Abstract
The objective of this experiment was to evaluate the influence of summer heat stress on physiological and behavioral responses of Osmanabadi, Salem Black, and Malabari goats. The study also evaluated the differences in heat shock protein 70 (HSP70) expression pattern between these breeds. The study was conducted over 45 days during summer (April–May) using 36 1-year-old female goats by randomly allocating them into six groups with six animals in each group: Osmanabadi control (Osmanabadi CON), Osmanabadi heat stress (Osmanabadi HS), Malabari control (Malabari CON), Malabari heat stress (Malabari HS), Salem Black control (Salem Black CON), and Salem Black heat stress (Salem Black HS). The Osmanabadi CON, Malabari CON, and Salem Black CON animals were housed in a shed while the Osmanabadi HS, Malabari HS, and Salem Black HS groups were subjected to heat stress by exposing them to outside environment between 1000 and 1600 h during the experimental period. All 36 animals were provided with ad libitum feed and water. The data generated were analyzed by general linear model (GLM) repeated measurement analysis of variance. Results indicated that the drinking frequency (DF) was higher (p < 0.01) in heat stress groups (12.58, 12.25, and 10.75 times for the Osmanabadi HS, Malabari HS, and Salem Black HS, respectively) as compared to their respective control groups (5.67, 6.25, 5.58 times for the Osmanabadi CON, Malabari CON, and Salem Black CON, respectively). Water intake (WI) also showed similar trend to DF. The urinating frequency also (UF) differed between breeds with lower value (p < 0.05) recorded in the Salem Black HS (1.5 times) compared to the Malabari HS (2.92 times). The highest (p < 0.05) rumination time (RuT) was recorded in the Malabari HS (48.00 min) than both the Osmanabadi HS (20.91 min) and Salem Black HS (23.67 min). The heat stress increased (p < 0.05) all physiological variables at 1400 h. The findings of this study suggest RR, RT, and PBMC HSP70 are reliable biological markers for evaluating thermo-tolerance capacity of indigenous goat breeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change has been forecasted to negatively influence the animal production in the coming decades (Rojas-Downing et al. 2017). The livestock in developing countries are more affected by the adverse effects of heat stress primarily due to the extensive rearing systems (Stocker 2014). In these regions, it is expected that animal productivity will reduce approximately 25% (Seguin 2008). This projected reduction in animal production by heat stress could be due to the reduced growth, milk and meat production, feed utilization, and performance (Rojas-Downing et al. 2017).
The genetic potential of individual animals determines their vulnerability to heat stress. Further, the variations in efficiency of the adaptive responses determine the ability of the animals to adapt to a particular adverse environmental condition (Silanikove and Koluman 2015). Breed difference was established in ruminant animals for thermo-tolerance which was specific to a particular agro-ecological zone (da Silva et al. 2017). Tropical and sub-tropical indigenous breeds are known to have a greater adaptive capacity to stressful conditions than non-indigenous breeds (Habibu et al. 2016). Therefore, selection of breeds that are adapted based on genotypic trait is a promising strategy to tackle the climate change-associated adverse consequences in livestock production.
Adaptation refers to the different mechanisms by which the animals cope with harsh climatic conditions (Ratnakaran et al. 2017). On encountering an environmental challenge, the animals respond to the situation by altering its behavioral, physiological, biochemical, neuroendocrine, and molecular mechanisms. The behavioral responses comprise reduced feed intake, decreased urinating and defecating frequencies, increased water intake, increased lying time, reduced standing time, increased drinking frequency, and shade-seeking behavior (Alam et al. 2013). Further, the animals withstand the adverse impact of heat stress by altering their physiological mechanisms such as respiration rate (RR), rectal temperature (RT), and skin temperature (ST) (Manjari et al. 2015; Shilja et al. 2016). Adaptation of animals based on different phenotypic traits primarily modifies their behavioral or metabolic response which helps them to survive the stress and imparts them the ability to cope with such subsequent challenges (Al-Haidary et al. 2012). Apart from these phenotypic traits, the animals also adapt to heat stress challenges by altering their genotypic traits, and a heat shock protein 70 (HSP70) gene is the classical heat stress-associated genetic marker in heat stressed goats (Gupta et al. 2013; Banergee et al. 2014). The HSPs are highly conserved stress proteins which are expressed in response to environmental stresses which impart the stress tolerance ability to goat. Although, there are studies on phenotypic traits and HSP70 role in livestock adaptation, there are limited studies investigating these adaptive traits in different indigenous breeds available.
