in the promoter sequences of RMP1, RMP2, and RMP5. Numeric values indicate the upstream position from ATG, start codon sequence. b LOGOs of consensus sequences in four CREs and summary information of these CREs collected from PlantPAN 2.0. Numeric values in position column indicate the position in the plus strand promoter, and “(−)” symbols indicate the position in the minus strand promoter
Discussion
We have identified nine RMP genes from rice and performed several experiments with a GUS reporter system to examine the patterns of tissue/developmental stage-specific expression when their promoters are used with Arabidopsis. All nine promoters exhibited GUS expression in anther tissues (Fig. 2a). For RMP2 and RMP6, GUS expression was first observed at the UC stage but then was arrested at the BC stage. In contrast, expression in the RMP7 and RMP8 transgenic lines was detected in the pollen only at the TC and MP stages (Fig. 2b). For the RMP1, RMP3, RMP4, RMP5, and RMP9 promoters, GUS was expressed during pollen development throughout all stages, from UC through TC and MP (Fig. 2b). Although expression was not detected in other tissue types from transgenic plants driven by the RMP1 and RMP2 promoters, it was found in immature leaves, cotyledons, and young roots of seedlings with the RMP3 through RMP9 promoters. These findings confirmed the conclusions predicted from microarray data, indicating that RMP genes are preferentially expressed at the microspore stages.
We have previously taken a transgenic approach to characterize the eight RMP promoters in rice and have verified that eight (RMP1 through RMP8) drive GUS expression in reproductive and vegetative tissues (Nguyen et al. 2016). All eight promoters exhibit similar patterns in the anthers, with GUS activity peaking from the UC through the MP stages of pollen development. In extending this research to a heterologous plant system, we have now confirmed that RMP promoters also confer distinct patterns of GUS activity in Arabidopsis (Fig. 2a, b). This strengthens earlier conclusion on the existence of evolutionarily conserved and divergent transcriptional machinery for pollen developmental program (Khurana et al. 2013a; Oo et al. 2014; Nguyen et al. 2015). Further detailed analysis to identify cis-acting elements, we found that RMP1, RMP2, and RMP5 contained four conserved CREs in the promoter sequences which were not be identified in the late pollen (OsLPS) promoters. Although these conserved CREs have not been reported for pollen expression, they might play an important role for early pollen expression of RMP genes.
We also demonstrated here that RMP1, RMP2, and RMP5 can be applied to this heterologous system, based on our findings from complementation analysis using Arabidopsis male-gametophytic mutant scp-2 homozygotes, which display developmental defects at the microspore stage (Oh et al. 2010). Our current results indicated that the occurrence of mutant pollen is critically diminished in most lines harboring proRMP-SCP:dHA in the T2 generation, when compared with frequencies calculated for non-transgenic scp-2 homozygous mutants (Fig. 3a). This provides evidence that the RMP promoters can effectively direct the expression of SCP to recover the scp-2 mutation at the precise time before the BC stage begins.
A protein member of the glycoside hydrolase (GH) 35 family is encoded by RMP1. Its deduced biological functions in plants are mainly the degradation of structural pectins and xyloglucans in cell walls, and family member genes such as OsBgals are expressed in rice anthers (Tanthanuch et al. 2008). Two other genes—At1g31740 and At5g20710—in the GH35 family encode β-galactosidases and share high identities with RMP1. Both function in the early stages of microspore and pollen development in Arabidopsis (Hrubá et al. 2005; Ahn et al. 2007) (Table S1). RMP2 encodes peptidase S9A in the prolyl oligopeptidase (POP) family. That group of serine peptidases has been implicated in the degradation of biologically important peptide hormones and neuropeptides (Rosenblum and Kozarich 2003). These POP members are involved in regulating the development of reproductive organs in rice, and they also confer tolerance to abiotic stresses in Escherichia coli (Yoshida and Kuboyama 2001; Tan et al. 2013). In this study, we found an Arabidopsis gene, At1g76160, that encodes SKU5 (monocopper oxidase-like protein) similar five protein and shares 65% amino acid identity with RMP2. Although no SKU5 functions have been reported in pollen, the eFP database (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi) has indicated that At1g76160 is expressed in flower tissues from stages 9 to 15. Another gene, At5g09570, encodes a Cox19-like coiled-coil-helix-coiled-coil-helix domain (CHCHD)-containing protein family similar to RMP5 (Table S1). However, the functions of this protein have previously been described primarily in humans (Banci et al. 2012; Darshi et al. 2012), but not in plants. Our results are the first to show that CHCHD protein is also accumulated in pollen grains.
