Abstract
Background and aims
Twenty-four species of eucalypts were studied regarding their ability to grow under low P and their responsiveness to P inputs.
Methods
Growth and photosynthesis-related parameters were evaluated.
Results
Growth of all species was influenced by low P availability. No significant correlation was found between leaf P concentration and biomass, indicating that P concentrations in leaves cannot be solely considered an indication of the responsiveness to P in eucalypts. Species responsive to P-input (high agronomic P efficiency values, APE) were those with low P use efficiency - PUE (here assessed as relative efficiency of P-use, REP) and low P uptake efficiency (PUpE). But, non-responsive species were related to higher P-efficiency under low soil P-availability. Eucalyptus tereticornis, E. cladocalyx, E. globulus and E. camaldulensis were efficient under low-P availability. Whereas, E. crebra and E acmenoides were the most responsive species, with high APE, suggesting that for these species P-inputs are needed to guarantee plant growth. The root:shoot ratio remained constant at different P availabilities, suggesting that biomass allocation towards the root in response to P and greater investment in roots were not correlated with greater PUE. Under limited P, E. robusta and E. botryoides exhibited low foliar P contents and higher root:shoot ratios than those of other species with higher P contents, indicating that greater root investment does not necessarily result in greater PUE.
Conclusion
The results suggest that the divergence among species is probably related to different mechanisms, which may improve P-use efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eucalyptus commercial forests occupy approximately 18 million ha, predominantly in India (45%), Brazil (17%) and China (7%) (Novaes 2010). Several eucalypt species are cultivated for different economic purposes, from the use of its wood to the extraction of essential oils for pharmaceutical and chemical industries. Although the cellulose and paper production industrial sectors have the greatest demand for this raw material, interest in eucalypts as an energy crop is increasing (Pérez-Cruzado et al. 2011).
The high desirability of eucalypt species for commercial plantations is due to their fast growth, straight trunk and highly adaptative capacity to a wide variety of climates and soils, resulting in high economic benefits (Grattapaglia and Kirst 2008; Moura et al. 2010). Eucalypts originated from the Australian continent, and most are widely distributed, demonstrating their adaptability to different environments.
Phosphorus (P) fertilization in eucalypt plantations has typically resulted in increased yields, but responses differ depending on plantation age, due to different demands of young and mature trees (Thomas et al. 2006). The physiological and molecular mechanisms behind such increased growth are not well understood for this group.
P is an essential element for plants, being a part of important molecules and, processes such as photosynthesis and respiration, or in the regulation of several enzymatic reactions (Vance et al. 2003). Especially in tropical soils, which are more weathered and have high P retention capacity, the Pi concentration in soil solution is typically low, between 2 and 10 μM (Friesen et al. 1997). As a consequence, P supply to roots by diffusion is commonly very low, with few exceptions (Huang et al. 2017). Thus, P is less available to plants in these soils, potentially limiting their growth (Marschner 1995).
Pi used as fertilizer comes from the phosphate extraction of rock deposits, and although reserves depletion may take longer than previously estimated, there is an urgent need to reduce agricultural P-use (Fixen and Johnston 2012; Chowdhury et al. 2017). Approximately one-third of global arable land is limited by P availability (Wang et al. 2010) and a large portion of the supplied Pi is immobilized in soil (Neumann 2016). Considering this scenario, identifying mechanisms that increase P-use efficiency is essential for more sustainable plant management (Lambers and Plaxton 2018).
In response to low P availability, through evolution, plants developed several mechanisms to acquire P from the soil and maximize its use, through metabolic recycling or metabolic bypasses that use smaller Pi amounts (Del-Saz et al. 2018). The joint effect of these mechanisms has been termed P-use efficiency (PUE) (Hammond et al. 2009) and defines the ability to efficiently using P for biomass synthesis (Clarkson and Hanson 1980). Ozturk et al. (2005) proposed a P efficiency index, calculated as the ratio of shoot biomass produced under low P to that under adequate P supply, as a way to rank wheat genotypes for P efficiency, and defined this ratio as REP (relative efficiency of P-use). Other authors used REP index to study coffee and cabbage cultivars (Hammond et al. 2009; Neto et al. 2016).
