Abstract
Key message
Eucalypt seedlings differently modulate root morphology in response to phosphorus availability, with changes in the length or density of root hairs being more common that changes in root length.
Abstract
Phosphorus (P) is an essential nutrient for plant growth and development and thus can restrict biomass accumulation when it is at low levels in the soil. Eucalypts cover large areas of planted forests in the world, including regions with naturally low P availability. This study was conducted to evaluate the morphological changes in the roots of seedlings of five eucalypt species: Eucalyptus acmenoides, E. globulus, E. grandis, E. tereticornis and Corymbia maculata in response to low P concentration. Seedlings were grown in pots with vermiculite and received a nutrient solution of low (25 μM), and sufficient concentration (500 μM) of P. Root hair length and density were evaluated in secondary root segments, and the production of plant biomass and P concentration in the shoots were determined. The species modulated root morphology differently in response to P limitation. E. tereticornis showed the lowest plasticity of these morphological traits in response to P concentration. The total root length increased in some species, but changes in the length and/or density of root hairs were the commonest response to low P concentration. P concentrations in the shoots and biomass production were not related to increase of root length or root hair density and length.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is an essential macronutrient for plant development. Besides being a component of nucleic acids and phospholipids, it is a critical component in physiological and molecular functions of plants, such as photosynthesis, energy transfer and carbohydrate metabolism (Vance et al. 2003). P is absorbed by plants in the form of inorganic P, predominantly as orthophosphate, H2PO4−. Its availability for plants is usually limited because H2PO4− can form stable and/or insoluble complexes with Fe and Al oxides and hydroxides or with Ca in the soil (Holford 1997). The mineralogical characteristics of the soil and the quantity and quality of soil organic matter are also intrinsically related to P availability for plants (Binkley and Vitousek 2000).
The concentrations of inorganic P in the soil solution are usually very low, ranging from 2 to 10 μM (Hinsinger and Gilkes 1996). This low level is particularly true in the acidic and weathered soils of tropical regions, which have high P retention ability, leading to plant deficiency (Friesen et al. 1997). Besides, P diffusion to plant roots is a slow process (Huang et al. 2017), which makes P one of the least available nutrients for plants (Marschner 2012). Therefore, understanding the response of plant roots to P restriction is essential for the development of plants for efficient P uptake (Lambers and Plaxton 2018).
Planted forests represent around 7% of global forests (Valadares et al. 2020). In Brazil, these forests cover around 7.83 million hectares, and although this area corresponds to less than 1% of the Brazilian territory, they supply approximately 91% of the country's timber market. Eucalypt plantations occupy 72.7% of the total area of planted forests in Brazil, and many of these areas have soils of low natural fertility (Valadares et al. 2020), showing the eucalypts ability to adapt to the edaphoclimatic conditions in Brazil (Grattapaglia and Kirst 2008; Pérez-Cruzado et al. 2011).
Australia and nearby islands are the centres of eucalypt diversification, in regions where P concentration in the soil is naturally very low (Flores et al. 2016; Rossel and Bui 2016). As a result, eucalypts may have evolved mechanisms to increase the efficiency of P uptake and use (Del-Saz et al. 2018).
Response to P availability varies according to the species and developmental stage of eucalypt plants (Thomas et al. 2006; Bulgarelli et al. 2019). Recently, a comparison of 24 eucalypt species showed contrasting responses to P availability in the soil, with varied efficiency and responsiveness (Bulgarelli et al. 2019). Species such as E. acmenoides and E. crebra were responsive to the addition of P and inefficient in P use. In contrast, other species, such as E. tereticornis and E. globulus, were efficient in biomass production under low P availability but had no response to the addition of P. Bulgarelli et al. (2019) did not find a correlation between P concentration in the leaves and biomass accumulation. They suggested that foliar P concentration is not a good indicator of the response of eucalypts to P, which is probably related to the varied P use efficiencies and mechanisms related to P acquisition.
Adaptive plant strategies to increase P uptake in conditions of low availability include: (i) mining strategies with an increase in the desorption of P adsorbed into soil colloids through root exudation of organic molecules, such as organic acids (Miguel et al. 2015); (ii) increased expression of genes encoding high-affinity phosphate transporter proteins located in the membranes (Tang et al. 2020); (iii) foraging strategies to increase soil exploration by promoting root growth, changes in root architecture or changes in root hair emergence and growth (Trindade and Araujo 2014); (iv) development of symbiotic associations with mycorrhizal fungi (Miguel et al. 2015). Therefore, plants can adapt to low P conditions, increasing the ability of soil exploration and uptake and optimizing the use of absorbed P (Tang et al. 2020).
Changes in root morphology and physiology are important in plant adaptation to P limitation (Tang et al. 2020). P exploration capacity is closely linked with the morphological and architectural plasticity of roots (Cao et al. 2013; Tang et al. 2020), considering plasticity as the ability of a given genotype to express different phenotypes under different environmental conditions (Palmer et al. 2012). In situations of P limitation, plants can adapt the morphological traits of roots to increase the volume of soil explored for the acquisition of P (Xia et al. 2020; Miguel et al. 2015). A study that analyzed root plasticity in eight commercial eucalypt hybrids under P limitation showed root morphology alteration in response to the varied supply of P in the soil (Zhou et al. 2017). Roots of some hybrids proliferated in places where P was more abundant, while the opposite was observed with other genotypes, with broader root proliferation in places where P availability was lower (Zhou et al. 2017). Plants of Cunninghamia lanceolata (Lamb.) Hook., Pinus massoniana Lamb., and Phoebe zhennan S. Lee changed root growth patterns to adapt to lower P availability in the soil, showing an increase in the root-shoot ratio (Yan et al. 2019).