Promoters that control expression at a specific stage during microspore/pollen development are ideal tools for the biotechnological manipulation of targeted genes involved in pollen functions. For example, these promoters can be potentially applied for modifying the hormonal biosynthesis pathway that generates male sterile plants or for improving other agronomic traits associated with fertility (Bae et al. 2010). Thus far, several microspore-specific promoters have been identified in plants, including TNM19, Bp4, BECLIN 1, and TA29 (Albani et al. 1991; Kriete et al. 1996; Oldenhof et al. 1996; Singh et al. 2010). When these promoters are combined with the cytotoxic barnase, TNM19- and Bp4-barnase plants clearly show lethal pollen phenotypes, and pollen from these plants does not germinate, causing sterility in species such as Nicotiana tabacum (Custers et al. 1997). However, the source of such promoters is quite restrictive for the development of elite GM rice. In our previously report, more than 410 RMP genes, which are explored by analyzing meta-expression data, showed that most of the genes (285 genes) have no GO terms and only two genes have been functionally characterized. In contrast, we found 23 out of 263 tapetum-preferred genes have been functionally investigated, and 52% had GO terms (Nguyen et al. 2016). These suggest that the functions of microspore genes are still in vague and need to be addressed. RMP genes could be new targets to understand male-gametophytic development.
Recently, several research groups have focused on male gametophyte development under stressful conditions including genomics and proteomics, suggesting that pollen is new target for development of plants adapting to environmental changes (reviewed by Grover et al. 2016). Microspores in plants are very sensitive to abiotic stresses, such as drought, heat, and cold, which are becoming serious challenges in agricultural regions. The introduction of stress during key reproductive stages can reduce or completely eliminate crop yields due to pollen sterility (Solomon et al. 2007; Zinn et al. 2010). The metabolic alterations involved in male reproductive development may be associated with abiotic stresses such as abscisic acid, gibberellic acid, and excess sugar (Sharma 2014; Burke and Chen 2015). Among the nine RMP promoters described in this paper, three promoters of RMP1, RMP2, and RMP5 which are active and preferential at the microspore stage in both monocotyledonous and dicotyledonous plant species will be applicable to manipulate tissue-specific transgenic expression in the pollen-preferential way.
Author contribution statement
TDN and SM performed the experiments and generated the data. SAO, KHJ, and SKP designed the experiments. TDN, SM, SAO, KHJ, and SKP wrote the manuscript.
References
Ahn YO, Zheng M, Bevan DR, Esen A, Shiu SH, Benson J, Peng HP, Miller JT, Cheng LI, Poulton JE, Shih MC (2007) Functional genomic analysis of Arabidopsis thaliana glycoside hydrolase family 35. Phytochemistry 68:1510–1520
Albani D, Altosaar I, Arnison PG, Fabijanski SF (1991) A gene showing sequence similarity to pectin esterase is specifically expressed in developing pollen of Brassica napus. Sequences in its 5′ flanking region are conserved in other pollen-specific promoters. Plant Mol Biol 16:501–513
Aya K, Suzuki G, Suwabe K, Hobo T, Takahashi H, Shiono K, Yano K, Tsutsumi N, Nakazono M, Nagamura Y, Matsuoka M, Watanabe M (2011) Comprehensive network analysis of anther-expressed genes in rice by the combination of 33 laser microdissection and 143 spatiotemporal microarrays. PLoS ONE 6:e26162
Bae HK, Kang HG, Kim GJ, Eu HJ, Oh SA, Song JT, Chung IK, Eun MY, Park SK (2010) Transgenic rice plants carrying RNA interference constructs of AOS (allene oxide synthase) genes show severe male sterility. Plant Breed 129:647–651