The superior genetic merits of indigenous animals in adapting to heat stress challenges as compared to non-indigenous and crossbred animals project them to be the future animals to ensure food security (Katiyatiya et al. 2017). Although the native breeds possess ability to survive in specific location, there could be differences in their capacity to adapt when they are shifted to other ecological zone. Further, there could be differences among the indigenous breeds to maintain their productive performance when relocated to new locations. Information and research attempts are therefore necessary to select the best indigenous breed which apart from adapting to a particular agro-ecological zone may also produce optimally. Therefore, a study was conducted to assess the resilience capacity of three indigenous (Osmanabadi, Malabari, and Salem Black) goat breeds to heat stress. The specific objective of the study was to compare the resilience capacity of the three breeds based on changes in their phenotypic traits and HSP70 gene expression patterns.
Materials and methods
Location
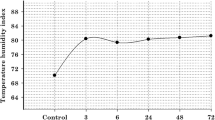
The study was conducted at the experimental livestock unit of the ICAR-National Institute of Animal Nutrition and Physiology, Bengaluru, India, during summer season (April–May). The maximum-minimum temperatures, relative humidity, dry and wet bulb temperature, and pen surface temperature during the study period (45 days) are listed in Table 1. From these data, THI was calculated as described by McDowell (1972) THI = 0.72 (Tdb + Twb) + 40.6, where Tdb = dry bulb temperature in degrees Celsius; Twb = wet bulb temperature in degrees Celsius. The THI during the study period was reflected in Fig. 1.
Description of average THI for the entire study duration between inside and outside the shed. The THI values were calculated as per method described by McDowell (1972). Accordingly, the formula used was THI = 0.72 (Tdb + Twb) + 40.6, where Tdb = dry bulb temperature in degrees Celsius; Twb = wet bulb temperature in degrees Celsius. The THI values 72 and less are considered comfortable, THI values between 75 and 78 are considered stressful, and THI above 78 is considered extreme distress
Animals and housing
Osmanabadi, Malabari, and Salem Black are the three goat breeds selected for the study. The study was conducted using 10- to 12-month-old 36 female goats (twelve each in Osmanabadi, Malabari, and Salem Black breeds). The animals were maintained as per standard management practices in well-ventilated sheds. The Osmanabadi breed is native to the experimental location. However, the other two breeds are brought from different hot humid zones in southern India. These two breed animals were brought to the experimental location and acclimatized for the feeding and handling procedures. The animals were considered acclimatized when they were feeding normally in addition to coping with the handling procedures during individual feeding.
At the start of study, the average body weights of Osmanabadi, Malabari, and Salem Black breeds are 16.92 ± 0.82, 13.44 ± 0.83, and 16.69 ± 0.99 kg, respectively. Similarly, the average BCS of these breeds in a scale of 1–5 points are 2.37 ± 0.16, 2.12 ± 0.13, and 2.37 ± 0.09, respectively. Malabari is a pure white color breed while both Osmanabadi and Salem Black goats are pure black in color (Thiruvenkadan et al. 2014).
The animals were housed in a well-ventilated shed which had an east-west orientation. The shed description along with standard management and prophylactic measures followed in the study are described by the previous published reports (Bagath et al. 2016; Archana et al. 2018).
Experimental design
The experimental animals were randomly allocated into six groups with six animals in each group: Osmanabadi control (Osmanabadi CON), Osmanabadi heat stress (Osmanabadi HS), Malabari control (Malabari CON), Malabari heat stress (Malabari HS), Salem Black control (Salem Black CON), and Salem Black heat stress (Salem Black HS). Before allocation into the specific groups, the body weight of all animals was recorded in each breed, and based on body weight of 12 available animals in each breed, grouping was done randomly by allocating the animals in such a way that their average body weights were similar between the groups in each breed. The duration of the study was 45 days. All 36 animals were provided with ad libitum feed and water. The animals were stall-fed with a diet consisting of 60% roughage which comprises Hybrid Napier and 40% concentrate comprising maize, wheat bran, soybean meal, mineral mixture, and common salt. The roughage and concentrate were provided as a mixed ration. The animals were fed twice at 800 h and 1800 h, and the residues were recorded prior to next feeding. The Osmanabadi CON, Malabari CON, and Salem Black CON animals were maintained in the shed in, while the Osmanabadi HS, Malabari HS, and Salem Black HS animals were subjected to heat stress by exposing them to outside environment between 1000 to 1600 h during the experimental period. The Osmanabadi CON, Malabari CON, and Salem Black CON animals were fed and watered inside the shed, while the Osmanabadi HS, Malabari HS, and Salem Black HS animals were fed and watered while they are exposed to summer heat stress in the outside environment. The Osmanabadi HS, Malabari HS, and Salem Black HS animals were also kept housed along with the control animals inside the shed between 16.00 and 10.00 h. The animals were housed in separate pen inside the shed where they were fed and watered individually as per control animals. Under these experimental conditions, the behavioral responses were recorded on days 0 and 45 of the study. Physiological responses were recorded twice daily (8.00 h and 14.00 h) at 14 days interval (day 0, day 15, day 30, and day 45). However, blood collection was carried out only on day 45 for peripheral blood mononuclear cell (PBMC) isolation for HSP70 expression.