Root hairs increase the surface area of roots, consequently increasing the contact between root and soil (Rongsawat et al. 2020; Ruiz et al. 2020; Bates and Lynch 2001). The emergence of root hair can be driven by low P availability, representing a strategy of lower metabolic cost for increasing the P uptake (Klamer et al. 2019; Foehse and Jungk, 1983). For several plant species, these two characteristics increase during P deficiency, as observed in Poncirus trifoliata Raf. orange (Cao et al. 2013) and Triticum aestivum L. wheat (Yuan et al. 2016).
However, studies have demonstrated that only longer hair length may not be a guarantee of higher P uptake by plants and that other morphological and physiological characteristics of roots significantly contribute to an efficient acquisition of this nutrient (Brown et al. 2013). Transgenic mutants of Brachypodium distachyon L. with shorter root hairs than wild-type plants showed lower growth and P uptake in conditions of low P availability than mutants of longer root hairs (Zhang et al. 2018).
Here, we analyzed the morphological changes in roots and root hair of seedlings of five eucalypt species in response to the low P concentration. We aimed to have a better understanding of the relationship among phenotypic plasticity of the root, P availability, and accumulation of biomass and P in eucalypts. We hypothesized that eucalypt genotypes have different root morphological traits in response to low P concentration and that such responses are related to the ability of P uptake and accumulation by plants.
Material and methods
Experimental design and biological material
The eucalypt species studied were: Eucalyptus acmenoides Schauer, E. globulus Labill., E. grandis W. Hill ex Maiden, E. tereticornis Sm., and Corymbia maculata (Hook.). These species were used in this study because previous research conducted by our group showed they differ regarding P uptake (Bulgarelli et al. 2019). Among 24 species, E. acmenoides was classified as non-efficient under low P availability in soil and responsive to added P, C. maculata was non-efficient and non-responsive, E. globulus and E. teretircornis were efficient and non-responsive, while E. grandis presented an intermediate response.
The seeds of the species used here were obtained from clonal garden plants (Caiçara Sementes, Brejo Alegre, São Paulo, Brazil; Instituto de Pesquisas e Estudos Florestais—IPEF, Piracicaba, São Paulo, Brazil). Seeds were germinated in trays with vermiculite. Since germination, seedlings received modified Hoagland solution, ½ strength (Hoagland and Arnon 1938), with low level (25 μM) and sufficient level (500 μM) of P (Bahar et al. 2018). When the seedlings had between four and five leaves, they were transplanted into 3 L pots containing vermiculite, with four seedlings per pot, and four pots per treatment. The seedlings continued to receive the same nutrient solutions according to the treatments (200 mL per pot and three times a week). Thus, the statistical experimental design was a 5 × 2 factorial scheme, with five eucalypt species and two P concentrations in the nutrient solution; with four replicates.
Plant harvest
After 21 days of transplantation, the seedlings were carefully removed from the pots, washed in running tap water, and had the shoots and roots separated. The shoots were dried at 60 °C, weighed, and ground. The roots were stored in 50% ethanol for the analysis of morphological traits.
Morphological traits of roots
The roots were scanned at a resolution of 600 dpi (Epson Expression 12000XL, Epson America), and the images were analyzed with WinRHIZO (Regent Instruments Inc., Canada). Data obtained were: average diameter (mm), total length (cm), volume (cm3), and surface area (cm2). After scanning, segments of root tips were reserved for root hair analysis. Data for length and surface area were used to calculate specific root length (SRL) and specific root area (SRA), dividing them by the root dry matter mass. Root tissue density (RTD) was calculated as the ratio between dry mass and root volume.
Root hair length and density
We used five 0.5 cm segments from each of all four treatment replicates to analyze root hair length and density. These segments were obtained from the apex of second-order roots. They were cut with a scalpel under a stereomicroscope (Leica LED5000SLI) and then stained with 2% trypan blue for 10 min and then used to obtain images on a microscope equipped with image system (Leica DM2700M). We obtained root hair length and abundance using the images processed in the ImageJ software (https://imagej.net/Fiji/Downloads). In each segment, the length of ten root hairs was measured. An area between 0.018 and 0.088 mm2 was delimited for determining the abundance of root hairs per area.
Determination of aerial and root masses
After the morphological analysis of the roots, roots and aerial parts of the plants were dried at 60 °C and weighed to obtain the dry masses.
Determination of the concentration of P and other nutrients
The dried shoots and roots were ground and submitted to nitric-perchloric acid digestion (HNO3–HClO4) to determine the contents of nutrients (P, K, Mg, Zn, Cu, B, Cu and Mn) by plasma emission spectrophotometry (ICP-OES) (JobinYvon, JY50P Longjumeau, France).
Statistical analysis
Data were analyzed by two-way ANOVA, Scott-Knott test for comparison of means at 5% significance using the SISVAR software (Ferreira 2011). Data related to counts and percentages were transformed into log (x + 1) and arcsine √(x/100)], respectively, before statistical analysis. Pearson's correlation was used to check the correlation between the main variables, and the concentration of P. Principal component analysis (PCA) was conducted to identify the variables that best explained the highest proportion of data set variance using Minitab software (Minitab Statistical version 17).
Results
Root morphology
In general, species and P concentration significantly influenced root morphological parameters. E. globulus was the species with higher values of root length and surface area, while E. acmenoides and E. tereticornis had the lowest values (Fig. 1). Regarding the response to P concentration, E. globulus and C. maculata showed higher values of root length and surface area in sufficient P concentration (Fig. 1a, b). E. grandis seedlings showed higher values for these parameters in low P concentration (Fig. 1a, b).