Banci L, Bertini I, Coffi-Baffoni S, Jaiswal D, Peruzzini R, Winkelman J (2012) Structural characterization of CHCHD5 and CHCHD7: two atypical twin CX9C proteins. J Struct Biol 180:190–200
Borg M, Brownfield L, Twell D (2009) Male gametophyte development: a molecular perspective. J Exp Bot 60:1465–1478
Burke JJ, Chen J (2015) Enhancement of reproductive heat tolerance in plant. PLoS ONE 10:e0122933
Chang WC et al (2008) PlantPAN: plant promoter analysis navigator, for identifying combinatorial cis-regulatory elements with distance constraint in plant gene groups. BMC Genom 9:561
Chen YC, McCormick S (1996) sidecar pollen, an Arabidopsis thaliana male gametophytic mutant with aberrant cell divisions during pollen development. Development 122:3243–3253
Cheong YH, Yoo CM, Park JM, Ryu GR, Goekjian VH, Nagao RT, Key JL, Cho MJ, Hong JC (1998) STF1 is a novel TGACG-binding factor with a zinc-finger motif and a bZIP domain which heterodimerizes with GBF proteins. Plant J 15:199–209
Chow CN, Zheng HQ, Wu NY, Chien CH, Huang HD, Lee TY, Chiang-Hsieh YF, Hou PF, Yang TY, Chang WC (2016) PlantPAN 2.0: an update of plant promoter analysis navigator for reconstructing transcriptional regulatory networks in plants. Nucleic Acids Res 44(D1):D1154–D1160
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Custers JBM, Oldenhof MT, Schrauwen JAM, Cordewender JHG, Wullems GJ, van Lookeren Campagne MM (1997) Analysis of microspore-specific promoters in transgenic tobacco. Plant Mol Biol 35:689–699
Darshi M, Trinh K, Murphy A, Taylor S (2012) Targeting and import mechanism of coiled-coil helix coiled-coil helix domain-containing protein 3 (ChChd3) into the mitochondrial intermembrane space. J Biol Chem 287:39480–39491
Fujita M, Horiuchi Y, Ueda Y, Mizuta Y, Kubo T, Yano K, Yamaki S, Tsuda K, Nagata T, Niihama M, Kato H, Kikuchi S, Hamada K, Mochizuki T, Ishimizu T, Iwai H, Tsutsumi N, Kurata N (2010) Rice expression atlas in reproductive development. Plant Cell Physiol 51:2060–2081
Grover A, Twell D, Schleiff E (2016) Pollen as a target of environmental changes. Plant Reprod 29:1–2
Gupta V, Khurana R, Tyagi AK (2007) Promoters of two anther-specific genes confer organ-specific gene expression in a stage-specific manner in transgenic systems. Plant Cell Rep 26:1919–1931
Han MJ, Jung KH, Yi G, Lee DY, An G (2006) Rice immature pollen 1 (RIP1) is a regulator of late pollen development. Plant Cell Physiol 47:1457–1472
Honys D, Oh SA, Renak D, Donders M, Solcova B, Johnson JA, Boudova R, Twell D (2006) Identification of microspore-active promoters that allow targeted manipulation of gene expression at early stages of microgametogenesis in Arabidopsis. BMC Plant Biol 6:31
Hrubá P, Honys D, Twell D, Capková V, Tupý J (2005) Expression of beta-galactosidase and beta-xylosidase genes during microspore and pollen development. Planta 220:931–940
Jeon JS, Chung YY, Lee S, Yi GH, Oh BG, An G (1999) Isolation and characterization of an anther-specific gene, RA8, from rice (Oryza sativa L.). Plant Mol Biol 39:35–44
Jung KH, Han MJ, Lee YS, Kim YW, Hwang I, Kim MJ, Kim YK, Nahm BH, An G (2005) Rice undeveloped tapetum 1 is a major regulator of early tapetum development. Plant Cell 17:2705–2722
Khurana R, Kapoor S, Tyagi AK (2013a) Spatial and temporal activity of upstream regulatory regions of rice anther-specific genes in transgenic rice and Arabidopsis. Transgenic Res 22:31–46
Khurana R, Kathuria H, Mukhopadhyay A, Kapoor S, Tyagi AK (2013b) 286 bp upstream regulatory region of a rice anther specific gene, OSIPP3, confers pollen-specific expression in Arabidopsis. Biotechnol Lett 35:455–462