Yet, agronomic P use efficiency (APE) is another measure of PUE that relates yield increases per unit of added P as fertilizer (Hammond et al. 2009). APE is then an index related to plant responsiveness to external P concentrations. At this respect, while an increase in the efficiency of a particular physiological/biochemical process may constitute an adaptative gain, from an agronomic point of view the increase in efficiency is commonly linked to gains in yield or harvested biomass for specific resource inputs (Blair 1993) or, better, the same yield output with a lower resource’s input.
Because different plants present different responses to P fertilization and different uptake efficiencies (Gerloff 1977), some studies have used these indices to differentiate four categories of response to P supply and plant efficiency in acquiring and converting P into biomass (Hammond et al. 2009; Neto et al. 2016): 1) efficient and non-responsive (E-NR), plants with high biomass yield at low P-level and which do not respond to nutrient addition; 2) efficient and responsive (E-R), plants with high yield at low P input and which respond to nutrient addition; 3) non-efficient and non-responsive (NE-NR), plant with low yield at low P-level and which does not respond to nutrient input; and 4) non-efficient and responsive (NE-R), plant with low yield at low P-level and which does respond to nutrient input.
PUE comprises a range of physiological, structural and phenological characteristics, which determine the efficiency in P-acquisition, its use and mobilization between different organs and tissues with diverse physiological roles and nutrient demands (Veneklaas et al. 2012). Important basic mechanisms of PUE are the plant’s capacity to acquire P from the soil and to remobilize/allocate P to support biomass production (van de Wiel et al. 2016).
Under P limitation, in addition to decreased photosynthesis and leaf respiration rates, young plants of different eucalypt species had reduced growth (Bahar et al. 2018). Plants modulate their growth under low P availability, with differential root growth, decreased shoot growth (Gomez-Roldan et al. 2008; Niu et al. 2013; Salazar-Henao et al. 2016) and increased PUE, with P remobilization between compartments and organs. This remobilization involves reprogramming gene expression, which leads to changes in P allocation between roots and shoot, the release of vacuolar Pi and the replacement of membrane phospholipids with sulfolipids and galactolipids (Dörmann et al. 2002; Kelly et al. 2003; Morcuende et al. 2007; Cheng et al. 2011). Another internal source of Pi during P deficiency is the remobilization from senescent leaves (Robinson et al. 2012).
In Eucalyptus, the macronutrient concentrations follow this order (g kg−1): Ca (0.5–1.5) > N (1.0–2.5) > K (0.5–1.5) > Mg (0.2–0.6) > P (0.15–0.60) (Raij et al., 1997). However, visual symptoms of deficiency and the highest responses to fertilization in the field follow the order: P > N > K > Ca > Mg, indicating that eucalypt plantations are most likely to demand P (Santana et al. 2002).
In the present study, we report on the response and efficiency of 24 eucalypt species to grow on low and sufficient soil P availability. Plant growth and physiological parameters, such as photosynthesis, stomatal conductance, nutrient concentrations and changes in foliar pigments, were analysed and based on these data we classified the species in terms of efficiency and responsiveness to P supply.
Material and methods
Experimental design
The experiment was performed in a greenhouse between September 2017 and June 2018, in Campinas, São Paulo, Brazil (22°49′10.38”S 47°04′12.88”W). A 24 × 2 factorial design was used, with the factors being 24 eucalypt species and two P levels, with five replicates per treatment.
Soil preparation
The two soil P levels used in this work were low P (3.7 mg kg−1 available P) and sufficient P (7.7 mg kg−1 available P). To find soil with this low P level we analysed the nutrient in different sample soils collected around University of Campinas and decided for a subsample collected from the 30 - 60 cm layer. This soil was from a native grassland area and was classified as a ferrosol (IUSS Working Group WRB 2015). In this study, “available P” refers to the soil resin extractable-P, which is considered the soil fraction accessible for plants.