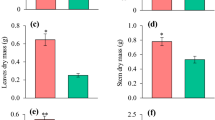
Morphological traits of the seedling root system of five eucalypt species grown with a nutrient solution of low (25 µM) and sufficient (500 µM) P concentration. a Total length (RL) of the root system, b surface area (RSA), c specific root length (SRL), d specific surface area (SRA), e root tissue density (RTD) and f average root diameter (ARD). Different letters indicate a significant difference among species for each P concentration, whereas the asterisk indicates a significant difference between P concentrations for each species, by the Scott-Knott test at 5% probability. Bars indicate standard error (n = 16)
For specific root length (SRL), the species showed a similar response and only C. maculata had significantly lower values when compared to the other species (Fig. 1c). Regarding the response of SRL to P, no significant difference was observed among plants at low and sufficient P concentration (Fig. 1c). For specific root area (SRA), E. globulus, E. grandis, and E. tereticornis had the highest values, followed by E. acmenoides and C. maculata (Fig. 1d). For this root trait, no significant difference was observed in response to the P concentration (Fig. 1d).
Regardless of the P concentration, E. globulus and E. grandis had the lowest root tissue density (RTD), and E. acmenoides and C. maculata showed the highest RTD. RTD did not respond significantly to the increase in P concentration in any of the studied species (Fig. 1e). Despite the average root diameter (ARD) did not respond to P concentration it was significantly different among eucalypt species (Fig. 1f). C. maculata showed the greatest ARD and E tereticornis and E. acmenoides the smallest ARD (Fig. 1f).
Root hair length and density
In general, root hair length varied significantly among species and with P concentration (Fig. 2a). E. globulus showed the longest root hairs, around three times those of E. acmenoides, which had the shortest root hairs (Fig. 2a). The other studied species, E. grandis, E. tereticornis and C. maculata, had intermediate root hair length (Fig. 2a). In response to P concentration, E. grandis and C. maculata had longer root hair length at low P concentration, and C. maculata seedlings developed root hairs up to twice the length of seedlings developed at sufficient P concentration (Fig. 2a, c).
Root hair length (a) and root hair density (b) on secondary roots of eucalypt species grown with a nutrient solution of low (25 µM) and sufficient (500 µM) P concentration. Different letters indicate a significant difference among species for each P concentration, whereas the asterisk indicates a significant difference between P concentrations for each species, by the Scott-Knott test at 5% probability. Bars indicate standard error (n = 20). c Photographs showing contrasting responses in root hair length and density at low and sufficient P concentration (scale bar: 100 μm)
Root hair density varied in response to P level and species (Fig. 2b). In general, E. grandis roots had the highest root hair density among all species that grew in low P conditions, followed, in decreasing order, by E. globulus, E. tereticornis, E. acmenoides and C. maculata. When the seedlings were grown in sufficient P concentration, E. grandis and E. tereticornis had a higher hair density than E. globulus and C. maculata, which, in turn, had a higher density when compared to E. acmenoides, which was the species with the lowest value (Fig. 2b). When comparing root hair density as a function of P concentration, seedlings of E. grandis, E. globulus and E. acmenoides had between 33 and 40% higher hair density at low P than those grown at sufficient P concentration (Fig. 2b). C. maculata and E. tereticornis did not show significant differences in root hair density in response to P concentration (Fig. 2b).
Biomass production
In general, shoot biomass accumulation varied among species in response to P concentrations (Fig. 3a). In particular, at sufficient P, E. globulus and C. maculata presented higher shoot biomass than the other species (Fig. 3a) and produced a significantly smaller amount of shoot biomass when grown at low P concentration, with around 40% less biomass accumulation than plants grown with sufficient P. The same growth pattern was found in E. tereticornis that produced around 50% less biomass at low P. In contrast, E. grandis accumulated 46% more shoot biomass when grown at low P compared to sufficient P (Fig. 3a).
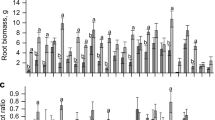
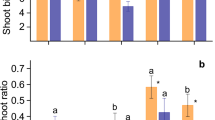
Biomass production of the shoot (a), root (b) and root to shoot ratio (c) of eucalypt species grown at low and sufficient P concentration. Different letters indicate a significant difference among species for each P concentration, whereas the asterisk indicates a significant difference between P concentrations for each species, by the Scott-Knott test at 5% probability. Bars indicate standard error (n = 4)
Regarding the root biomass, at sufficient P C. maculata stood out among all species, producing around three times more root biomass than E. acmenoides; the latter species, along with E. grandis and E. tereticornis, showed the lowest root biomass production. At low P, E. globulus, E. grandis and C. maculata had higher root biomass than E. acmenoides and E. tereticornis (Fig. 3b). When comparing the response to P, E. grandis had higher root biomass at low P, and C. maculata had higher root biomass at sufficient P (Fig. 3b).
The root-shoot (R:S) ratio varied between species (Fig. 3c). At sufficient P concentration, E. grandis and C. maculata had the highest R:S ratio, while at low P, E. acmenoides had the lowest ratio. E. tereticornis was the only species with a significant increase of R:S ratio at low P (Fig. 3c).
P concentration and accumulation in the shoots
The concentration of P in the shoots varied significantly depending on the concentration of P in the nutrient solution. Species that grew at sufficient P showed higher shoot P concentrations than species grown at low P (Fig. 4a). At sufficient P, E. tereticornis presented higher P concentration in the shoots when compared to all other species, being 155% higher than in E. acmenoides plants, which presented the lowest concentration of P (Fig. 4a). At low P, the five species showed similar shoot P concentrations (Fig. 4a).
