Abstract
This study was conducted to investigate the effects of heat stress on the behavioral and physiological patterns in Small-tail Han sheep housed indoors in summer without climate control. Sixteen adult animals were allocated into two groups of eight animals, based on sex: one group of eight rams and one group of eight ewes. Temperature-humidity index (THI) was used to assess the degree of heat stress. All sheep were subjected to a 10-day pre-experimental period of habituation to the experimental feed and environment. Physiological parameters monitored were respiratory rate (RR), rectal temperature (RT), and heart rate (HR). Blood chemistry parameters were also recorded, including plasma minerals and blood metabolites, from jugular vein blood samples. Behavioral parameters were lying, standing, excreting, drinking, foraging, walking, and ruminating. The research findings showed that there were some significant differences of behavior (standing, P = 0.001; walking, P = 0.049; ruminating, P = 0.010), physiology (RR, P = 0.0001; HR, P = 0.002; RT, P = 0.03;) and plasma minerals and blood metabolites (sodium, P = 0.047; phosphorus, P = 0.002; T4, P = 0.041; cortisol, P = 0.0047; triglyceride, P = 0.009) between ram and ewe and that heat stress also significantly affected (P < 0.05) standing, lying, foraging and drinking behavior, all of the physiological parameters and some of the blood chemistry parameters (chlorides, sodium, phosphorus, total protein, tetraiodothyronine, cholesterol, triglyceride, creatinine, cortisol, and glucose). These results indicate that ewe has better high-temperature tolerance than ram, and heat stress can alter behavioral and physiological patterns in Small-tail Han sheep housed indoors. These changes may allow the sheep to adapt better to the ambient temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heat stress occurs when the core body temperature of a given species exceeds its range specified for normal activity resulting from a total heat load (internal production and environment) exceeding the capacity for heat dissipation (Bernabucci et al. 2010). Sheep are homoeothermic animals, which under thermoneutral conditions can maintain body temperature within a normal range utilizing sensible heat loss (convection, conduction, and radiation) to dissipate body heat to the surrounding environment (Fadare et al. 2012). This is necessary for sheep to remain healthy, survive, and maintain productivity (Marai et al. 2007). Generally, sheep have good adaptation ability and are resistant to hard environment conditions and were widely farmed all over the world, especially in developing countries (Krzysztof et al. 2014). However, heat stress is considered a limiting factor for animal welfare and sheep production and sheep reproduction (McManus et al. 2009; Bernabucci et al. 2010) as it can lead to a dramatic decrease in the production and reproduction of sheep. This is especially so in China, where reduced performance of sheep housed indoors during summer months can be largely due to elevated ambient air temperature (Zang et al. 2006; Bernabucci et al. 2009). These reduced performances can be further compounded when elevated ambient temperature is coupled with high humidity, exacerbating heat stress.
The performance which high temperatures strongly affected involves a series of neuroendocrinological, physiological, and behavioral responses, which act to equilibrate animal functions (Marai et al. 2007). Such responses can promote alterations in the level of blood metabolites and metabolic hormones (Marai et al. 2008; Sejian et al. 2010; Macias-Cruz et al. 2013). Keim et al. (2002) reported that homeostatic mechanisms are controlled by the hypothalamus via various neuroendocrine pathways, leading to different endogenous and behavioral responses that are measurable. Therefore, an animal’s response to heat stress can be measured by: respiratory rate, heart rate, rectal temperature, activity level, hematological, and other physiological traits (Marai et al. 2007; McManus et al. 2009).
A good indicator of thermal stress in animals is the temperature-humidity index (THI), a value which allows the integration of temperature and humidity and can give an objective comparison of environmental conditions (Krzysztof et al. 2014). Therefore, the THI allows us to better understand the effects of a high temperature and humidity environment.
The Small-tail Han sheep is a predominant indigenous breed in China which originated from Mongolia and has a large number of breeding in northern China. The Small-tail Han sheep has notable physical and sexual vigor and robustness that enables them to withstand stress related to the harsh environment, disease, and irregular feeding. Relatively speaking, the Small-tail Han sheep has better cold but poorer high-temperature resistance. So, most of Small-tail Han sheep raised in farming or pastoral areas in northern China grow slowly or even stop growing under high temperature and result in a lot of economic losses (Zang et al. 2006; Srikandakumar et al. 2003). Thus, it is quite important to study the behavior and physiological changes of Small-tail Han sheep under heat stress, though there is a lot of research about the effect of heat stress on the behavior and physiology of sheep; but for this breed, information is lacking up to now. The aim of this study was to investigate the effects of heat stress on the behavior and physiology in Small-tail Han sheep housed indoors and to provide theoretical guidance for sheep breeding.