The substrate used to grow plants was a 1:1 mix of soil and coarse sand (v:v), prepared using a concrete mixer. The data from the chemical characterization of the low P soil (Supplementary Table 1) was used to calculate the P applications needed to reach the P levels required for each treatment, as well as the Cu, Mg, Zn, Mn and B concentrations recommended for forest species at a medium-fertility level in the state of São Paulo, Brazil (Raij et al., 1997). The amounts added (mg kg−1 soil) were: B (H3BO3), 2.25; Cu (CuCl2), 2.7; Mn (MnSO4), 15.6; Zn (ZnCl2), 52.7; and Mg (MgSO4), 418.3. The sufficient P level was achieved by adding potassium phosphate (KH2PO4). For the low P treatment, no P was added to the soil because it already had very low available P concentration. Water solutions of the above nutrients were prepared and added to the substrate when the soil was mixed with sand. This soil-sand substrate was stored for 20 days and a sample was taken for soil chemical analysis (Supplementary Table 1). Ion-exchange resin method was used for determination of soil available P and exchangeable bases Ca, K and Mg (Raij et al. 2001). Boron was extracted with hot water and metal micronutrients with 0.005 mol L−1 DTPA solution at pH 7.3. The nutrient concentrations in the extracts were determined by inductively coupled plasma-optical emission spectrometry (ICP-OES; JobinYvon, JY50P Longjumeau, France). Soil pH was determined in CaCl2.
Plant material
Seeds of 24 eucalypt species were obtained from the Caiçara Sementes company (Brejo Alegre, SP) or from the Instituto de Pesquisas e Estudos Florestais (IPEF, Piracicaba, SP), all originating from clonal garden plants. The species used were: Eucalyptus acmenoides Schauer, E. amplifolia Naudin, E. brassiana S.T. Blake, E. camaldulensis Dehnh, Corymbia citriodora (Hook.) K.D. Hill & L.A.S. Johnson, E. deanei Maiden, E. cladocalyx F. Muell., E. dunnii Maiden, E. exserta F. Muell., E. globulus Labill., E. grandis W. Hill ex Maiden, C. maculata (Hook.) K.D. Hill & L.A.S. Johnson, E. microcorys F. Muell., E. pellita F. Muell., C. henryi (S.T. Blake) K.D. Hill & L.A.S. Johnson, E. propinqua Maiden Deane, E. resinifera Sm., E. robusta Sm., E. saligna Sm., E. tereticornis Sm., E. urograndis (E. grandis × E. urophylla), E. urophylla S.T. Blake, E. crebra F. Muell. and E. botryoides Sm (Supplementary Table 2 and Supplementary Fig. 1).
Experimental design and growth conditions
Seeds were germinated in a mix of a commercial garden substrate and soil (1:1, v:v). When the seedlings had four leaf pairs, they were transferred to 7 L pots containing the soil-sand substrate and maintained under micro-sprinkler irrigation. The pots remained in a greenhouse for 23 weeks, at an average maximum temperature of 38.9 °C and average minimum temperature 19.2 °C. The photoperiod varied between 12 and 10 h of light, with a maximum photon flux density of 1800 μmol m−2 s−1. Nitrogen (N) fertilization was applied as NH4NO3 and/or KNO3, on five occasions during the experiment, totalizing 125 mg N per pot.
Growth analysis
Plant height and main stem base diameter were evaluated every two weeks. At harvest, plants were separated into leaves, stems and roots (carefully washed in running tap water), and subsequently used for nutrient analysis.
Determination of leaf and stem macro- and micronutrient concentrations
Nutrients were analysed in leaves and stems of plants from each treatment. P, K, S, Ca, Mg, Fe, Mn, Cu, Zn and B concentrations were determined by inductively coupled plasma optical emission spectrometry (ICP-OES; Varian Vista MPX, Palo Alto, CA, USA) following nitro-perchloric acid digestion of the samples. N concentration was determined using the Kjeldahl method, after sulphuric acid digestion (Bremner 1965). The relative efficiency of P use (REP, %) was calculated according to Neto et al. (2016), where DM is plant dry matter produced either at low or sufficient P:
Agronomic P use efficiency (APE, g DM g−1 P) was calculated using the following equation (Neto et al. 2016):
P uptake efficiency (PUpE, g PShoot g−1 PSoil) was calculated relative to the soil with low P availability using the following equation:
Chlorophyll, anthocyanins and flavonols in leaves
Epidermal chlorophyll, anthocyanin and flavonol indices and the nitrogen balance index (NBI, chlorophyll to flavanols ratio) were determined one week before plant collection for the five plants from each treatment with a Dualex Scientific+™ portable sensor (ForceA, France), using the adaxial side of fully expanded leaves (mature leaves) and the third pair of leaves under the apex (young leaves). These measurements were not performed in E. crebra and E. exserta, which have small and narrow leaves, not compatible with the equipment’s measuring area.
