Abstract
Kacang (K) and Kacang Etawah (KE) cross goats are the major goat breeds that are important for farming livelihood and income and recognized for their tolerance to hot and humid tropical climates. As global warming progresses, the daily maximum temperature (Tmax) is predicted to be continuously increased, which will challenge goat production in the future. The objective of this study was to evaluate the physiological and behavioral responses of the goats to the elevated Tmax. Six K and six KE female goats were housed in a normal environment (average Tmax: 33°C; temperature humidity index (THI): 76 to 86) for 6 weeks and then in a hot environment (average Tmax: 38°C; THI: 76 to 92) for 7 weeks. During hot conditions, rectal, rectal surface, and skin temperature, respiration rate, hemoglobin, and cholesterol increased (p < 0.05), whereas glucose blood levels decreased (p < 0.01). Dry matter (DM) intake was lowered (p < 0.01), and DM digestibility was elevated (p < 0.01), whereas drinking water and body water retention were lowered but varied (p < 0.05) during hot weeks. Lying time increased during the hot weeks in both breeds (p < 0.01), whereas lying and ruminating as well as total ruminating time was longer (p < 0.05) in KE goats compared to K goats, which explain the greater (p < 0.05) DM digestibility in KE goats. The effect of the elevated Tmax might be less severe since it also depends on the duration of the Tmax and the variation of daily THI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Population and economic growth in many developing countries have increased the demand for livestock products (World Bank 2009). Goat farming is a suitable alternative livestock farming business for smallholder farmers in Southeast Asia since it contributes significantly to farmers’ livelihoods and agricultural sustainability. Goat farming provides nutritious food and a source of cash income for products such as meat, milk, and manure. Furthermore, farmers prefer goats over large ruminants due to their low investment and ability to survive and reproduce with simple feeding and rearing management (Devendra 2011).
The Kacang goat is a small breed of goat that spread widely throughout the islands of Indonesia and the Malay Peninsula and is found sporadically in other Southeast Asian countries (Lin et al. 2013). Genetic improvement through crossbreeding with the imported Etawah (Jamnapari) breed has been programmed to improve the growth rate and milk yield (Payne and Wilson 1999). However, the sustainability of goat farming is challenged by a wide range of environmental issues, including climate change.
As global warming progresses, the increase in daily maximum temperature (Tmax) and extreme events in the regions have been documented. The average daily maximum temperature had increased by 0.18 to 0.21°C per decade (Siswanto et al. 2016; Supari et al. 2017), which seriously challenges future livestock production. Moreover, the proportion of livestock potentially exposed to heat stress was predicted to double by 2050 and be much greater in tropical regions compared to temperate regions (Thornton et al. 2021). The negative effects of heat stress on goat performance, physiological and behavioral adaptations have been fully studied elsewhere (Ribeiro et al. 2018; Gupta and Mondal 2021). However, information on the behavioral and physiological responses of the native and cross-breed goats to increased global warming impacts in tropical and humid regions is limited. High temperatures and relative humidity are serious obstacles to the optimal performance of livestock in the region since the animal’s heat dissipation is severely limited.
Temperature humidity index (THI) values of 79 or 89 are defined as high or extreme heat stress for the animals, respectively (Serradilla et al. 2017). When Tmax exceeds 34°C at 70% relative humidity (RH), for instance, the daily THI in the lowlands of the tropics is greater than 86. As a result, the goats are subject to heat stress throughout the year, and their responses to the increased Tmax are important for future adaptation options and the breeding program. The purpose of this study was to establish the effects of heat stress on physiological changes and behavioral responses in Kacang (K) and Kacang Etawa (KE) cross goats.
Materials and methods
Location
The experiment was approved by the Faculty of Agriculture, Universitas Sriwijaya (No. KPPHP-2022-1). The site is located at 104°39’30.5"E longitude and 3°11’38.4"S latitude with an average elevation of 6 m. The mean temperature (°C) is between 25.4 ± 0.31 and 30.0 ± 0.59 and the RH (%) ranges from 76.3 ± 1.06 to 93.5 ± 0.93 on an annual basis.