Variables studied
Behavioral responses
Standing time (ST; min), lying time (LT; min), drinking frequency (DF; no. of times), defecation frequency (DeF; no. of times), urination frequency (UF; no. of times), and rumination time (RuT; min) are the behavioral variables recorded in the study. These variables were recorded by observing closely the individual animals for 6 h (1000–1600 h) continuously, on day 0 and day 45 of the experiment. All the behavioral responses were recorded manually using a stopwatch. Standing time refers to the average time that the animals were standing, walking, running, and playing until sitting or lying down. Lying time is defined as the total time spent by each group of animals in lying position. Drinking frequency refers to the number of times the animals were near the water trough with the intension to drink water during the 6-h duration. Water intake was measured based on the total water intake of the animals. Rumination time was determined by observing the jaw movement continuously by close supervision and recording the duration using a stopwatch. Urine and fecal exertion frequency refers to the number of times the animals excreted urine and feces, respectively. All the behavioral variables were recorded as per the method described by Alam et al. (2013). The water was measured and offered to the animals, and the residues were recorded to calculate the water intake (WI; L/day) at every 14-day interval.
Physiological responses
Respiration rate (RR), pulse rate (PR), rectal temperature (RT), and body surface temperature (BST) are the physiological variables recorded in the study. All the physiological variables were recorded as per the standard method as described by Shilja et al. (2016).
Blood collection
Blood samples of 8 mL were collected from the external jugular vein on day 45 using 20 gauge sterilized needles and a plastic syringe in ethylenediaminetetraacetic acid (EDTA)-coated vials at 1100 h from all animals. The HSP70 mRNA expression was carried out only from the day 45 blood samples. The purpose of this is to get the cumulative effect of heat stress on day 45. Immediately after collection, the blood samples were transferred to a tray containing ice cubes to maintain cold chain until transported to a laboratory. In the laboratory, the blood samples were stored at 4 °C refrigeration and the samples were processed on the same day for isolation of PBMC.
Expression patterns of PBMC HSP70
The PBMC was isolated using RBC lysis buffer. The protocol used for total RNA isolation, cDNA synthesis, primer sequences for amplifying HSP70 and GAPDH gene, and relative expression of these genes by real-time qPCR are as per the protocol described by Shilja et al. (2016). The primer sequences for both HSP70 and GAPDH genes are described in Table 2. The GAPDH gene was used as housekeeping gene to serve as internal control, and the relative expression HSP70 gene was quantified as per the formula 2ΔΔCT (Shilja et al. 2016).
Statistical analysis
Prior to subjecting for statistical analysis, all the variables were subjected to Shapiro-Wilk normality test. The data generated from the study was analyzed by general linear model (GLM) repeated measurement analysis of variance employing only the main effects in the model (SPSS 18.0). The fixed factors breed effect (Osmanabadi, Malabari, Salem Black) and treatment effect (control, heat stress) were taken as between subject factor, and days of study (day 0, day 15, day 30, and day 45) were taken as within subject factor in the model. The model also included breed × treatment interaction. The relative expression of HSP70 gene in relation to the reference gene was analyzed by one-way analysis of variance (ANOVA) using SPSS (18.0) software. To compare the means of different subgroups of all variables studied, Tukey’s post hoc test was performed.
Results
Behavioral responses
The behavioral responses after heat stress exposure in Osmanabadi, Malabari, and Salem Black goat breeds are described in Table 3. Breed did not influence the behavioral variables ST, LT, DF, DeF, and WI. However, breed influenced both UF (p < 0.05) and RuT (p < 0.01). The UF was 1.83, 2.17, and 1.08 in Osmanabadi, Malabari, and Salem Black breeds, respectively. Similarly, the RuT was 37.25, 65.50, and 40.83 in Osmanabadi, Malabari, and Salem Black breeds, respectively. Heat stress increased (p < 0.01) both DF and WI in all Osmanabadi HS, Malabari HS, and Salem Black HS as compared to their respective control animals. Although water intake was recorded at 24.00 h, still the higher rate of water intake during heat stress exposure period (1000 to 1600 h) in all HS groups was enough to bring in the difference in 24.00 h water intake between the control and heat stress groups. However, in all three breeds, both UF and RuT did not differ between their control and heat stress groups. Furthermore, day of study influenced all the behavioral variables (ST (p < 0.01), LT (p < 0.05), DF (p < 0.01), UF (p < 0.01), RuT (p < 0.01), and WI (p < 0.01)) except DeF.