Phosphorus concentration (a) and content (b) in the shoot of eucalypt species grown at low and sufficient P concentration. Different letters indicate a significant difference among species for each P concentration, whereas the asterisk indicates a significant difference between P concentrations for each species, by the Scott-Knott test at 5% probability. Bars indicate standard error (n = 4)
As observed for P concentration in the shoots, P content (P concentration multiplied by shoot biomass) was highest in plants that grew at sufficient P (Fig. 4b). At sufficient P conditions, E. globulus accumulated 2.8- and 4.6-times higher P contents than E. grandis and E. acmenoides, respectively; the later species presented the lowest P content (Fig. 4b). However, at low P, no significant differences were observed among the species in P accumulation in the shoots (Fig. 4b).
Regarding other nutrients, Online resource Table 1 shows the concentrations of potassium (K), magnesium (Mg), boron (B), manganese (Mn), iron (Fe), copper (Cu), molybdenum (Mo), and zinc (Zn) in the shoots. In general, seedlings grown at sufficient P had a higher concentration of the evaluated nutrients, except for Zn and B, which had higher concentrations in seedlings grown at low P. For B and Mg, E. acmenoides and E. tereticornis presented significantly higher concentrations than the other species (Online resource Table 1).
Principal component analysis and correlations
Principal component analysis (PCA) was performed separately for P levels (Fig. 5). At low P, the first (PC1) and the second (PC2) components explained 59.2% and 26.8% of the total variation, respectively. For P sufficiency, PC1 and PC2 explained 59.8% and 22.8% of the total variation, respectively. At low P, the root morphological and root hairs traits contributed to the separation of E. grandis and E. globulus (Fig. 5a, c). These variables were grouped with shoot P content, indicating that for these species, P accumulation and biomass had a strong correlation with the characteristics of roots and root hairs. On the other hand, E. tereticornis, at both low and sufficient P, segregated for the loading plot quadrants without the presence of root characteristics, indicating that these characteristics were not so determinant for the responses to P (Fig. 5a). E. acmenoides was separated mainly due to the high variance of root tissue density, and oppositely, due to the morphological traits of root hairs (Fig. 5). B and Mg concentrations strongly contributed to the separation of E. acmenoides and E. tereticornis in the PC2 axis; as these species showed the higher concentrations of these nutrients, while in PC1 axis they were in opposite quadrants influenced mainly by P concentrations, that were low in E. acmenoides and high in E. tereticornis (Fig. 5).
Principal component analysis (PCA) from five eucalypt species grown at low (a and c) and sufficient (b and d) P concentration, score plots (a and b) and loading plots (c and d) of the analyzed variables dispersed in the first (PC1) and second (PC2) components. Numbers in parentheses indicate the variation percentage explained by PC1 and PC2. Text colours indicate the cluster to which parameters have been assigned: blue, root morphology; red, nutrient concentrations; green, P content; orange, biomass. shoot biomass (SDW), root biomass (RDW), root length (RL), average root diameter (ARD), root surface area (RSA), root hairs length (RHL), root hairs density (RHD), phosphorus content (PC), root tissue density (RTD) and phosphorus (P), potassium (K), magnesium (Mg), boron (B), manganese (Mn), iron (Fe), copper (Cu), molybdenum (Mo), and zinc (Zn) concentrations in the shoots
Discussion
The adverse effects of P limitation are significant in the early stages of eucalypts development (Graciano et al. 2005). Here, we observed that the negative impact of low P on shoot growth was not observed for E. grandis, which produced the larger biomass, and for E. acmenoides, which had no significant changes. These results support our previous findings on the response variability of eucalypt species to P availability (Bulgarelli et al. 2019). These responses may be related to changes in biomass allocation, with increased investment in root growth, changes in root architecture and morphology, the emergence of more prolonged or more abundant root hairs, among others (Kramer-Walter and Laughlin 2017; Poorter et al. 2012). Physiological adaptations of the root system can also contribute to more efficient phenotypes in environments of low P availability (Wang et al. 2020). So, the characteristics that determine how plants will cope with low P concentration represent different strategies of plant phenotype (White and Hammond 2008).
In our study, changes were observed in root morphology of seedlings of all five eucalypt species in response to P supply, but the response varied depending on the species. We observed that biomass was preferentially distributed in the shoots than in the roots, a fact that has already been reported by other authors (Wu et al. 2014). However, changes in characteristics that could contribute to improving the uptake of P were not associated with higher shoot P concentration, although in P deficiency, root biomass production, length, and root surface area correlated positively and significantly with accumulated P in the shoots (Online resource Fig. 1).
Among the morphological traits evaluated, only average root diameter was not altered by P deficiency in any species. Some species showed changes in root length and/or surface area in response to low P concentration, but differently, depending on the species. Compared with sufficient P, while E. globulus and C. maculata had longer roots and larger root surface area at sufficient P, E. grandis had these characteristics increased at low P. The association between an increase of the root length with low P availability have been observed in Zea mays (Wang et al. 2020), Rosa multiflora Thunb. ex Murr. (Ma et al. 2020), Fagus sylvatica (Loew et al. 2020), and Eucalyptus dunii (Dias et al. 2017).
E. acmenoides and E. tereticornis did not present significant changes in the main root morphology traits evaluated, although SRL and SRA tended to increase at low P in E. tereticornis. Longer SRL has been related to improved efficiency in P uptake in tree species (Lugli et al. 2019; Li et al. 2017) and several herbaceous species (Haling et al. 2018; Sandral et al. 2018).