Experimental animal, feeding management, and treatments
Six female K and six female KE goats between 7 and 9 months old were used in the present experiment. On an electronic balance, the goats were weighed at the beginning of the experiment and then every Sunday morning before feed offering. The live weights (LW) of K and KE were 9 ± 0.6 and 13 ± 0.7 kg, respectively, at the beginning of the experiment. The goats were then treated with anthelmintics and placed in individual cages (1.5 m × 0.75 m) in a house (3.5 m height) with an asbestos roof. Each cage is equipped with food and water containers and a urine-feces separator beneath the cage.
The diet consisted of chopped Guinea hay and a concentrate mixture (53% corn meal, 30% copra meal, 13% fish meal, 2% molasses, 1% mineral mix, and 1% common salt). The concentrate was offered at 3% of LW (Dry matter (DM) basis) at 9:00, and the hay was offered ad libitum in equal portions at 9:00 and 16:00. The goats had free access to drinking water. Subsequently, the goats were accustomed to the feeding and measurement methods for 30 days.
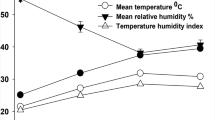
The entire experiment was conducted from April to August 2022, and the treatment period (May to August 2022) was divided into two periods: normal (week 1 to 6) and hot weeks (week 7 to 13). During normal weeks, the daily maximum indoor temperature (Tmax) and RH followed the pattern of the daily variation in outdoor temperature and RH. During the hot weeks, the Tmax was increased from 10:00 to 16:00 with air heaters. The Tmax was gradually increased to 35°C within 2 days and then to 36°C at the end of week 7. The Tmax was then maintained at ≤ 39.5°C during the hot weeks period using a digital thermostat (XH W3001). During treatment, THI ranged from 76.4 to 86.4 in the normal weeks and 76.3 to 92.1 in the hot weeks (Table 1). Referring to Serradilla et al. (2017) and Thornton et al. (2021), we assumed that the maximum THI levels were sufficient to induce heat stress on the goats.
Experimental measurements, sample collection, and analysis
The indoor temperature and RH were recorded continuously at 10-min intervals throughout the experiment using a climate data logger (Benetech G1365). The recorded temperature and RH were then used to calculate the THI (THI= 0.8×Temperature + ((RH/100) × (Temperature – 14.3)) + 46.4) according to Thom (1959).
Body temperature and respiration rate were measured twice a week when THI levels peaked (13:30 to 14:30). Rectal temperature (RT) was measured using a digital thermometer (Omron MC-343F), while rectal skin temperature (RST) and skin temperature (ST, in shaved areas at the right shoulder) were measured with an infrared thermometer (Beureur FT 90) at a distance of ±2 cm. Furthermore, a stopwatch was used to record the respiration rate (RR) by counting a flank movement when the goats were lying down. In addition, blood samples were collected once a week on the day following the measurement of body temperature and RR. A blood sample (± 2 ml) was collected between 13:30 and 14:30 through the jugular vein into EDTA tubes and immediately placed in an ice box. The samples were then transferred to the laboratory for hematological analysis. Hemoglobin, erythrocytes, leukocytes, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) levels were measured with Sysmex XP-100 while glucose and cholesterol concentrations were measured with Humastar 100.
To determine DM digestibility, samples of offered and refused feed as well as feces were collected over 6 consecutive days. The concentrate mixture (±100 g) and Guinea grass hay (±100 g) were sampled twice a week. Refused hay and concentrate, as well as feces, were sampled daily. Subsample of 100 g of refused hay and 100 g of refused concentrate were collected per animal per day, and ±10% of the daily defecation amount was collected. Afterward, all samples were oven-dried at 45°C for 3 consecutive days and then pooled per animal at the end of the week. Dried feed and fecal samples were ground to pass a 1-mm mesh for DM analysis (AOAC 2005). The DM intake was calculated as the difference between the DM amount of feed offered and refused, whereas DM digestibility was calculated as the difference between the amount of DM intake and fecal excretion (Ali et al. 2022).