Materials and method
All the experimental procedures were performed according to the authorization granted by the Chinese Ministry of Agriculture. All procedures involving animals were approved by the animal care and use committee at the institution where the experiment was conducted (LYU20150603).
Location of study
The study was conducted at a sheep farm of the BaShaBu, Lan-Shan District, Linyi city, Shandong province, China, which is located in the semi-moist climate region of the country at longitude 118.35° E and the latitude of 35.05° N and at an altitude of 300 m above mean sea level. The climate is temperate monsoon region continental climate and in summer has high temperature and high humidity. The annual rainfall in this area ranges from 800 to 1000 mm. The minimum and maximum ambient temperature ranges from − 11.1 to 37 °C, respectively.
Animals, management, diet, and environment
Sixteen adult Small-tail Han sheep, of an average 7 ± 1 months of age and 32.41 ± 5.28 kg in weight, were allocated to two groups of eight sheep based on sex (one ram-group and one ewe-group). Each group was housed in a space of 5 m × 6 m, in semi-open barns (the south side of the barn was open), with soil flooring. There was no cross-ventilation in the sheepfold, which aggravated heat load for the sheep in summer. The sheep were fed concentrate (300 g per sheep, 12% crude protein and 2300 kcal ME/kg) and hay (about 200 CP per kg dry mass) ad libitum at 8:00h and 17:00h respectively daily; fresh water was available at all times. Sheep were sheared on May 10, 2015, and at the beginning of the test (August 2, 2015), the wool was about 2 cm long on average. For recognition, all sheep had different colors painted on their flanks and heads using aerosol paints. Sheep were subjected to 10 days of a pre-experimental habituation period to acclimate them to their new feeding regime, micro-environment, and experimental measurement. Data was collected between 14:00h and 15:00h over the experimental period, which followed a repetitive pattern (test cycle) of two consecutive sampling days followed by one rest day, where no data was collected, from August 2nd to August 31st and from October 5th to October 13th in 2015.
The environment survey data included air temperature and relative humidity, which were recorded by a portable temperature and humidity meter (Shanghai Huayan instrument equipment co., LTD, NANNA HI8564/HI93649). The meter was placed 0.8 m above the ground (relative to the sheep pens) to record the ambient temperature and relative humidity four times at 15-min intervals from 14:00h to 15:00h in each barn. The temperature-humidity index (THI) was calculated using the air temperature and relative humidity using the following formula (Marai et al. 2001):
where db °C is the dry bulb temperature (°C), RH the relative humidity (%). The THI values obtained were used to define four categories of heat stress as follows: < 22.2 = absence of heat stress; 22.2 to 23.3 = moderate heat stress; 23.3 to 25.6 = severe heat stress and > 25.6 = extreme severe heat stress (Marai et al. 2001).
Physiological data collection and analysis
Rectal temperature (RT), respiratory rate (RR), and heat rate (HR) were measured in the afternoon during the experimental period, after the sheep was restrained in the pens and while blood was collected from 15:00h to 15:30h. Every time, only half of the sheep (four sheep) in one barn were measured; therefore, each individual sheep was tested 13 times over the experimental period. RT was taken using a digital thermometer. RR and HR were measured using a stethoscope after blood samples were collected.
Animal behavioral observation
In each barn, a four-channel video camera monitoring system (Huaya Ltd., Shenzhen, China) was installed on the wall. Every sheep was observed from 14:00h to 15:00h during the experimental period, and all the video data were stored by mobile HDD and were taken to the laboratory. The data were collected using continuous focal observations in the laboratory. Before observation, the observers were trained for 10 days with pre-existing video recordings. Sheep behaviors observed were foraging, drinking, ruminating, standing, resting, and walking (Table 1).