Physiological responses
Physiological responses after heat stress exposure both at morning (8.00 h) and afternoon (14.00 h) in Osmanabadi, Malabari, and Salem Black goat breeds are described in Table 4. Breed influenced (p < 0.01) both pulse rate morning (PRM) and rectal temperature morning (RTM), while the respiration rate morning (RRM) did not show any variation. In addition, heat stress reduced (p < 0.01) RRM only in Osmanabadi HS (22.04) as compared to Osmanabadi CON group (26.02). Further, breed influenced all the physiological responses (p < 0.01) at 14.00 h. In addition, heat stress (p < 0.01) increased respiration rate afternoon (RRA) and rectal temperature afternoon (RTA) in all the three breeds. The RRA in Osmanabadi HS, Malabari HS, and Salem Black HS groups were 116.31, 110.02, and 87.42, respectively. Further, the RTA in Osmanabadi HS, Malabari HS, and Salem Black HS groups were 40.05, 40.35, and 39.88, respectively. Furthermore, day of study (p < 0.01) influenced all the physiological responses except RTA. However, pulse rate afternoon (PRA) was higher (p < 0.01) only in Malabari HS as compared to Malabari CON group.
Body surface temperature
Body surface temperature at the head, shoulder, and flank after heat stress exposure of the Osmanabadi, Malabari, and Salem Black goat breeds both during morning and afternoon are described in Table 5. Breed influenced all the body surface temperature variables (body surface temperature head morning (BSTHM; p < 0.05), body surface head afternoon (BSTHA; p < 0.01), body surface temperature shoulder afternoon (BSSTSA; p < 0.01), body surface temperature flank morning (BSTFM; p < 0.01), and body surface temperature flank afternoon (BSTFA; p < 0.01) except body surface temperature shoulder morning (BSTHM). Further, heat stress treatment reduced (p < 0.01) BSTFM only in the Osmanabadi HS group. Furthermore, heat stress increased (p < 0.01) BSTHA and BSTFA in all three breeds. However, BSTSA was higher only in the Osmanabadi HS and Salem Black HS groups as compared to their respective control animals. Among the heat stress groups, the Malabari breed showed lowest value for BSTHA, BSTSA, and BSTFA as compared to both the Osmanabadi and Salem Black breeds. In addition, the experimental period highly influenced all the body surface temperature variables (p < 0.01).
PBMC HSP70 gene expression
Relative PBMC HSP70 mRNA transcript expression after heat stress exposure in the Osmanabadi, Malabari, and Salem Black goat breeds are depicted in Fig. 2. The results obtained indicated PBMC HSP70 expression in the Osmanabadi CON, Osmanabadi HS, Malabari CON, Malabari HS, Salem Black CON, and Salem Black HS to be 1.07-, 2.3-, 1.04-, 2.13-, 1.11-, and 1.13-fold respectively. Comparatively, both the Osmanabadi and Malabari breed heat stress groups showed (p < 0.05) higher expression of PBMC HSP70 mRNA compared to their respective control animals. However, PBMC HSP70 expression did not differ between the control and heat stress groups in the Salem Black breed. Also, the level of HSP70 mRNA expression was lower in the Salem Black HS as compared to the Osmanabadi HS and Malabari HS groups.
Means (± s.e.m) for PBMC HSP70 mRNA expression patterns in three goat genotypes (n = 36, 12 per genotype and 6 per treatment) exposed to inside and outside environmental conditions at 12° N and 77° E. Values bearing different alphabets both within and between breed differ significantly with each other at p < 0.05
Discussion
Climate resilience in livestock production requires multifaceted approach in identifying the appropriate breeds that can adapt and maintain production in challenging environments. This study attempts to provide some evidence regarding the resilience capacity of three indigenous goat breeds exposed to heat stress. Breed differences were established for reliable adaptive variables such as RRM, RRA, and HSP70 gene expression. Both respiration rate (Marai et al. 2007; Indu et al. 2014) and HSP70 gene (Banerjee et al. 2014; Bharati et al. 2017) are considered reliable biological markers for quantifying heat stress response in livestock. The differences obtained for these variables in this study clearly indicated that even extremely adapted indigenous breeds in their native track can influence their ability to survive in a new location. This shows breed differences do exist among the indigenous breeds in their ability to cope with heat stress challenges.
The THI above 75 as per McDowell (1972) model was considered extremely severe heat stress to animals. With the THI value of 86.5 recorded during outside exposure during entire experimental period clearly indicated that the heat-stressed animals experienced extreme severe heat stress. Thus, the findings based on THI justifies studying the growth performance of the Malabari goats as the animals were exposed to extreme heat stress.