The volume of daily drinking water intake (DWI) was recorded before feed offerings and then converted to weight at the end of the experiment. Water refusal was discharged, and the buckets were cleaned and then refilled. Subsequently, to estimate the daily evaporative loss, two buckets of water were placed, and the values were used to correct the DWI. The feed water intake was calculated from the water content and the amount of feed intake (Ali et al. 2022).
Daily urinary water excretion was determined by weighing the urine after filtering and homogenizing. Then, a 40-ml of urine sample was taken and stored in a freezer at -20°C. At the end of the experiment, the samples were pooled per animal, and 3 ml of the sample was dried at 60°C for 24 h to calculate the water content of the urine. The water in the fecal excretion was obtained from the water content of the feces. Body water retention was calculated from the difference between total water intake and total water excretion in the urine and feces (Hamzaoui et al. 2013).
The behavioral responses of each animal were observed twice a week with a camera system for 9 h (10:00 to 19:00). The recordings were scanned at 5-min intervals for behavior activities (eating, standing idle, standing and ruminating, lying idle, lying and ruminating, urination, and defecation frequency).
Statistical analysis
The data were analyzed using the proc mixed procedure of the statistical analysis system (SAS Inst., Inc., Cary, NC), where the week was included in the model together with the breed and their interactions as fixed effects, whereas the animal was included as a random effect. The Tukey post hoc test was applied to compare differences among means. The significance was determined at p < 0.05.
Results
During the control week, the ambient temperature and hourly RH fluctuated. The minimum temperature and maximum RH were found at the dawn (4:30 to 5:30) and conversely, the maximum temperature and the minimum RH were found in the afternoon (13:00 to 15:00). The fluctuation also occurred during hot weeks, except during rainy periods, when temperature decreased while the RH increased (Table 1).
There was a significant (p < 0.01) increase in RT, RST, ST, and RR during the hot weeks (weeks 7–13) compared to the control week (week 6). No significant difference in RT and ST between week 7 and 13, but RST was significantly (p < 0.01) lower in week 11 compared to week 7 and 9. Moreover, Hemoglobin and cholesterol concentrations were higher (p < 0.05) in the hot weeks compared to the control week while glucose concentrations were significantly (p < 0.01) lowered during the hot weeks. During the hot weeks, glucose concentration was higher (p < 0.01) in week 7 compared to week 9, 11, and 13. Meanwhile, no significant difference was observed in the levels of erythrocytes, leukocytes, hematocrit, MCV, MCH, and MCHC during control and hot weeks. Moreover, no significant effect of the different breeds was seen in all parameters of thermo-physiological and hematological responses while the interaction effect of breed and the week was seen in the leukocyte concentration (Table 2).
The hay intake was higher (p < 0.01) in week 13 compared to week 6 and 11 while the concentrate intake was lower (p < 0.01) after week 9 compared to the intake in week 6. A similar difference was also noted in the total feed intake where feed intake was decreased during hot weeks. Meanwhile, concentrate intake was higher (p < 0.01) in the K than in the KE goat. Moreover, dry matter digestibility was elevated (p < 0.01) during the hot weeks, whereas, DM digestibility in KE goat was higher (p < 0.01) compared to the K goat. Further, there was no significant influence of breed and week interaction on intake and digestibility parameters (Table 3).
The total water intake showed significant (p < 0.01) variation during the experimental weeks, the highest intake was seen in the control THI (week 6) and the lowest intake was in week 11 while no significant influence of goat genotype and breed and week interaction on the water intake parameters. The fecal and total water excretion and the body water retention were significantly (p < 0.05) influenced by the experimental weeks, while the urinary water excretion did not differ. The fecal and total water excretions decreased (p < 0.01) as the increased THI in week 7 and were the lowest in week 11, while water retention was the lowest at week 9 (Table 3). In addition, the urinary and total water excretion was influenced (p < 0.01) by the breed and week interaction.