E. acmenoides and C. maculata had the highest RTD at low P and E. globulus and E. grandis the lowest, with RTD presenting negative correlation with root length and root hair length (Online resource Fig. 1). Under limited P, low root tissue density may reduce the metabolic costs of producing new roots (Eissenstat et al. 2000), mainly in those species with short root length. These results might be related to lower dependence on root proliferation and the existence of other physiological strategies for P uptake, which may be metabolically more economical than investing in the development of new tissues (Xia et al. 2020; Funayama-Noguchi et al. 2015). However, RTD can also be a plant genotype characteristic, as reported for different coffee genotypes (Silva et al. 2019).
Improved contact between roots and soil particles can be achieved with longer or more numerous root hairs (Haling et al. 2013), considering it promotes a larger specific surface of the roots (Raghothama and Karthikeyan 2005). Changes in root hairs characteristics have been associated with a strong ability to absorb nutrients of low diffusivity in the soil, as seen with P (Ma et al. 2001; Miguel et al. 2015). Eucalypt species showed different responses to P availability in terms of morphological plasticity of root hairs, which in general were longer and more abundant at low P. E. grandis was the only species with an increase in both length and root hairs density at low P. Higher root hairs abundance was the commonest characteristic in the studied species. However, although some studies relate an increase in the number and length of root hairs under low P availability (Ruiz et al. 2020), our study did not find a correlation between longer and/or most abundant root hairs with higher P concentration or accumulation in the shoots of plants (Online resource Fig. 1).
Because our study was carried with plants growing in vermiculite and receiving nutrient solution, one may argue that it does not reflect the potential benefit of more abundant or longer root hairs in P uptake. Although root hairs can be more effective in P uptake when plants grow in the soil, where P-depletion areas can form around roots (Ruiz et al. 2020) our results show that eucalyptus genotypes responded solely to the low concentration of P in the nutrient solution.
E. tereticornis was the species with the lowest plasticity of root morphological traits. However, it was the only species that increased the root-shoot ratio at low P. Such behaviour indicates that biomass allocation was a response to low P in this species. On the contrary, an increase in biomass allocation to the roots has been widely reported in young tree plants growing with limited P (Santiago et al. 2012; Yan et al. 2019). On the other hand, E. grandis was the species with the highest root plasticity at low P, since it had an increase in root hair density and length, as well as in root length and surface area. E. grandis was also the only species that accumulated more biomass in the shoots at low P than at sufficient P. Such results suggest that for this species changes in the root traits may be related to higher biomass production, even not leading to higher P accumulation in the shoots.
Root morphological response to P limitation may be dependent on plant genotype (Zhou et al. 2017). In a study with eucalypt hybrids, the responses to P limitation varied depending on the genotype/hybrid, with an overall increase in root length and volume, and an increase in root hairs length and density at low P (Zhou et al. 2017). For C. lanceolata plants grown at P low concentrations, an increase in P uptake was strongly related to root plasticity, and plants with longer roots were more efficient in P absorption (Yan et al. 2019). This behaviour suggests the pattern of root growth may change so that plants can adapt to a lower P availability in the soil, increasing the root to shoot ratio to improve P uptake.
Increases in root hairs length and density can act synergistically in response to a low P availability (Brown et al. 2013), favouring P influx in plants and indicating an intrinsic relationship between root hairs and P uptake (Bates and Lynch 2001). This type of response to low P availability in the soil has been reported for Eucalyptus spp. (Zhou et al. 2017), Oryza sativa (Kakade et al. 2017), Phaseolus vulgaris (Miguel et al. 2015), and Zea mays (Zhu et al. 2010). However, this relationship between root hairs length and density and increased P uptake was not observed in different genotypes of Zea mays L. (Tang et al. 2020; Yuan and Chen 2015). Mutants of Brachypodium distachyon grass also did not show this relationship, involving other traits to improve P uptake (Zhang et al. 2018). Therefore, root hairs plasticity does not seem to be a universal trait, but specific to some species and/or genotypes to increase P uptake from soil (Vandamme et al. 2013). However, for species presenting such response to low P, this strategy may be beneficial for P uptake, offering a relatively low metabolic cost when compared to exudation of organic acids and protons, association with soil microorganisms, or development of new roots (Lynch and Ho 2005, Van de Wiel et al. 2016). Some studies showed that arbuscular mycorrhizal symbiosis affect the length and density of root hairs by inducing changes in the metabolism of cell wall and phytohormones, such as auxins (Liu et al. 2020; Ma et al. 2021). In this context, further studies under natural soil conditions are needed to elucidate the effects of the interaction between mycorrhizae and P availability on root morphology and root hairs characteristics.
In conclusion, our results showed that seedlings of eucalypt species present different abilities to adapt their root morphological traits in response to P limitation. However, this response did not reflect positively on P uptake and accumulation under our experimental conditions. Root morphological parameters, such as length and surface area, differed between species, showing root plasticity in eucalyptus regarding P availability. Plasticity, in response to P availability, reveals the extent to which growth processes and interactions can be altered and how environmental factors can be perceived and translated into morphological changes by plants (Gruber et al. 2013). Thus, our results strongly support that root morphological traits, such as long and dense root hairs, are important for plant growth, especially in agroforestry systems under limited P conditions.
Author contribution statement
PM and SALA conceptualized and designed the study. SB conducted the experiment, supervised by SALA, and wrote the first manuscript draft. PM and SALA edited draft manuscripts and PM obtained the funding to conduct the research.
Availability of data and materials
Data are available on reasonable request to the corresponding author.