Chlorophyll a fluorescence and gas exchange
Chlorophyll a fluorescence was measured one week before collection, using a portable fluorimeter (FluorPen FP100, Photon Systems Instruments, Czech Republic). Fluorescence was recorded before and after applying a saturating pulse [λ < 710 nm, PPFD ~10,000 μmol photons m−2 s−1, 0.7 s], following PSII excitation with a light pulse [λ = 735 nm, PPFD <15 Wm−2, 3.0 s]. Photochemical variables were measured in leaves that were dark-adapted for 30 min (using a “clamp”) and in light-adapted leaves. For dark-adapted leaves, the following parameters were determined: maximum (Fm), minimum (F0) and variable fluorescence (Fv = Fm - F0), potential quantum efficiency (Fv/fm) and non-photochemical quenching (NPQ). For light-adapted leaves, the effective quantum yield of photosystem II (ФPSII) and fluorescence quantum efficiency (Qy) were determined.
The photosynthetic rate (A), stomatal conductance (gs) and intercellular CO2 concentration (Ci) were measured in young and fully expanded leaves, between 9 h and 14 h, using an infrared gas analyser, IRGA (LI-6400, LI-COR, Lincoln, NE, USA). The measurements were performed under ambient CO2 concentration (≅400 ppm) and light intensity of 1000 μmol photons m−2 s−1, with 10% blue light. Gas exchange measurements and chlorophyll a fluorescence analysis were not performed in E. creba and E. exserta because of their small and narrow leaves.
Statistical analysis
The overall effects of soil P availability and eucalypt species on the analysed growth variables, leaf and stem nutrient concentration, gas exchange and pigment indices were evaluated using a two-factor analysis of variance (two-way ANOVA). Averages were compared using a Tukey test, or the t-Student test, at p ≤ 0.05, using the SISVAR software (Ferreira 2011). A Pearson correlation analysis and the correlation network of the studied variables were performed at p ≤ 0.05, using a t-test to analyse the relationships between variables. Also, principal component analysis (PCA) was performed to reduce the dataset and identify the variables that best explained the highest proportion of total variance, using Rbio (Bhering 2017) and Minitab® 17 (Minitab 17 statistical software) software.
Results
Growth response to soil P availability
Overall, plant growth (shoot biomass, and stem height and diameter) was higher with higher soil P availability (Fig. 1a, and Supplementary Table 3 and Supplementary Figs. 2 and 3). However, the low P availability did not affect the growth of E. camaldulensis, E. cladocalyx, E. globulus, E. tereticornis, E. urograndis and C. maculata, which presented similar shoot biomass production with both soil P availabilities (Fig. 1a). In soil with low P, E. camaldulensis, E. globulus and E. tereticornis presented the highest shoot biomass production and E. crebra and E. acmenoides presented the lowest (Fig. 1a). Considering the root, stem and leaf growth separately, the overall response was similar to that observed for the total shoot mass, with plants grown with higher P availability presenting higher biomass production (Fig. 1a and Supplementary Table 3). E. crebra and E. tereticornis presented the lowest and highest root biomass production under low soil P availability conditions, respectively (Fig. 1b).
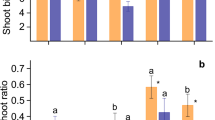
Biomass of shoot (a), root (b) and root to shoot ratio (c) of plants of 24 eucalypt species cultivated in soil with low (dark grey) and sufficient (light grey) availability of P. When displayed, different letters indicate significant difference between means of P levels by the Tukey test (P < 0.05). Bars represent standard error (n = 5)
In 20 of the species studied, soil P availability did not affect the root:shoot ratio (R:S) or the root mass fraction (RMF), which relate biomass allocation to above- and below-ground organs (Fig. 1c and Supplementary Table 3). Contrary to expectation, in some species, such as E. amplifolia, E. deanei, E. microcorys and E. urophylla, the R:S was lower in soil with low P than with sufficient soil P (Fig. 1c). In soil with low P availability, C. henryi and E. crebra presented the lowest R:S, and E. robusta and E. botryoides presented the highest, indicating that these two species allocate more biomass to their roots than other species (Fig. 1c and Supplementary Table 3).