The effects of heat stress on behavioral responses are shown in Table 4. The ruminating times while lying and standing were not influenced (p > 0.05) by the elevated THI. However, the standing idle time was lowered while lying idle time was increased during hot weeks (p < 0.01). During hot weeks, the highest total lying time (idle + ruminating) and the lowest total standing time (idle + ruminating) were seen in week 11. Drink frequencies in week 6 and 7 were similar and then significantly (p < 0.01) lowered during week 9 to 13. A significant variation between breeds was observed at the lying & ruminating time where the time in the KE breed was significantly (p < 0.01) higher than in the K breed.
Discussion
In the present study, the daily THI was ≥ 79 from 8:00 to 20:00 in the normal and hot weeks, and the maximum THI levels in the hot weeks (91) were higher than in the control week (86). According to Thornton et al. (2021), also using the equation of Thom (1959), a THI of 79 indicates high heat stress, and a THI above 89 is classified as extreme heat stress for the goats. Exposing K and KE goats to the elevated heat stress level had a pronounced impact on their thermoregulatory systems. The increase in RT, RST, ST, and RR during the hot weeks illustrated the physiological acclimatization of the goats to cope with the elevated heat stress challenge due to the increased THI, though the animals had already experienced the high daily THI stressor during the control week. In addition, during the hot weeks, the thermoregulatory responses did not significantly differ during the long-term assessment (7 weeks), which bears no evidence of adaptation to cope with the elevated heat stress.
The high body temperature of the goats due to the increased THI was broadly reported in the previous studies (Ribeiro et al. 2018; Gupta and Mondal 2021). Moreover, The lowered glucose level during the hot weeks could be associated with a decrease in propionate level or higher insulin activity when nutrient intake decreased (Rhoads et al. 2009). The glucose levels dropped to the lowest level at week 11 whereas cholesterol levels were elevated and reached the highest level at week 9. The lowered glucose levels were also reported in previous studies (Pandey et al. 2012; Hooda and Upadhyay 2014; El-Tarabany et al. 2017); however, Hamzaoui et al. (2013) showed a non-significant difference in the glucose levels when DM intake was reduced at elevated ambient temperatures.
The increased cholesterol level could be attributed to the mobilization of triglycerides during heat stress (Ribeiro et al. 2018). Joy et al. (2020) also reported a similar result, while others reported the opposite result that heat stress decreased cholesterol levels (Pandey et al. 2012; El-Tarabany et al. 2017; Cardoso et al. 2021). The lower cholesterol levels may be due to increased total body water, decreased thyroid activity, and reduced dietary cholesterol intake (Pandey et al. 2012; Gupta and Mondal 2021). The increase in cholesterol levels in the present study could also be attributed to the significant reduction in body water retention (Table 3).
In this study, the increase in hemoglobin level with increasing daily heat stress may be related to the increase in oxygen consumption due to the increase in RR (Ribeiro et al. 2018). However, a previous study (Sivakumar et al. 2010) reported a decline in hemoglobin levels during heat stress. Moreover, in the present study, the levels of erythrocytes, leukocytes, hematocrit, MCV, MCH, and MCHC did not change (p > 0.05). The different responses of hematological parameters in heat-stressed goats were reported from previous studies: lower levels of hemoglobin and MCH were reported in dry summer compared to winter in Nubian goats, while the levels of erythrocytes, leukocytes, MCV, and MCHC were not different (Abdelatif et al. 2009). On the other hand, a study in a humid tropical region of Bangladesh (Alam et al. 2011) reported elevated levels of hemoglobin, erythrocytes, and leukocytes in heat-stressed goats. The differences in hematological responses may be due to the differences in the physiological state, breed, duration, and severity of stress (Ribeiro et al. 2018; Gupta and Mondal 2021).