Blood collection and analysis
Blood samples were collected in the afternoon from 15:00h to 15:30h while the RT was measured. Approximately 4–5 ml of blood was collected, by jugular venipuncture from each animal using 5-mL vacutainer tubes coated with heparin sodium, an anticoagulant. The samples were collected within 2 min and placed immediately on ice before being taken to the laboratory. Plasma samples were prepared by centrifugation of whole blood (3000 rpm) for 10 min and stored at − 80 °C until further analysis. Plasma concentrations of Na, K, Cl, P, Ga, total protein, triiodothyronine, tetraiodothyronine, cholesterol, triglyceride, creatinine, urea nitrogen, cortisol, and glucose were analyzed using a commercial kit (America BD import packing, Shanghai Yuping Biological Technology co., LTD) according to the manufacturer’s instructions. The intra-assay coefficients of variations of all indicators are listed in Table 2.
Statistical analysis
Physiological and behavioral parameters were obtained from individual sheep in each group during experimental day, and then the data of each sheep in the same group were combined at the different THI levels according to the value of THI of the sampling period during experimental day (1 = absence of heat stress; 2 = moderate heat stress; 3 = severe heat stress and 4 = extreme severe heat stress). Data of ram or ewe at different levels were analyzed by analysis of variance procedure using IBM.
Results
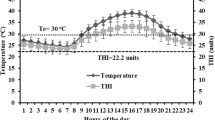
Environmental temperature and air relative humidity were all important factors which affected THI (Fig. 1). According to the THI formula (Marai et al. 2001), during the whole experimental period, there were only 2 days (10/9 and 10/11) which means an absence of heat stress; 2 days (10/8 and 10/12) which means moderate heat stress; 2 days (8/7 and 10/6) which means severe heat stress; and 20 days which means extreme severe heat stress (Fig. 1).
There were some significant behavioral differences between ram and ewe. The ruminating and walking of ewe were significantly higher than that of ram (P < 0.05), while the standing of ram was significantly higher than that of ewe (p < 0.01) during extreme severe heat stress (Table 3). When the THI was equal to 2, the foraging and lying of ram were significantly higher than that of ewe (P < 0.05). Environmental temperature also significantly affects behavior, the duration of standing behavior decreased with the increasing levels of THI, but only for ewe; and under the condition of extreme severe heat stress, there was a significant decrease (P < 0.05). The duration of foraging behavior decreased with the increasing levels of THI (P < 0.05), but the duration of lying behavior increased with the increasing levels of THI (P < 0.05).
The effect of heat stress on respiratory rate, heart rate, and rectal temperature in the Small-tail Han sheep are presented in Table 4. There were some significant differences between ram and ewe. When the THI is equal to 4, the RR of ram was significantly faster than that of ewe (P = 0.0001) while the HR of ewe was significantly higher than that of ram (P = 0.002), and the RT of ewe was significantly higher than that of ram (P = 0.03). Environmental temperature also significantly affects RR, HR, and RT. RR increased with the increasing levels of THI for rams and ewes and was fastest in extreme severe heat stress (P < 0.05). The HR of rams was fastest when they were in moderate heat stress (P < 0.05), but the HR of ewes was fastest when they were in extreme severe heat stress (P < 0.05). Only for rams and in moderate heat stress the RT was significantly different to that in the other levels of THI (P < 0.05).
The effect of different THI levels on plasma mineral and metabolite concentrations in the Small-tail Han sheep are presented in Table 5. There were some significant differences between ram and ewe. The sodium of ram were significantly higher than that of ewe except in extreme severe heat stress (P < 0.05), and the phosphorus and T4 of ram were significantly higher than that of ewe in extreme severe heat stress (P < 0.05); the cortisol of ram was higher than that of ewe under high-temperature environments but only in moderate heat stress was there a significant difference (P = 0.047). Environmental temperature also significantly affects plasma mineral and metabolite concentrations. Table 4 shows that plasma chlorides increased and plasma T4, triglyceride, and creatinine decreased with the levels of THI for rams and ewes (P < 0.05). But plasma sodium increased and plasma cortisol decreased with the increasing levels of THI only for the ewes (P < 0.05). The concentrations of phosphorus, total protein, cholesterol, urea nitrogen, glucose, and the ratio of urea nitrogen:creatinine changed significantly (P < 0.05) with the increasing levels of THI only for rams. To be specific, the phosphorus, cholesterol, urea nitrogen, and the ratio of urea nitrogen:creatinine increased, the total protein decreased, and the glucose initially decreased and then increased with the increasing levels of THI for rams.