P concentrations in leaves and stems
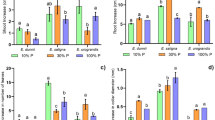
Overall, leaf P concentration was higher for plants grown with sufficient soil P than with low soil P (Fig. 2a). However, a significant number of species (E. amplifolia, E. cladocalyx, E. crebra, E. dunnii, E. exserta, E. globulus, E. microcorys, E. pellita, E. tereticornis, C. citriodora and C. henry) exhibited no significant differences in leaf P concentration between the two soil P availabilities (Fig. 2a). With low soil P, E. botryoides, E. deanei, E. robusta, E. urophylla, E. pellita and E. grandis presented the lowest leaf P concentrations (0.27 to 0.38 g kg−1), and E. amplifolia presented the highest leaf and stem P concentrations (0.86 and 1.15 g kg−1, respectively) (Fig. 2a, B and Supplementary Tables 4 and 5). Under sufficient soil P conditions, E. robusta presented the highest leaf P concentration (1.83 mg kg−1), approximately five times higher than observed for the same species under low soil P conditions (0.38 g kg−1) (Fig. 2a and Supplementary Table 4).
Phosphorus (a, b) and nitrogen (c, d) concentrations and N:P ratio (e, f) in leaves and stems, respectively, of plants of 24 eucalypt species cultivated in soil with low (dark grey) and sufficient (light grey) availability of P. When displayed, different letters indicate significant difference between means of P levels by the Tukey test (P < 0.05). Bars represent standard error (n = 3)
Total P content accumulated per plant (mg P plant−1) was highest for plants growing at sufficient soil P conditions (Fig. 3). Under low soil P conditions, shoot P content varied between 0.62 and 5.13 mg plant−1 and there were no significant differences between species. Variability between species was higher with sufficient soil P, and P content varied between 3.8 and 15.9 mg plant−1, with E. robusta presenting significantly higher shoot P accumulation than the other species (Table 1 and Fig. 3).
Phosphorus (P) content in the shoot of plants of 24 eucalypt species cultivated in soil with low (red) and sufficient (green) availability of P. When displayed, different letters indicate significant difference between means of P levels by the Tukey test (P < 0.05). Bars represent standard error (n = 3)
Uptake of other nutrients
Leaf N concentration was, overall, higher for plants grown under low P conditions, except for E. amplifolia, E. camaldulensis, E. crebra, E. dunnii, E. propinqua, E. resinifera, E. robusta, C. maculata and E. urophylla, which had higher leaf N when grown in sufficient soil P conditions (Fig. 2c). Under low soil P conditions, E. crebra and C. maculata presented the highest leaf N concentrations (14 and 16 g kg−1 N, respectively), whereas E. saligna, E. pellita, E. urophylla, E. botryoides and E. globulus presented N concentrations of approximately 8.5 mg kg−1 (Fig. 2c and Supplementary Table 4). The highest NBI values were observed for plants with more N and grown in soil with low P concentrations (Supplementary Fig. 4).
Most species presented a higher leaf N:P ratio with low soil P than with sufficient soil P, and these ratios were high, between 14 and 34. E. amplifolia, E. exserta, E. microcoris and E. urophylla presented no significant differences in leaf N:P ratios between the two P treatments (Fig. 2e). For the remaining nutrients, soil P availability had a significant effect on leaf Ca, Mg, Cu and Zn concentrations, which were higher with low soil P; no significant effect was observed for S, K, Fe and Mn concentrations (Table 1 and Supplementary Table 4).
The species factor had a significant effect on leaf concentration of all nutrients analysed, except S (Table 1). Under low P, E. crebra presented the highest N, Mn and B concentrations and lowest Ca and Mg concentrations, whereas C. maculata displayed the highest leaf Cu and Fe concentrations (Supplementary Table 4), nutrients that play an important role in photosynthesis. E. camaldulensis was especially efficient in K uptake, presenting one of the highest leaf K concentrations, independent of soil P availability. Leaf Mn concentration varied between 257 mg kg−1 for C. maculata and 597 mg kg−1 for E. robusta (Supplementary Table 4).