The studies (Ribeiro et al. 2018; Gupta and Mondal 2021) also related the reduced nutrient intake as an adaptive response to reduced metabolic heat during heat stress. This is because heat gain from feeding accounts for the majority of total heat production. Additionally, heat-stressed goats showed higher DM digestibility. As shown in Table 3, DM digestibility increased significantly between week 9 to 13. To the best of our knowledge, there are no data comparing digestibility under heat stress in tropical goat breeds. The higher digestibility during hot weeks in this study is consistent with previous studies conducted with lactating Murciano-Granadina goats (Hamzaoui et al. 2013) and male Saanen goats (Hirayama et al. 2004). This could be related to the elevated retention time of ingested feed in the gastrointestinal tract as the reduction in DM intake (Ali et al. 2019). The greater digestibility during the hot weeks might have compensated for the reduced DM intake and could partially explain the lack of adverse effects of heat stress on the LW gain.
In this study, the increasing Tmax decreased total water intake from the DWI and dietary water intake. The lowered DWI (Table 3) with the decrease in DM intake was consistent with the decrease in drink frequency (Table 4). The decline in DWI was different from a previous study in which Tmax increased the DWI (Ali et al. 2022). The difference in response could be partially explained by the severity of heat stress. The THI reached 92 in this study, whereas the maximum THI value was 85 in the previous study. The increased water intake in heat-stressed goats as a result of the higher loss of evaporative water (Ribeiro et al. 2018; Gupta and Mondal 2021). However, as the level of heat stress increases, the reduction in feed intake in the current study may have resulted in a reduction in the drinking water intake.
The increase in daily maximum temperature significantly (p < 0.05) lowered fecal and total water excretion. The lowest level of the water excretion was found at week 11, while the lowest level of body water retention was observed at week 9. Contrary to that, Hamzaoui et al. (2013) showed that heat-stressed goats had three times higher water retention/evaporation than non-stress goats in a fully controlled environment. Moreover, the study showed insignificant differences in urinary water excretion with increasing THI. Also, in this study, urinary frequency did not differ between normal and increased THI. The reduced urinary frequency in heat-stressed goats has been reported and could relate to the behavioral acclimatization to maintain body fluid balance, as evaporative water loss from the body increased (Alam et al. 2011; Shilja et al. 2016).
The treatments had a significant (p < 0.01) effect on the observed standing and lying parameters, with standing and lying times showing an inverse trend between normal and hot weeks. Lying time was significantly (p < 0.01) longer during the hot weeks, which might be an adaptive mechanism to prevent additional heat load from body movements. Heat stress has been found to decrease standing time and increase lying time in sheep (Li et al. 2018) and goats (Darcan et al. 2008; Salama et al. 2021; Hartmann et al. 2021). However, Shilja et al. (2016) reported that higher THI levels shortened laying time in Osmanabadi goats. The different responses may occur due to the severity of stress, adaptability, and breed differences.
Total ruminating time was numerically longer during the hot weeks and could be associated with the higher values of DM digestibility. The result is different from that observed in goats (Darcan et al. 2008) and cows (Moretti et al. 2017), where heat stress shortened ruminating time. Additionally, ruminating time (ruminating while lying) was significantly longer in KE goats than in K goats, which partly explains the greater DM digestibility in KE goats (Table 2). Further, as the increase Tmax, the cross-breeding of the KE breed might have an advantage since the Etawa breed is native to Uttar Pradesh, which has a higher daily maximum temperature (Payne and Wilson 1999).
Different goat performances during normal and heat stress environments were evidenced in the present work and could be considered for breeding programs under the changing climate conditions (Thornton et al. 2021). The advantage associated with crossbreeding (Payne and Wilson 1999) was shown when KE goats had higher DM digestibility than K goats, although there was no significant difference in their growth during the treatment weeks.