References
Bahar NHA, Gauthier PPG, O’Sullivan OS, Brereton T, Evans JR, Atkin OK (2018) Phosphorus deficiency alters scaling relationships between leaf gas exchange and associated traits in a wide range of contrasting Eucalyptus species. Funct Plant Biol 45(8):813–826. https://doi.org/10.1071/FP17134
Bates TR, Lynch JP (2001) Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 236(2):243–250. https://doi.org/10.1023/A:1012791706800
Binkley D, Vitousek P (2000) Soil nutrient availability. In: Pearcy RW, Ehleringer J, Mooney HA, Rundel PW (eds) Plant physiological ecology field methods and instrumentation. Chapman & Hall, London, pp 75–96. https://doi.org/10.1007/978-94-010-9013-1
Brown LK, George TS, Dupuy LX, White PJ (2013) A conceptual model of root hair ideotypes for future agricultural environments: what combination of traits should be targeted to cope with limited P availability? Ann Bot-London 112(2):317–330. https://doi.org/10.1093/aob/mcs231
Bulgarelli RG, Silva FMO, Bichara S, Andrade SAL, Mazzafera P (2019) Eucalypts and low phosphorus availability: between responsiveness and efficiency. Plant Soil 445(1–2):349–368. https://doi.org/10.1007/s11104-019-04316-2
Cao X, Chen C, Zhang D, Shu B, Xiao J, Xia R (2013) Influence of nutrient deficiency on root architecture and root hair morphology of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under sand culture. Sci Hortic 162:100–105. https://doi.org/10.1016/j.scienta.2013.07.034
Del-Saz NF, Romero-Munar A, Cawthray GR, Palma F, Aroca R, Baraza E, Florez-Sarasa I, Lambers H, Ribas-Carbó M (2018) Phosphorus concentration coordinates a respiratory bypass, synthesis and exudation of citrate, and the expression of high-affinity phosphorus transporters in Solanum lycopersicum. Plant Cell Environ 41(4):865–875. https://doi.org/10.1111/pce.13155
Dias LPR, Gatiboni LC, Brunetto G, Arruda B, Costa MM (2017) Distribuição e morfologia do sistema radicular de Eucalyptus dunnii em resposta à aplicação de fósforo. Rev Cienc Agrovet 16(3):203–213. https://doi.org/10.5965/223811711632017203
Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147(1):33–42. https://doi.org/10.1046/j.1469-8137.2000.00686.x
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Cienc Agrotec 35(6):1039–1042. https://doi.org/10.1590/S1413-70542011000600001
Flores T, Alvares CA, Souza V, Stape JL (2016) Eucalyptus no Brasil: zoneamento climático e guia para identificação. Piracicaba, IPEF, p. 488
Foehse D, Jungk A (1983) Influence of phosphate and nitrate supply on root hair formation of rape, spinach and tomato plants. Plant Soil 74(3):359–368. https://doi.org/10.1007/BF02181353
Friesen DK, Rao IM, Thomas RJ, Oberson A, Sanz JI (1997) Phosphorus acquisition and cycling in crop and pasture systems in low fertility tropical soils. Plant Soil 196(2):289–294. https://doi.org/10.1023/A:1004226708485
Funayama-Noguchi S, Noguchi K, Terashima I (2014) Comparison of the response to phosphorus deficiency in two lupin species, Lupinus albus and L. angustifolius, with contrasting root morphology. Plant Cell Environ 38(3):399–410. https://doi.org/10.1111/pce.12390
Graciano C, Guiamét JJ, Goya JF (2005) Impact of nitrogen and phosphorus fertilization on drought responses in Eucalyptus grandis seedlings. For Ecol Manag 212(1–3):40–49. https://doi.org/10.1016/j.foreco.2005.02.057
Grattapaglia D, Kirst M (2008) Eucalyptus applied genomics: from gene sequences to breeding tools. Plant Physiol 179(4):911–929. https://doi.org/10.1111/j.1469-8137.2008.02503.x
Gruber BD, Giehl RFH, Friedel S, von Wirén N (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol 163(1):161–179. https://doi.org/10.1007/s10681-015-1572-3
Haling RE, Brown LK, Bengough AG, Young IM, Hallett PD, White PJ, George TS (2013) Root hairs improve root penetration, root-soil contact, and phosphorus acquisition in soils of different strength. J Exp Bot 64(12):3711–3721. https://doi.org/10.1093/jxb/ert200
Haling RE, Brown LK, Stefanski A, Kidd DR, Ryan MH, Sandral GA, George TS, Lambers H, Simpson RJ (2018) Differences in nutrient foraging among Trifolium subterraneum cultivars deliver improved P-acquisition efficiency. Plant Soil 424(1–2):539–554. https://doi.org/10.1007/s11104-017-3511-7
Hinsinger P, Gilkes RJ (1996) Mobilization of phosphate from phosphate rock and alumina-sorbed phosphate by the roots of ryegrass and clover as related to rhizosphere pH. Eur J Soil Sci 47(4):533–544. https://doi.org/10.1111/j.1365-2389.1996.tb01853.x
Hoagland DR, Arnon DI (1938) The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn 347:32
Holford ICR (1997) Soil phosphorus: its measurement, and its uptake by plants. Aust J Soil Res 35(2):227–239
Huang G, Hayes PE, Ryan MH, Pang J, Lambers H (2017) Peppermint trees shift their phosphorus-acquisition strategy along a strong gradient of plant-available phosphorus by increasing their transpiration at very low phosphorus availability. Oecologia 185(3):387–400. https://doi.org/10.1007/s00442-017-3961-x