Discussion
Acclimation to heat stress imposes behavioral, physiological, and metabolic adjustments to reduce the strain and enhances the likelihood of surviving the stress (Bernabucci et al. 2010). In this study, the duration of standing and lying behavior changed significantly with increasing levels of THI, with standing decreasing and lying increasing. Lying down may reduce energy consumption and increase individual comfort under high-temperature environments: lying down may help to reduce heat load by providing a ready conduit for heat to transfer to the floor, which has greater conductivity than air (Silanikove 2000). Studies have shown that high temperatures can increase the baseline energy needs of sheep, while decreasing blood flow to the rumen, and reducing ruminal motility, rumination, and appetite (da Costa et al. 1992; Silanikove 1992). This supports the results of the current study, which found that the duration of foraging behavior decreased with the increasing levels of THI for rams and ewes. High-temperature environments can considerably increase water and ion losses of ruminants and hence increases their requirements (Beede and Collier 1986). However, there was no clear variation trend of drinking behavior under high temperature in the current study. A possible reason for this unexpected result may be that with reduced foraging, or increased lying down, i.e., the sheep might be comforted and need not to reduce heat stress by the way of changing drinking behavior. In this study, we defined ruminating as sheep’s lying awake with inversing, chewing, and swallowing of the food; this also showed the comfort level. So, we could conclude that the ewes had better resistance capability through the behavioral differences of ram and ewe in heat stress. And we also can estimate the degree of heat stress by observing behavior changes of Small-tail Han sheep under high temperature and take corresponding measures to resist heat stress because the effect of heat stress on sheep first changes in behavior.
Alamer and Al-Hozab (2004) stated that respiration rate (RR) can be used as an indicator of heat stress, and to estimate the adverse effects of environmental temperature. In sheep, panting is the major evaporation heat loss mechanism and respiratory frequencies tend to follow closely heat loss by evaporation (Marai et al. 2007). In the current study, the RR increased with the increasing levels of the THI for rams and ewes, but only for rams did the RR rise above 80 breaths/min, when the THI level was equal to 4, which indicated that the rams were in high heat stress (Silanikove 2000). Considered alone, compared with Australian Merino, this result suggests that Small-tail Han Sheep have a high thermal tolerance capacity, because Srikandakumar et al. (2003) reported that the RR of white wooly Australian Merino rose to 128 breaths/min under severe heat stress (dry bulb temperature of 35.5–43.9 °C and relative humidity 95–35%). The observed accelerated heart rate could be due to the reported redistribution of blood to peripheral tissues during heat exposure in sheep (Silanikove 2000a). These findings support the previous reports on other sheep breeds (Marai et al. 2009; McManus et al., 2009). Increased rectal temperature has also been considered a good indicator of the level of heat stress of animals (Alamer and Al-Hozab 2004). Anderson and Jónasson (1996) showed that in sheep the RT begins to rise when the environmental temperature reaches 32 °C and the RH is below 65%. The current study shows the similar trend, the difference is that RT increased initially but decreased at the highest level of the THI, and the RT of rams and ewes was highest when the THI level was equal to 2 (P < 0.05). The lower core body temperatures under extreme severe heat stress might similarly be because the sheep could adapt physiologically and behaviorally with reduced water intake, reduced foraging, and increased lying down. We also could conclude that the ewes had better resistance capability through the differences of RR, HR, and RT between ram and ewe in extreme severe heat stress. Compared to HR and RT, RR is an increasing indicator with the increasing of THI under high temperature for Small-tail Han sheep, and we can use it as an important indicator of heat stress in sheep farm management.
Srikandakumar et al. (2003) reported plasma Ca was affected by total plasma protein concentration as approximately 45–50% of the total plasma Ca is bound to plasma proteins, in the current experiment heat stress also decreased total plasma protein and Ca. The observed reduction in plasma Ca and total protein concentration could be attributed to the reduction in the duration of foraging, as reduced dietary intake has been reported under heat stress conditions (Marai et al. 2007). Heat stress also increased plasma Na and P in ewes and rams, but only the concentration of plasma Na in ewes and plasma P in rams markedly increased with the levels of THI (P < 0.05). Okoruwa (2014) also observed an increase in concentrations of Na under high-temperature environments and thought this attributed to dehydration, which has been reported to occur as a result of increased breathing rate. More et al. (1980) reported that sheep and goats exposed to 42 °C for 5 h showed increased values for plasma inorganic phosphorus; this is similar to our results. But there are differing reports in previous studies. Rai et al. (1983) reported that in experimental thermal stress of sheep, serum inorganic phosphorus showed a decreasing trend. Perhaps this was related to ingesting ration type and feed consumption, for higher feed conversion ratio contribute to increase plasma inorganic phosphorus (Chiericato et al. 1994). Through the above analysis, we also could conclude that the ewes had better resistance capability through the differences of Na and P between ram and ewe in heat stress.