The evaluation of breed differences for ST, LT, DF, and DeF did not differ indicating the indigenous natures these three breeds depicting the similarity in their approaches to adapt to harsh climatic conditions. Further, the non-significant influence of heat stress on ST, LT, and DeF proves the superiority of these breeds to heat stress challenges. The THI described extreme heat stress exposure for these animals in the study. Similar result of non-significant change in ST and LT was also reported in Osmanabadi bucks exposed to heat stress in the same agro-ecological zone (Shilja et al. 2016). The reason for the non-significant changes in these behavioral parameters in the current study on all three breeds indicated that they did not rely on these behavioral responses to cope with heat stress. However, there are reports indicating increased ST and decreased LT in Osmanabadi (Panda et al. 2016) and Black Bengal goat breeds (Alam et al. 2013) during heat stress conditions. This difference observed in the results with our study could be attributed to the magnitude of heat stress in different agro-ecological zones. The recorded weather variables clearly showed higher magnitude of heat stress in the former region (AT—43.6 °C, RH—88%) compared to the current region (AT—39.9 °C, RH—29.1%) during afternoon hours (Panda et al. 2016). This change in behavior of indigenous breeds also signifies their wider adaptability to different harsh climatic regions. The DF refers to number of times the animal had access to water resources. The results obtained from the present study exhibited increased DF in heat-stressed groups than control groups in all the three breeds. The result was in accordance with the previous experiment conducted in Osmanabadi bucks which also showed increased DF during heat stress condition (Shilja et al. 2016). This result could be due to severe dehydration in goats as a result of enhanced evaporative cooling mechanisms through both respiratory tract as well as skin in heat-stressed animals (Shilja et al. 2016). Consequently, the heat-stressed animals tend to show higher DF in an effort to increase the WI to cool their body and also to restore their normal body water level (Garner et al. 2017). There were breed differences for UF with lower UF reported in the Salem Black heat-stressed goats. The lower UF in the Salem Black goats could suggest the better thermo-tolerance capability of this breed in conserving the body water during heat stress condition. The highest value for RuT was exhibited by the Malabari breed compared to both the Osmanabadi and Salem Black breeds. The complete absence of RuT in both the Osmanabadi and Salem Black breeds on day 45 could be a metabolic adaptive mechanism in these breeds to reduce their internal metabolic heat production (Panda et al. 2016). The higher value for RuT in Malabari breed as compared to the Osmanabadi and Salem Black could be attributed to the breed difference. The heat stress treatment increased WI level in all the three breeds. The result obtained was in accordance with other studies which also indicated higher WI in heat-stressed animals (Indu et al. 2014; Shiljai et al. 2016). Generally, ruminants exposed to heat stress environments tend to increase their total WI as a compensatory mechanism to restore the higher fluid loss from their body through respiratory and cutaneous evaporative cooling activities (Indu et al. 2014; Shilja et al. 2016). Apart from maintaining the body water status, the increased ingestion of water also help the heat-stressed animals to cope up to the adverse conditions by immediate rumen-reticular cooling which reduces their core body temperature effectively (Garner et al. 2017). Further, higher WI in the Osmanabadi HS as compared to the Malabari HS could be attributed to breed difference in addition to their differences in coat color. Similarly, Valente et al. (2015) also reported breed variation for WI between heat-stressed cattle breeds, and they attributed the higher WI in Angus breeds to their lower thermo-tolerance ability. The white coat color of the Malabari goats might have helped this breed to reduce the direct effects of the heat stress by reflecting more incident solar radiation throughout the study period (Katiyatiya et al. 2017). However, the WI level of the Salem Black HS group was recorded to be similar to both the Osmanabadi HS and Malabari HS, which also indicated their superior adaptive capability in the current agro-ecological zone compared to the other two breeds.