Conclusions
In conclusion, the increased daily maximum temperature affected the performances of K and KE goats. Their physiological changes and behavioral responses to heat stress were similar. However, nutrient utilization might be better in KE goats, which was correlated with the longer duration of ruminating time. The variety of the responses during the hot weeks indicates that the severity of the effects of heat stress might also depend on the duration of the daily maximum temperature and the fluctuation of hourly temperature and humidity.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdelatif A, Ibrahim M, Hassan Y (2009) Seasonal variation in erythrocytic and leukocytic indices and serum proteins of female Nubian goats. Mid East J Sci Res 4
Alam M, Hashem MA, Rahman M et al (2011) Effect of heat stress on behavior, physiological and blood parameters of goat. Progress Agric 22. https://doi.org/10.3329/pa.v22i1-2.16465
Ali AIM, Sandi S, Sahara E et al (2022) Effects of acid drinking water on nutrient utilization, water balance, and growth of goats under hot-humid tropical environment. Small Rumin Res 210:106689. https://doi.org/10.1016/j.smallrumres.2022.106689
Ali AIM, Wassie SE, Korir D et al (2019) Digesta passage and nutrient digestibility in Boran steers at low feed intake levels. J Anim Physiol Anim Nutr 103:1325–1337. https://doi.org/10.1111/jpn.13158
AOAC (2005) Official Methods of Analysis of AOAC International. Arlington, VA, USA
Cardoso EA, Furtado DA, Ribeiro NL et al (2021) Biochemical and hormonal parameters of goats kept in a controlled environment consuming water with different levels of salinity. Arq Bras Med Veterinária e Zootec 73:853–860. https://doi.org/10.1590/1678-4162-12186
Darcan N, Cedden F, Cankaya S (2008) Spraying effects on some physiological and behavioural traits of goats in a subtropical climate. Ital J Anim Sci 7:77–85. https://doi.org/10.4081/ijas.2008.77
Devendra C (2011) Integrated tree crops-ruminants systems in South East Asia: advances in productivity enhancement and environmental sustainability. Asian-Australas J Anim Sci 24:587–602. https://doi.org/10.5713/ajas.2011.r.07
El-Tarabany MS, El-Tarabany AA, Atta MA (2017) Physiological and lactation responses of Egyptian dairy Baladi goats to natural thermal stress under subtropical environmental conditions. Int J Biometeorol 61:61–68. https://doi.org/10.1007/s00484-016-1191-2
Gupta M, Mondal T (2021) Heat stress and thermoregulatory responses of goats: a review. Biol Rhythm Res 52:407–433. https://doi.org/10.1080/09291016.2019.1603692
Hamzaoui S, Salama AAK, Albanell E et al (2013) Physiological responses and lactational performances of late-lactation dairy goats under heat stress conditions. J Dairy Sci 96:6355–6365. https://doi.org/10.3168/jds.2013-6665
Hartmann E, Högberg M, Olsson K, Dahlborn K (2021) Physiological and behavioural responses of Swedish domestic goats and their kids (Capra hircus) to 15 days of heat exposure. Acta Agric Scand Sect A —. Anim Sci 70:41–49. https://doi.org/10.1080/09064702.2020.1869817
Hirayama T, Katoh K, Obara Y (2004) Effects of heat exposure on nutrient digestibility, rumen contraction and hormone secretion in goats. Anim Sci J 75:237–243. https://doi.org/10.1111/j.1740-0929.2004.00182.x
Hooda OK, Upadhyay RC (2014) Physiological responses, growth rate and blood metabolites under feed restriction and thermal exposure in kids. J Stress Physiol & Biochem 10:214–227
Joy A, Sejian V, Krishnan G et al (2020) Heat stress impact on blood biochemical response and plasma aldosterone level in three different indigenous goat breeds. J Anim Behav Biometeorol 8:266–275. https://doi.org/10.31893/jabb.20034