Thyroid hormones, mainly thyroxine T4, play an important role in an animal’s adaptation to environmental changes (Koluman and Daskiran 2011). They stimulate oxygen consumption and heat production in cells, which increases the basal metabolic rate, enhances glucose utilization, modifies lipid metabolism, and stimulates cardiac and neural functions (Todini et al. 2007). Nazifi et al. (1999) reported that low ambient temperatures tend to increase thyroid activity, whereas high temperatures depress it. In the current study, we had the similar trend that thyroxine concentration was lowest for rams and ewes when the THI level was equal to 3 (P < 0.05). The concentration of cholesterol increased with increasing levels of THI for rams but there was no significant change for ewes. Perhaps this is due to the decreasing of thyroid hormone, as thyroid hormone can promote transport and excretion of cholesterol (Ma et al. 1992).
Cortisol is secreted by the adrenal glands and can stimulate physiological changes in the body, which allow the animal a better tolerance to the stress caused by high temperatures (Christison and Johnson 1972). However, in the current study, the concentration of plasma cortisol decreased with the increasing levels of THI for rams and ewes. Perhaps this was because the sheep could better accommodate the high environmental temperature by changing behaviors, and the decline of plasma cortisol also contributed to reduce metabolic rate and then reduce heat production because one of the main function of cortisol is to increase metabolic rate. Silanikove (2000) also found that the levels of cortisol initially increased and then gradually decreased during long-term exposure as the animals adapt to acute thermal conditions (Silanikove 2000), which could explain the results of the chronically thermally challenged sheep in the current study. And we also could find that the ewes had better resistance capability through the difference of plasma cortisol between ewe and ram in heat stress.
The increase in blood urea nitrogen (BUN) due to heat stress may indicate that kidneys experience reduced blood flow during heat stress conditions; heat stress is known to cause peripheral vasodilation to expel body heat and reduce the blood flow to the internal organs (Srikandakumar et al. 2003). In the current study, we also found the same tendency, but only for rams was this significant (P < 0.05). BUN can originate from hepatic deamination of amino acids mobilized from the skeletal muscle, and CR was a better circulating indicator of muscle catabolism. CR decreased in both rams and ewes in this study. Ganong (1977) reported that the rate of excretion of creatinine (CR) is influenced by the glomerular filtration rate such that CR is eliminated more easily than BUN. The ratio between urea and creatinine increased with the increasing levels of THI for rams and ewes. This may be due to the reduced muscular activity (increased lying down) as well as protein compensation (Kulkarni et al. 2010).
There was no effect of heat stress on plasma glucose (Glu) in ewes (P > 0.05) but heat stress markedly decreased plasma Glu in rams (P < 0.05), with the lowest concentration occurring when the THI level was equal to 3. It was reported that Glu metabolism was largely influenced by nutritional and physiological conditions (e.g., energy intake, energy demand, and mobilization of body fat reserves) (Sano et al. 1983; Srikandakumar et al. 2003), so the changing of plasma Glu might be related partially with the ingestion of feed and body fat reserves. Under high temperature, energy demand of sheep should increase because of higher respiratory rate. Sano et al. (1983) reported that Glu metabolism was reduced during heat exposure, but some reported that the plasma Glu concentrations were unchanged during heat exposure (Al-Mamun et al. 2007); this may partly be due to different ingestion of feed; in our studies, there were more effects of heat stress for rams than that for ewes. Among blood chemistry parameters which were recorded only plasma chlorides, triglyceride and creatinine changed steadily with the increasing of THI, and we can use it as an important indicator of heat stress in Small-tail Han sheep farm management under high-temperature environments.
Conclusion
Unlike other sheep, the behavioral, physiological, and blood parameters of Small-tail Han sheep changed under high-temperature environments. With the increasing levels of THI, the sheep changed their normal behavior to adapt to the external conditions. There were significant differences in some of the physiological and behavioral parameters between male and female sheep, and by contrast, the ewe has better high-temperature tolerance than the ram. Heat stress also significantly altered some behaviors (standing, lying, feeding, and drinking), all of the physiological parameters, and some blood chemistry parameters (chlorides, sodium, phosphorus, total protein, tetraiodothyronine, cholesterol, triglyceride, creatinine, cortisol, and glucose) in sheep. These results indicate that heat stress can partly alter the behavioral and physiological patterns in Small-tail Han sheep housed indoors in China, and also, to some extent, Small-tail Han sheep housed indoors can change some of their behavioral and physiological patterns to better accommodate the high-temperature environment.