Mechanism of physiological adaptation in the livestock is generally indicated by RR, PR, and RT (Gupta et al. 2013). Banerjee et al. (2015) also reported breed differences in Indian goat breeds for physiological variables with RR, PR, and RT being lower in breeds adapted to hot climates. Lower respiratory activity (RRM) in the Osmanabadi HS could be an adaptive mechanism for keeping their physiological activities to a minimum level to cope to the extreme stressful condition in the afternoon (Marai et al. 2007; Shilja et al. 2016). The RRA is the only parameter in the study which was influenced by all the independent variables (breed, treatment, and experimental period). This shows the significance of RR during afternoon in determining the adaptive ability of goats irrespective of breeds. The higher RR in heat-stressed animals could be directly related to the increased evaporative cooling mechanisms in restoring their thermal balance (Habibu et al. 2016). Similarly, increased RR were also reported in Black Bengal, Sokoto, and Sahel goats during afternoon (Alam et al. 2013; Habibu et al. 2016). Moreover, a higher RR could also be an adaptive mechanism in heat-stressed animals to meet the increased oxygen demand of the vital organs for the adaptation processes (Panda et al. 2016). In addition, Shilja et al. (2016) stated the RR to be a reliable indicator to measure magnitude of heat stress in small ruminants. In comparison to the other two breeds, the Salem Black HS group showed lower RR which also indicates the superior adaptability of this breed to cope with hot environments. This could be attributed to the much severe tropical climate exposure in their place of origin. Further, RR was influenced by day of study. This also indicates that the response of the groups varied over time for RR for adapting to the heat stress challenges depicting the higher thermo-tolerant ability of these indigenous goat breeds to harsh climatic conditions. Therefore, RR can serve as a reliable indicator to evaluate the thermo-tolerant capacity of different indigenous goat breeds. The most noticeable effect of heat stress on heart and blood vessels are evident from increased PR in goats during summer (Gupta et al. 2013). Between the breeds, Malabari breed showed highest PR value during both morning as well as afternoon. Further, Popoola et al. (2014) also established a direct correlation between heart rate and general metabolic status in goats. In addition, the non-significant change in PR in heat-stressed Osmanabadi and Salem Black compared to their control animals also establishes their higher adaptive capacity to the heat stress challenges by maintaining their metabolic and circulation status even during high stressful conditions. The RT is also considered as the most common indicator of heat stress in farm animals and also used as a simple and reliable tool for monitoring animal welfare in hot environment (Al-Tamimi 2007). The RT also showed similar trend to that of PR with higher value in the Malabari HS both during morning and afternoon. Higher RT value in the Malabari goats indicates their increased vulnerability to the hot environments. Further, the lower RT in the Salem Black HS compared to the Salem Black CON shows the superior adaptive capability of Salem Black goats to keep themselves cool in morning hours to cope to the stressful condition in the afternoon (Shilja et al. 2016). However, heat stress treatment increased the RT in all the three heat stress groups compared to their respective control animals. Similar results were also reported in goats by Habibu et al. (2016) and Shilja et al. (2016). The increased RT in heat-stressed group indicates the inefficient thermoregulatory mechanisms in these goats to maintain thermal equilibrium (Marai et al. 2007).
All body surface parameters showed significant variation for the heat stress treatment. Further, the body surface temperatures in different area during morning in heat stress groups was generally lower as compared to the respective control animals while reverse trend was observed during afternoon. These highly significant changes in body surface temperatures between the groups indicate their significance for assessing the adaptive nature of these breeds. The similar results of increased body surface temperature due to heat stress were also reported in goats by Hooda and Upadhyay (2014) and Shilja et al. (2016). This higher body surface temperature could be directly attributed to the vasodilatation of skin capillary bed to enhance the blood flow to the skin periphery for facilitating heat transfer to the surroundings (Shilja et al. 2016). Although Malabari breed showed highly significant changes for most of the physiological indicators, body surface temperatures in various regions showed reverse trend in this breed indicating lower body surface temperature during heat stress than both the Osmanabadi and Salem Black goats. This difference could be attributed to the coat color as the Malabari breed is pure white while both the Osmanabadi and Salem Black goats are pure black in color. Similar results of low temperature in white color goats were also reported by Hagan et al. (2012). The goats with light colored coat have greater advantage over the dark-colored coat regarding thermoregulation mechanisms in hot environments, as the white color reflects most of the direct solar radiation imparting better thermo-tolerance to the light coat-colored breed (Hagan et al. 2012). Further, the significant influence of experimental period on both BSTHA and BSTFA signifies the importance of these parameters on goat adaptation.
The HSP70 is recognized as one of the most abundant and important proteins in the HSP family playing a critical role in goats during thermal adaptation (Gupta et al. 2013). Additionally, HSP70 is also identified as a confirmatory cellular marker for heat and humidity stress in ruminants (Shilja et al. 2016; Bharati et al. 2017). Both the Osmanabadi and Malabari goats showed a different trend to that of the Salem Black goats with higher HSP70 expression in the heat-stressed groups compared to their respective control animals. The result obtained was in accordance with other studies which also indicated higher HSP70 expression in heat-stressed goats as compared to the control goats (Banerjee et al. 2014; Shilja et al. 2016). Further, the increased HSP70 in the heat-stressed Osmanabadi and Malabari goats also indicates the higher magnitude of stress experienced by these two breeds as compared to Salem Black breed. The result also emphasizes the higher HSP70 requirements in these two breeds to counteract the deleterious effects of hyperthermia at the cellular level (Banerjee et al. 2014). The non-significant difference in HSP70 expression between the Salem Black CON and Salem Black HS groups indicated that the stressful condition was not severe enough to create cellular responses in this breed. Further, the level of HSP70 mRNA expression in the Salem Black HS group was lower than the Osmanabadi HS and Malabari HS groups. These findings clearly indicate the superior adaptive capability of the Salem Black goats to tropical environments. From these results, it may be inferred that HSP70 gene can be used effectively as a biomarker for comparatively assessing the superior thermo-tolerant ability of the extremely adapted goat breeds.