Kakade D, Singh J, Wallalwar M, Janjal A, Gupta A, Raghuvanshi R, Kongbrailatpam M, Verulkar S, Banerjee S (2017) Differential response of root morphology of rice (Oryza sativa L.) genotypes under different phosphorus conditions. Int J Curr Microbiol Appl Sci 6(7):149–160. https://doi.org/10.20546/ijcmas.2017.607.018
Klamer F, Vogel F, Li X, Bremer H, Neumann G, Neuhäuser B, Hochholdinger F, Ludewig U (2019) Estimating the importance of maize root hairs in low phosphorus conditions and under drought. Ann Bot 124(6):961–968. https://doi.org/10.1093/aob/mcz011
Kramer-Walter KR, Laughlin DC (2017) Root nutrient concentration and biomass allocation are more plastic than morphological traits in response to nutrient limitation. Plant Soil 416:539–550. https://doi.org/10.1007/s11104-017-3234-9
Lambers H, Plaxton WC (2018) Phosphorus: back to the roots. Annu Plant Rev 48:3–22. https://doi.org/10.1002/9781119312994
Li D, Nan H, Liang J, Cheng X, Zhao C, Yin H, Yin C, Liu Q (2017) Responses of nutrient capture and fine root morphology of subalpine coniferous tree Picea asperata to nutrient heterogeneity and competition. PLoS ONE 12(11):e0187496. https://doi.org/10.1371/journal.pone.0187496
Liu CY, Zhang F, Zhang DJ, Zou YN, Shu B, Wu QS (2020) Transcriptome analysis reveals improved root hair growth in trifoliate orange seedlings by arbuscular mycorrhizal fungi. Plant Growth Regul 92:195–203. https://doi.org/10.1007/s10725-020-00630-3
Loew CAE, Schack-Kirchner H, Fink S, Lang F (2020) Fine root size and morphology of associated hyphae reflect the phosphorus nutrition strategies of european beech forests. Front Glob Change 3:1–15. https://doi.org/10.3389/ffgc.2020.00095
Lugli LF, Andersen KM, Aragão LEOC, Cordeiro AL, Cunha HFV, Fuchslueger L, Meir P, Mercado LM, Oblitas E, Quesada CA, Rosa JS, Schaap KJ, Valverde-Barrantes O, Hartley IP (2019) Multiple phosphorus acquisition strategies adopted by fine roots in low-fertility soils in Central Amazonia. Plant Soil 450:49–63. https://doi.org/10.1007/s11104-019-03963-9
Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269:45–56. https://doi.org/10.1007/s11104-004-1096-4
Ma Z, Bielenberg DG, Brown KM, Lynch JP (2001) Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ 24(4):459–467. https://doi.org/10.1046/j.1365-3040.2001.00695.x
Ma Q, Chen L, Du M, Zhang Y, Zhang Y (2020) Localized and moderate phosphorus application improves plant growth and phosphorus accumulation in Rosa multiflora thunb. ex murr. via efficient root system development. Forests 11(5):570. https://doi.org/10.3390/f11050570
Ma X, Li X, Ludewig U (2021) Arbuscular mycorrhizal colonization outcompetes root hairs in maize under low phosphorus availability. Ann Bot-London 127(1):155–166. https://doi.org/10.1093/aob/mcaa159
Marschner H (2012) The mineral nutrition of higher plants. Academic Press, London, p 158
Miguel MA, Postma JA, Lynch JP (2015) Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiol 167(4):1430–1439. https://doi.org/10.1104/pp.15.00145
Palmer CM, Bush SM, Maloof JN (2012) Phenotypic and developmental plasticity in plants. In: eLS. Wiley, Chichester. https://doi.org/10.1002/9780470015902.a0002092.pub2
Pérez-Cruzado C, Merino A, Rodríguez-Soalleiro R (2011) A management tool for estimating bioenergy production and carbon sequestration in Eucalyptus globulus and Eucalyptus nitens grown as short rotation woody crops in north-west Spain. Biomass Bioenerg 35(7):2839–2851. https://doi.org/10.1016/j.biombioe.2011.03.020
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193(1):30–50. https://doi.org/10.1111/j.1469-8137.2011.03952.x
Raghothama KG, Karthikeyan AS (2005) Phosphate acquisition. Plant Soil 274:37–49. https://doi.org/10.1007/s11104-004-2005-6
Rongsawat T, Peltier J, Boyer J, Véry A, Sentenac H (2020) Looking for root hairs to overcome poor soils. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2020.09.001
Rossel RAV, Bui EN (2016) A new detailed map of total phosphorus stocks in Australian soil. Sci Total Environ 542:1040–1049. https://doi.org/10.1016/j.scitotenv.2015.09.119
Ruiz S, Koebernick N, Duncan S, Fletcher DM, Scotson C, Boghi A, Marin M, Bengough AG, George TS, Brown LK, Hallett PD, Roose T (2020) Significance of root hairs at the field scale—modelling root water and phosphorus uptake under different field conditions. Plant Soil 447:281–304. https://doi.org/10.1007/s11104-019-04308-2
Sandral GA, Haling RE, Ryan MH, Price A, Pitt WM, Hildebrand SM, Fuller CG, Kidd DR, Stefanksi A, Lambers H, Simpson RJ (2018) Intrinsic capacity for nutrient foraging predicts critical external phosphorus requirement of 12 pasture legumes. Crop Pasture Sci 69(2):174–182. https://doi.org/10.1071/CP17276
Santiago LS, Wright SJ, Harms KE, Yavitt JB, Korine C, Garcia MN, Turner BL (2012) Tropical tree seedling growth responses to nitrogen, phosphorus and potassium addition. J Ecol 100(2):309–316. https://doi.org/10.1111/j.1365-2745.2011.01904.x