Li FK, Yang Y, Jenna K et al (2018) Effect of heat stress on the behavioral and physiological patterns of Small-tail Han sheep housed indoors. Trop Anim Health Prod 50:1893–1901. https://doi.org/10.1007/s11250-018-1642-3
Lin BZ, Odahara S, Ishida M et al (2013) Molecular phylogeography and genetic diversity of East Asian goats. Anim Genet 44:79–85. https://doi.org/10.1111/j.1365-2052.2012.02358.x
Moretti R, Biffani S, Chessa S, Bozzi R (2017) Heat stress effects on Holstein dairy cows’ rumination. Animal 11:2320–2325. https://doi.org/10.1017/S1751731117001173
Pandey N, Kataria N, Kataria AK, Joshi A (2012) Ambient stress associated variations in metabolic responses of Marwari goat of arid tracts in India. J Stress Physiol Biochem 8:120–127
Payne WJA, Wilson RT (1999) An introduction to animal husbandry in the tropics. Blackwell Science, Oxford
Rhoads ML, Rhoads RP, VanBaale MJ et al (2009) Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin1. J Dairy Sci 92:1986–1997. https://doi.org/10.3168/jds.2008-1641
Ribeiro MN, Ribeiro NL, Bozzi R, Costa RG (2018) Physiological and biochemical blood variables of goats subjected to heat stress – a review. J Appl Anim Res 46:1036–1041. https://doi.org/10.1080/09712119.2018.1456439
Salama AAK, Hamzaoui S, Albanell E et al (2021) Metabolic and behavior responses of lactating goats under heat stress. Small Rumin Res 203:106496. https://doi.org/10.1016/j.smallrumres.2021.106496
Serradilla JM, Carabaño MJ, Ramón M et al (2017) Characterisation of Goats’ Response to Heat Stress: Tools to Improve Heat Tolerance. In: Kukovics S (ed) IntechOpen, Rijeka p Ch. 15
Shilja S, Sejian V, Bagath M et al (2016) Adaptive capability as indicated by behavioral and physiological responses, plasma HSP70 level, and PBMC HSP70 mRNA expression in Osmanabadi goats subjected to combined (heat and nutritional) stressors. Int J Biometeorol 60:1311–1323. https://doi.org/10.1007/s00484-015-1124-5
Siswanto S, van Oldenborgh GJ, van der Schrier G et al (2016) Temperature, extreme precipitation, and diurnal rainfall changes in the urbanized Jakarta city during the past 130 years. Int J Climatol 36:3207–3225. https://doi.org/10.1002/joc.4548
Sivakumar AVN, Singh G, Varshney VP (2010) Antioxidants Supplementation on Acid Base Balance during Heat Stress in Goats. Asian-Australasian J Anim Sci 23:1462–1468. https://doi.org/10.5713/ajas.2010.90471
Supari TF, Juneng L, Aldrian E (2017) Observed changes in extreme temperature and precipitation over Indonesia. Int J Climatol 37:1979–1997. https://doi.org/10.1002/joc.4829
Thom EC (1959) The Discomfort Index. Weatherwise 12:57–61. https://doi.org/10.1080/00431672.1959.9926960
Thornton P, Nelson G, Mayberry D, Herrero M (2021) Increases in extreme heat stress in domesticated livestock species during the twenty-first century. Glob Chang Biol 27:5762–5772. https://doi.org/10.1111/gcb.15825
World Bank (2009) Minding the stock: bringing public policy to bear on livestock sector development. The World Bank, Washington DC, USA
Acknowledgements
This work was supported by the Ministry of education, culture, research, and technology, Indonesia (Grand number 0148.013/UN9.3.1/PL/2022). We greatly appreciate the support of Akbar Jalil, Muhammad Irvan, and Fatkhur Rohman for technical support during the experiment and Eli Ermawati and Rise Juliana during the behavioral analysis. We would also like to thank I Komang Gede Wiryawan for correcting our English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, A.I.M., Sandi, S., Fariani, A. et al. Physiological changes and behavioral responses in heat-stressed goats under humid tropical environment. Int J Biometeorol 67, 1757–1764 (2023). https://doi.org/10.1007/s00484-023-02536-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-023-02536-x