Conclusion
This study has provided further insights regarding the resilience capacity of three indigenous goat breeds subjected to heat stress. The differences in response of the three breeds were established in variables such as RRM, RRA, and HSP70 gene expression. The study also established that both respiration rate and HSP70 gene may be considered reliable biological indicators to reflect heat stress impact in indigenous goats. The significantly lower respiration rate and HSP70 gene in the Salem Black HS group as compared to the Osmanabadi HS and Malabari HS groups point towards the better adaptive ability of the Salem Black breed as compared to the other two breeds. However, more detailed research efforts are required involving large population of these breeds to strengthen the findings of this study. Based on these preliminary observations on most of the variables studied, it was evident that the Salem Black breed showed better resilience capacity to cope with heat stress challenges. Therefore, promoting the Salem Black breed among the local farmers may prove beneficial in ensuring their livelihood securities. It is also essential to develop new marker-assisted selection breeding program using the biological markers identified in the Salem Black breed to improve other breeds’ adaptive ability.
References
Alam MM, Hashem MA, Rahman MM, Hossain MM, Haque MR, Sobhan Z, Islam MS (2013) Effect of heat stress on behavior, physiological and blood parameters of goat. Progress Agric 22(1&2):37–45
Al-Haidary AA, Aljumaah RS, Alshaikh MA, Abdoun KA, Samara EM, Okab AB, Alfuraiji MM (2012) Thermoregulatory and physiological responses of Nazdi sheep exposed to environmental heat load prevailing in Saudi Arabia. Pak Vet J 32:515–519
Al-Tamimi HJ (2007) Thermoregulatory response of goat kids subjected to heat stress. Small Rumin Res 71(1):280–285
Archana PR, Sejian V, Ruban W, Bagath M, Krishnan G, Aleena J, Manjunathareddy GB, Beena V, Bhatta R (2018) Comparative assessment of heat stress induced changes in carcass traits, plasma leptin profile and skeletal muscle myostatin and HSP70 gene expression patterns between indigenous Osmanabadi and Salem Black goat breeds. Meat Sci 141:66–80
Bagath M, Sejian V, Archana SS, Manjunathareddy BG, Parthipan S, Selvaraju S, Mech A, David ICG, Ravindra JP, Bhatta R (2016) Effect of dietary intake on somatotrophic axis-related gene expression and endocrine profile in Osmanabadi goats. J Vet Behav 13:72–79
Banerjee D, Upadhyay RC, Chaudhary UB, Kumar R, Singh S, Ashutosh, Tapan KD, De S (2015) Seasonal variations in physio-biochemical profiles of Indian goats in the paradigm of hot and cold climate. Biol Rhythm Res 46(2):221–236
Banerjee D, Upadhyay RC, Chaudhary UB, Kumar R, Singh S, Ashutosh GJM, Polley S, Mukherjee A, Das TK, De S (2014) Seasonal variation in expression pattern of genes under HSP70. Cell Stress and Chaperones 19(3):401–408
Bharati J, Dangi SS, Chouhan VS, Mishra SR, Bharti MK, Verma V, Bag S (2017) Expression dynamics of HSP70 during chronic heat stress in Tharparkar cattle. Int J Biometerol 61(6):1017–1027
da Silva WE, Leite JHGM, de Sousa JER, Costa WP, da Silva WST, Guilhermino MM, Façanha DAE (2017) Daily rhythmicity of the thermoregulatory responses of locally adapted Brazilian sheep in a semiarid environment. Int J Biometerol 61(7):1221–1231
Garner JB, Douglas M, Williams SRO, Wales WJ, Marett LC, DiGiacomo K, Hayes BJ (2017) Responses of dairy cows to short-term heat stress in controlled-climate chambers. Anim Prod Sci 57(7):1233–1241
Gupta M, Kumar S, Dangi SS, Jangir BL (2013) Physiological, biochemical and molecular responses to thermal stress in goats. Int J Livest Res 3(2):27–38
Habibu B, Kawu MU, Makun HJ, Aluwong T, Yaqub LS (2016) Seasonal variation in body mass index, cardinal physiological variables and serum thyroid hormones profiles in relation to susceptibility to thermal stress in goat kids. Small Rumin Res 145:20–27
Hagan JK, Apori SO, Bosompem M, Ankobea G, Mawuli A (2012) Morphological characteristics of indigenous goats in the coastal savannah and forest eco-zones of Ghana. J Anim Sci Adv 2(10):813–821