Kim DW, Lee SH, Choi SB, Won SK, Heo YK, Cho M, Park YI, Cho HT (2006) Functional conservation of a root hair cell-specific cis-element in angiosperms with different root hair distribution patterns. Plant Cell 18:2958–2970
Kriete G, Niehaus K, Perlick AM, Pühler A, Broer I (1996) Male sterility in transgenic tobacco plants induced by tapetum-specific deacetylation of the externally applied non-toxic compound N-acetyl-l-phosphinothricin. Plant J 9:809–818
Li N, Zhang DS, Liu HS, Yin CS, Li XX, Liang WQ, Yuan Z, Xu B, Chu HW, Wang J, Wen TQ, Huang H, Luo D, Ma H, Zhan DB (2006) The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 18:2999–3014
Liu X, Shangguan Y, Zhu J, Lu Y, Han B (2013) The rice OsLTP6 gene promoter directs anther-specific expression by a combination of positive and negative regulatory elements. Planta 238:845–857
Luo H, Lee JY, Hu Q, Nelson-Vasilchik K, Eitas TK, Lickwar C, Kausch AP, Chandlee JM, Hodges TK (2006) RTS, a rice anther-specific gene is required for male fertility and its promoter sequence directs tissue-specific gene expression in different plant species. Plant Mol Biol 62:397–408
Mi H, Muruganujan A, Casagrande JT, Thomas PD (2013) Large-scale gene function analysis with the PANTHER classification system. Nat Protoc 8:1551–1556
Nguyen TD, Oo MM, Moon S, Bae HK, Oh SA, Soh MS, Song JT, Kim JH, Jung KH, Park SK (2015) Expression analysis of two rice pollen-specific promoters using homologous and heterologous systems. Plant Biotechnol Rep 9:297–306
Nguyen TD, Moon S, Nguyen VNT, Gho Y, Soh MS, Song JT, An G, Oh SA, Park SK, Jung KH (2016) Genome-wide identification and analysis of rice genes preferentially expressed in pollen at an early developmental stage. Plant Mol Biol 92:71–88. doi:10.1007/s11103-016-0496-1
Oh SA, Park KS, Twell D, Park SK (2010) The SIDECAR POLLEN gene encodes a microspore-specific LOB/AS2 domain protein required for the correct timing and orientation of asymmetric cell division. Plant J 64:839–850
Oh SA, Jeon J, Park HJ, Grini PE, Twell D, Park SK (2016) Analysis of gemini pollen 3 mutant suggests a broad function of AUGMIN in microtubule organization during sexual reproduction in Arabidopsis. Plant J 87:188–201
Oldenhof MT, Groot PF, Visser JH, Schrauwen JA, Wullems GJ (1996) Isolation and characterization of a microspore-specific gene from tobacco. Plant Mol Biol 31:213–225
Oo MM, Bae HK, Nguyen TD, Moon M, Oh SA, Kim JH, Soh MS, Song JT, Jung KH, Park SK (2014) Evaluation of rice promoters conferring pollen-specific expression in a heterologous system, Arabidopsis. Plant Reprod 27:47–58
Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, Childs K, Thibaud-Nissen F, Malek RL, Lee Y, Zheng L, Orvis J, Haas B, Wortman J, Buell CR (2007) The TIGR rice genome annotation resource: improvements and new features. Nucleic Acids Res 35:D883–D887
Park SK, Howden R, Twell D (1998) The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development 125:3789–3799
Park JI, Hakozaki H, Endo H, Takada Y, Ito H, Uchida M, Okabe T, Watanabe M (2006) Molecular characterization of mature pollen-specific genes encoding novel small cysteine-rich proteins in rice (Oryza sativa L.). Plant Cell Rep 25:466–474
Rosenblum JS, Kozarich JW (2003) Prolyl peptidases: a serine protease subfamily with high potential for drug discovery. Curr Opin Chem Biol 7:496–504
Rutley N, Twell D (2015) A decade of pollen transcriptomics. Plant Reprod 28:73–89
Sharma KD (2014) Pollen development under cold stress: a molecular perspective. Austin J Genet Genom Res 1:4
Singh SP, Pandey T, Srivastava R, Verma PC, Singh PK, Tuli R, Sawant SV (2010) BECLIN1 from Arabidopsis thaliana under the generic control of regulated expression systems, a strategy for developing male sterile plants. Plant Biotechnol 8:1005–1022
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB (2007) Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, p 996