Silva IM, Schiavon NC, França AC, Franco MHR, Farneza MM (2019) Respostas de genótipos de Coffea arabica à aplicação de fósforo em substrato com ácido cítrico. Rev Cienc Agrar 62:1–7. https://doi.org/10.22491/rca.2019.2768
Tang H, Chen X, Gao Y, Hong L, Chen Y (2020) Alteration in root morphological and physiological traits of two maize cultivars in response to phosphorus deficiency. Rhizosphere 14:100201. https://doi.org/10.1016/j.rhisph.2020.100201
Thomas DS, Montagu KD, Conroy JP (2006) Leaf inorganic phosphorus as a potential indicator of phosphorus status, photosynthesis and growth of Eucalyptus grandis seedlings. For Ecol Manag 223:267–274. https://doi.org/10.1016/j.foreco.2005.11.006
Trindade RS, Araujo AP (2014) Variability of root traits in common bean genotypes at different levels of phosphorus supply and ontogenetic stages. Rev Bras Cienc Solo 38(4):1170–1180. https://doi.org/10.1590/S0100-06832014000400013
Valadares SV, César J, Leite HG, Barros NF, Cropper WP, Gerber S (2020) Predicting phosphorus use efficiency and allocation in eucalypt plantations. For Ecol Manag 460:117859. https://doi.org/10.1016/j.foreco.2019.117859
Van de Wiel CCM, Gerard WC, Olga L, Scholten E (2016) Improving phosphorus use efficiency in agriculture: opportunities for breeding. Euphytica 207(1):1–22. https://doi.org/10.1007/s10681-015-1572-3
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157(3):423–447. https://doi.org/10.1046/j.1469-8137.2003.00695.x
Vandamme E, Renkens M, Pypers P, Smolders E, Vanlauwe B, Merckx R (2013) Root hairs explain P uptake efficiency of soybean genotypes grown in a P-deficient ferralsol. Plant Soil 369(1–2):269–282. https://doi.org/10.1007/s11104-012-1571-2
Wang XX, Li H, Chu Q, Feng G, Kuyper TW, Rengel Z (2020) Mycorrhizal impacts on root trait plasticity of six maize varieties along a phosphorus supply gradient. Plant Soil 448:71–86. https://doi.org/10.1007/s11104-019-04396-0
White PJ, Hammond JP (2008) The ecophysiology of plant–phosphorus interactions (vol. 7). Springer Science & Business Media, Dordrecht
Wu P, Ma X, Tigabu M, Huang Y, Zhou L, Cai L, Hou X, Oden PC (2014) Comparative growth, dry matter accumulation and photosynthetic rate of seven species of Eucalypt in response to phosphorus supply. J for Res 25(2):377–383. https://doi.org/10.1007/s11676-014-0465-y
Xia Z, He Y, Yu L, Miao J, Korpelainen H, Li C (2020) Root traits and rhizosphere processes reflect differential phosphorus acquisition strategies in contrasting Populus clones. For Ecol Manag 457:117750. https://doi.org/10.1016/j.foreco.2019.117750
Yan XL, Wang C, Ma X, Wu P (2019) Root morphology and seedling growth of three tree species in southern China in response to homogeneous and heterogeneous phosphorus supplies. Trees Struct Funct 33(5):1283–1297. https://doi.org/10.1007/s00468-019-01858-x
Yuan ZY, Chen HYH (2015) Decoupling of nitrogen and phosphorus in terrestrial plants associated with global changes. Nat Clim Change 5(5):465–469. https://doi.org/10.1038/NCLIMATE2549
Yuan HM, Blackwell M, McGrath S, George TS, Granger SH, Hawkins JMB, Dunham S, Dunham JM, Shen JB (2016) Morphological responses of wheat (Triticum aestivum L.) roots to phosphorus supply in two contrasting soils. J Agric Sci 154(1):98–108. https://doi.org/10.1017/S0021859615000702
Zhang C, Simpson RJ, Kim CM, Warthmann N, Delhaize E, Dolan L, Byrne ME, Wu Y, Ryan PR (2018) Do longer root hairs improve phosphorus uptake? Testing the hypothesis with transgenic Brachypodium distachyon lines overexpressing endogenous RSL genes. New Phytol 217(4):1654–1666. https://doi.org/10.1111/nph.14980
Zhou C, Jiang W, Li Y, Hou X, Liu A, Cai L (2017) Morphological plasticity and phosphorus uptake mechanisms of hybrid Eucalyptus roots under spatially heterogeneous phosphorus stress. J for Res 28(4):713–724. https://doi.org/10.1007/s11676-016-0335-x
Zhu J, Zhang C, Lynch JP (2010) The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Funct Plant Biol 37(4):313–322. https://doi.org/10.1071/FP09197
Acknowledgements
The authors thank Espaço da Escrita—Pró-Reitoria de Pesquisa—UNICAMP for the language services provided. We also would like to thank anonymous reviewer #1 for helpful comments and generous feedback.
Funding
This study was supported by the São Paulo Research Foundation (FAPESP—Grant Number 2016/25498-0). We thank Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brazil (CNPq) and FAPESP for a MSc fellowship to SB (FAPESP Grant Number 2019/03165-7) and a research fellowship to PM (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there are no conflicts of interest/competing interests in this study.
Additional information
Communicated by V. De Micco.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bichara, S., Mazzafera, P. & de Andrade, S.A.L. Root morphological changes in response to low phosphorus concentration in eucalypt species. Trees 35, 1933–1943 (2021). https://doi.org/10.1007/s00468-021-02161-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-021-02161-4