Hooda OK, Upadhyay RC (2014) Physiological responses, growth rate and blood metabolites under feed restriction and thermal exposure in kids. J Stress Physiol Biochem 10(2):214–227
Indu S, Sejian V, Naqvi SMK (2014) Impact of simulated heat stress on growth, physiological adaptability, blood metabolites and endocrine responses in Malpura ewes under semiarid tropical environment. Anim Prod Sci 55(6):1314–1323
Katiyatiya CLF, Bradley G, Muchenje V (2017) Thermotolerance, health profile and cellular expression of HSP90AB1 in Nguni and Boran cows raised on natural pastures under tropical conditions. J Therm Biol 69:85–94
Manjari R, Yadav M, Uniyal S, Rastogi SK, Sejian V, Hyder I (2015) HSP70 as a marker of heat and humidity stress in Tarai Buffalo. Trop Anim Health Prod 47:111–116
Marai IFM, El-Darawany AA, Fadiel A, Abdel-Hafez MAM (2007) Physiological traits as affected by heat stress in sheep—a review. Small Rumin Res 71(1):1–12
McDowell RE (1972) Improvement of livestock production in warm climate. WH Freeman and Co., San Francisco, pp 51–53
Panda R, Ghorpade PP, Chopade SS, Kodape AH, Palampalle HY, Dagli NR (2016) Effect of heat stress on behaviour and physiological parameters of Osmanabadi goats under katcha housing system in Mumbai. J Livest Sci 7:196–199
Popoola MA, Bolarinwa MO, Yahaya MO, Adebisi GL, Saka AA (2014) Thermal comfort effect on physiological adaptation and growth performance of west African dwarf goats raised in Nigeria. Eur Sci J 3:275–281
Ratnakaran AP, Sejian V, Jose VS, Vaswani S, Bagath M, Krishnan G, Beena V, Indira Devi P, Varma G, Bhatta R (2017) Behavioural responses to livestock adaptation to heat stress challenges. Asian J Anim Sci 11(1):1–13
Rojas-Downing MM, Nejadhashemi AP, Harrigan T, Woznicki SA (2017) Climate change and livestock: impacts, adaptation, and mitigation. Clim Risk Manag 16:145–163
Seguin B (2008) The consequences of global warming for agriculture and food production. In: Rowlinson P, Steele M, Nefzaoui A (Eds) Proceedings in International Conference “Livestock and Global Climate Change” (Hammamet, Tunisia 17–20 9–11)
Shilja S, Sejian V, Bagath M, Mech A, David ICG, Kurien EK, Varma G, Bhatta R (2016) Adaptive capability as indicated by behavioral and physiological responses, plasma HSP70 level and PBMC HSP70 mRNA expression in Osmanabadi goats subjected to combined (heat and nutritional) stressors. In J Biometeorol 60:1311–1323
Silanikove N, Koluman N (2015) Impact of climate change on the dairy industry in temperate zones: predications on the overall negative impact and on the positive role of dairy goats in adaptation to earth warming. Small Rumin Res 123(1):27–34
Stocker T (Ed) (2014) Climate change 2013: the physical science basis: working group I contribution to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press
Thiruvenkadan AK, Jayakumar V, Kathiravan P, Saravanan R (2014) Genetic architecture and bottleneck analyses of Salem Black goat breed based on microsatellite markers. Vet World 7(9):733–737
Valente ÉEL, Chizzotti ML, Oliveira CVR, Galvão MC, Domingues SS, Rodrigues AC, Ladeira MM (2015) Intake, physiological parameters and behavior of Angus and Nellore bulls subjected to heat stress. Semina: Ciências Agrárias 36:4565–4574
Acknowledgements
The authors sincerely thank the Director of ICAR-National Institute of Animal Nutrition and Physiology for extending all research facilities.
Funding
The authors thank the Indian Council of Agricultural Research (ICAR) for funding this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The institute ethical committee has provided necessary permission for conducting this study (NIANP/IAEC/2/2017), and they approved the methodologies and techniques used. The experimental protocol was in strict accordance with international guidelines for exposing the animals to heat stress and for the use of animals in research.
Rights and permissions
About this article
Cite this article
Aleena, J., Sejian, V., Bagath, M. et al. Resilience of three indigenous goat breeds to heat stress based on phenotypic traits and PBMC HSP70 expression. Int J Biometeorol 62, 1995–2005 (2018). https://doi.org/10.1007/s00484-018-1604-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-018-1604-5