References
Alamer A., Al-Hozab A., 2004. Effect of water deprivation and season on feed intake, body weight and thermoregulation in Awassi and Najdi sheep breeds in Saudi Arabia. Journal of Arid Environments, 59, 71–84.
Al-Mamun M., Tanaka C., Hanai Y., Tamura Y., Sano H., 2007. Effects of plantain (Plantago lanceolata L.) herb and heat exposure on plasma glucose metabolism in sheep. Asian-Australasian Journal of Animal Sciences, 6, 20, 894–899.
Anderson, B.E., Jónasson, H.,1996. Regulação da temperatura e fisiologia ambiental. Dukes–fisiologia dos animais domésticos, 11, 805–841.
Beede D.K., Collier R.J., 1986. Potential nutritional strategies for intensively managed cattle during heat stress. Journal of Animal Science, 62,543–550.
Bernabucci U., Lacetera N., Danieli P.P., Bani P., Nardone A., Ronchi B., 2009. Influence of different periods of exposure to hot environment on rumen function and diet digestibility in sheep. International Journal of Biometeorology, 53, 387–395.
Bernabucci U., Lacetera N., Baumgard L.H., Rhoads R. P., Ronchi B., Nardone A.,2010. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal, 4, 1167–1183.
Chiericato, G.M., Ravarotto, L., Rizzi, C., 1994. Study of the metabolic profile of rabbits in relation to two different environmental temperatures. World Rabbit Science, 2, 153–160. https://riunet.upv.es/handle/10251/10533
Christison G.I., Johnson H.D., 1972. Cortisol turnover in heat stressed cows. Journal of Animal Science, 53, 1005–1010.
Mateus J.R. Paranhos da Costa, Roberto Gomes da Silva, Roberto Carlos de Souza, 1992. Effect of air temperature and humidity on ingestive behaviour of sheep. International Journal of Biometeorology, 36(4), 218–222.
Fadare, A.O., Peters, S.O., Yakubu, A., Sonibare, A.O., Adeleke, M.A., Ozoje, M.O., Imumorin, I.G., 2012. Physiological and haematological indices suggest superior heat tolerance of white-coloured West African Dwarf sheep in the hot humid tropics. Tropical animal health and production, 45(1), 157–165.
Ganong, W.F., 1977. Energy balance, metabolism and nutrition. In: Review of Medical Physiology, 8th ed. Lange Medical Publications, Los Altos, CA, 199–235.
Keim S.M., Guisto J.A., Sullivan Jr, J.B., 2002. Environmental thermal stress. Annals of Agricultural and Environmental Medicine, 9, 1–15.
Nazan Koluman, Irfan Daskiran, 2011. Effects of ventilation of the sheep house on heat stress, growth and thyroid hormones of lambs. Tropical Animal Health and Production, 43, 1123–1127.
Wojtas Krzysztof, Przemyslaw Cwynar, Roman Kolacz, 2014. Effect of thermal stress on physiological and blood parameters in merino sheep. Bulletin of the Veterinary Institute in Pulawy, 58, 283–288.
Kulkarni, S.S., Gaikwad, N.Z., Bapat, S.T., 2010. Effect of summer season on certain biochemical parameters in Deccani sheep. Indian Veterinary Journal, 87(7), 729–730.
Ma Weidong, Kang Mengsong, Gao Hong, Huang Guanghui, Zhou Fuzha, 1992. Effect of high ambient temperature on sheep in subtropical plains. Chinese Journal of Applied Ecology (In Chinese), 3, 2,155–159.
Macias-Cruz U., Alvarez-Valenzuela F.D., Correa-Calderon A., DiazMolina R., Mellado M., Meza-Herrera C.A., 2013. Thermoregulation of nutrient-restricted hair ewes subjected to heat stress during late pregnancy. Journal of Thermal Biology 38, 1–9.