Suwabe K, Suzuki G, Takahashi H, Shiono K, Endo M, Yano K, Fujita M, Masuko H, Saito H, Fujioka T, Kaneko F, Kazama T, Mizuta Y, Kawagishi-Kobayashi M, Tsutsumi N, Kurata N, Nakazono M, Watanabe M (2008) Separated transcriptomes of male gametophyte and tapetum in rice: validity of a laser microdissection (LM) microarray. Plant Cell Physiol 49:1407–1416
Swapna L, Khurana R, Vijaya-Kumar S, Tyagi AK, Rao KV (2011) Pollen-specific expression of Oryza sativa indica pollen allergen gene (OSIPA) promoter in rice and Arabidopsis transgenic systems. Mol Biotechnol 48:49–59
Tan CM, Chen RJ, Zhang JH, Gao XL, Li LH, Wang PR, Deng XJ, Xu ZJ (2013) OsPOP5, a prolyl oligopeptidase family gene from rice confers abiotic stress tolerance in Escherichia coli. Int J Mol Sci 14:20204–20219
Tanthanuch W, Chantarangsee M, Maneesan J, Ketudat-Cairns J (2008) Genomic and expression analysis of glycosyl hydrolase family 35 genes from rice (Oryza sativa L.). BMC Plant Biol 8:84
Twell D, Park SK, Hawkins TJ, Schubert D, Schmidt R, Smertenko A, Hussey PJ (2002) MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nat Cell Biol 4:711–714
Wang ZY, Kenigsbuch D, Sun L, Harel E, Ong MS, Tobin EM (1997) A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell 9:491–507
Wei LQ, Xu WY, Deng ZY, Su Z, Xue Y, Wang T (2010) Genome-scale analysis and comparison of gene expression profiles in developing and germinated pollen in Oryza sativa. BMC Genom 11:338
Yang T, Poovaiah BW (2002) A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. Biol Chem 277:45049–45058
Yokoi S, Tsuchiya T, Toriyama K, Hinata K (1997) Tapetum-specific expression of the Osg6B promoter-β-glucuronidase gene in transgenic rice. Plant Cell Rep 16:363–367
Yoshida KT, Kuboyama T (2001) A subtilisin-like serine protease specifically expressed in reproductive organs in rice. Sex Plant Reprod 13:193–199
Zhang H, Liang W, Yang X, Luo X, Jiang N, Ma H, Zhang D (2010) Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell 22:672–689
Zinn KE, Tunc-Ozdemir M, Harper JF (2010) Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exp Bot 61:1959–1968
Acknowledgements
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science and Technology Development (Project Nos. PJ01182602 to KHJ; PJ01194201 to SKP),” Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by David Twell.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Complementation analysis of scp-2 mutant using SCP gene expression under the control of RMP promoters in a heterologous system. a Schematic diagram of vectors used for complementation analysis. b Silique production in complementing lines (proRMP-SCP) compared with scp-2 hm mutant background (scp-2) and wild-type plants (EPS 8787 kb)
Fig. S2
Confirmation of T-DNA insertion in ProRMP-SCP:dHA lines. a 1316-bp SCP-dHA fragment was amplified from DNA of 14 transgenic lines (Lanes 1–14), using gene-specific primers SCP forward and reverse. b scp-2 allele was detected using LBb1 and LOBRP primer set. c SCP wild-type gene was checked in scp-2 hm background and transgenic lines using LBb1 and 47870-R primers. scp-2 hm and Col ecotype were used as controls (EPS 5240 kb)
Fig. S3
Percentages of aberrant pollen grains from non-transformed scp-2 homozygotes (scp-2) and transformed scp-2 hm harboring proRMP-SCP:dHA were calculated at mature pollen stage in T1 generation (EPS 3269 kb)
Rights and permissions
About this article
Cite this article
Nguyen, T.D., Moon, S., Oo, M.M. et al. Application of rice microspore-preferred promoters to manipulate early pollen development in Arabidopsis: a heterologous system. Plant Reprod 29, 291–300 (2016). https://doi.org/10.1007/s00497-016-0293-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-016-0293-7