Marai I.F.M., Ayyat M.S., Abd El-Monem U.M., 2001. Growth performance and reproductive traits at first parity of New Zealand White female rabbits as affected by heat stress and its alleviation, under Egyptian conditions. Tropical Animal Health and Pro, 33, 451–462.
Marai I.F.M., El-Darawany A.A., Fadiel A., Abdel-Hafez M.A.M., 2007. Physiological traits as affected by heat stress in sheep—a review. Small Ruminant Research. 71, 1–12.
Marai, I.F.M., El-Darawany, A.A., Fadiel, A., Abdel-Hafez, M.A.M., 2008. Reproductive performance traits as affected by heat stress and its alleviation in sheep. Tropical and Subtropical Agroecosystems, 8(3), 209–234.
Marai, I.F.M., El-Darawany, A.H., Ismail, E., & Abdel-Hafez, M.A.M., 2009. Reproductive and physiological traits of Egyptian Suffolk rams as affected by selenium dietary supplementation and housing heat radiation effects during winter of the sub-tropical environment of Egypt. Archives Animal Breeding, 52(4), 402–409.
Concepta McManus, Giane Regina Paludo, Helder Louvandini, Rosilene Gugel, Luiz Cláudio Bastos Sasaki, Samuel Rezende Paiva, 2009. Heat tolerance in Brazilian sheep: physiological and blood parameters. Tropical Animal Health and Production, 41, 95–101.
More, T., Rai, A.K., Singh, M., 1980. Note on certain biochemical responses of sheep and goats exposed to thermal stress. Indian Journal of Animal Sciences, 50(11), 1012–1014.
Nazifi, S., Gheisari, H.R., Poorabbas, H., 1999. The influences of thermal stress on serum biochemical parameters of dromedary camels and their correlation with thyroid activity. Comparative Haematology International, 9(1), 49–54.
Okoruwa, M.I., 2014. Effect of heat stress on thermoregulatory, live bodyweight and physiological responses of dwarf goats in southern Nigeria. European Scientific Journal, ESJ, 10(27),255–264.
Rai, A.K., Singh, M., More, T.,1983. Experimental thermal stress and past-stress responses of sheep. Indian Journal of Animal Sciences, 53, 1104–1106.
Sano H., Takahashi K., Ambo K., Tsuda T., 1983. Turnover and oxidation rates of blood glucose and heat production in sheep exposed to heat. Journal of Dairy Science, 66, 856–861.
Sejian, V., Maurya, V.P., Naqvi, S.M., 2010. Adaptive capability as indicated by endocrine and biochemical responses of Malpura ewes subjected to combined stresses (thermal and nutritional) in a semi-arid tropical environment. International Journal of Biometeorology, 54(6), 653–661.
Silanikove N., 1992. Effects of water scarcity and hot environment on appetite and digestion in ruminants: a review. Livestock Production Science, 30, 175–194
Silanikove N., 2000. Effects of heat stress on the welfare of extensively managed domestic ruminants, Livestock Production Science, 67, 1–18.
Silanikove N., 2000a. The physiological basis of adaptation in goats to harsh environments. Small Ruminant Research, 35, 181–193.
Srikandakumar A., Johnson E.H., Mahgoub O., 2003. Effect of heat stress on respiratory rate, rectal temperature and blood chemistry in Omani and Australian Merino sheep. Small Ruminant Research, 49, 193–198.
Todini L., Malfatti A., Valbonesi A., Trabalza-Marinucci M., Debenedetti A., 2007. Plasma total T3 and T4 concentrations in goats at different physiological stages, as affected by the energy intake, Small Ruminant Research, 68, 285–290.
Zang Qiang, Li Banming, Shi Zhengxiang, Han Jing, Zhao Yang, 2006. Effects of sheepcot shade on behavior and physiological indexes of Chinese little fat-tailed sheep in housing system during summer, Transactions of the CSAE (In Chinese), 22, 143–147.
Acknowledgements
We thank Ziming Jiang, Xin Shi, and Jianfu Fan for sheep care, handling, and blood sampling.
Funding
This study was funded by the National Natural Science Foundation of China (Grant Nos. 31572448, 31272480) and Domesticated Animal Germ Plasm Resources Platform of China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, F.K., Yang, Y., Jenna, K. et al. Effect of heat stress on the behavioral and physiological patterns of Small-tail Han sheep housed indoors. Trop Anim Health Prod 50, 1893–1901 (2018). https://doi.org/10.1007/s11250-018-1642-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-018-1642-3