P use efficiency and responsiveness to soil P availability
The relative efficiency of P-use (REP) was calculated as the ratio between biomass produced at low P and sufficient P. Among the 24 studied species, E. acmenoides and E. crebra presented the lowest REP (average REP between 12 and 15%) and E. tereticornis and E. globulus the highest (average REP of 90% and 79%, respectively). This wide range of REP reflects the contrasting response of these species regarding P utilization (Table 2). This suggests that REP is a good indicator of PUE, as the species with the highest REP also presented the highest biomass production under low P conditions (Table 2).
Agronomic P use efficiency (APE), which considers both the difference in biomass production between soils with sufficient and low P availability and the difference in available soil P concentration, was highest for E. acmenoides and E. resinifera (313 and 285 g mg−1 P, respectively) and lowest for E. tereticornis and E. globulus (31 and 68 g mg−1 P, respectively) (Table 2). Correlating APE (y-axis) with shoot biomass production in soils with low P availability (x-axis) and defining the average shoot biomass production (6.6 g) and APE (145 g mg−1 P), the eucalypt species were separated depending on their PUE and responsiveness to soil P availability (Hammond et al. 2009, Neto et al. 2016). Species with a higher APE than average may be considered “responsive” to P addition, and those with lower APE than average are regarded as “non-responsive” (Fig. 4). Species with above-average biomass production were considered “efficient” and species with below-average production were “non-efficient” in P utilization.
Relationship between shoot biomass production and the agronomic P-use efficiency (APE) of 24 eucalypt species grown in a soil with low P availability (n = 5). Solid lines indicate the mean value for each axis. NE-R, non-efficient and responsive; E-R, efficient and responsive; E-NR, efficient and non-responsive; NE-NR, non-efficient and non-responsive
Two species, E. grandis and E. saligna, were therefore efficient and responsive (E-R); nine species were non-efficient and responsive (NE-R: E. acmenoides, E. resinifera, E. microcorys, E. dunnii, C. citriodora, E. brassiana, E. deanei, E. crebra and E. propinqua); 11 were efficient and non-responsive (E-NR: E. exserta, E. robusta, E. pellita, E. urophylla, C. henryi, E. botryoides, E. urograndis, E. camaldulensis, E. globulus, E. cladocalyx and E. tereticornis); and two were non-efficient and non-responsive (NE-NR: C. maculata and E. amplifolia). However, the closer species were to the lines dividing the quadrants, the less clear the species behaviour was regarding P efficiency and responsiveness, as observed for quadrant E-R, where no species were prominent, and NE-NR, where only C. maculata was distinct (Fig. 4).
Shoot P content was used to calculate P uptake efficiency (PUpE) under low P availability (Fig. 3). Of the 24 species studied, E. crebra and E. acmenoides presented the lowest PUpE (0.17 and 0.38 g PShoot g−1 PSoil, respectively) and E. exserta and E. globulus exhibited the highest PUpE (1.40 and 1.37 mg PShoot g−1 PSoil, respectively) (Table 2).
REP and PUpE were positively correlated, with the species classified as E-NR presenting the highest REP and PUpE but the lowest APE. According to REP and PUpE, E-NR species were therefore efficient in P uptake at low soil P availability, but with no significant biomass gain at sufficient P (Table 2). However, species classified as NE-R presented the lowest REP and PUpE and the highest APE, presenting an inability to grow under low P availability but responding to sufficient soil P by increasing their biomass production (Table 2).
Photosynthetic traits
The CO2 assimilation rate (A), stomatal conductance (gs), intracellular CO2 concentration (Ci) and carboxylation efficiency (A/Ci) were not significantly affected by soil P availability (Table 1). However, the studied species presented significant differences in all gas exchange parameters (Supplementary Table 6). Noteworthy is that C. maculata presented the highest A (approximately 22 μmol CO2 m−2 s−1), accompanied by high gs values, and E. botryoides and E. dunnii exhibited the lowest A (approximately 6 μmol CO2 m−2 s−1) and low A/Ci and gs values (Supplementary Table 6).
Of the chlorophyll a fluorescence parameters, for fluorescence quantum efficiency (Qy), only E. pellita and E. propinqua responded significantly to soil P variation, where both presented lower Qy with sufficient soil P than with low soil P (Supplementary Table 7). Furthermore, E. deanei presented lower Qy under low P, being the only species with Qy lower than 0.6 (Supplementary Table 7), suggesting impairment of the photochemical capacity of PSII. From NPQ and ФPSII parameters, only E. microcorys displayed significant differences between the two P treatments (Supplementary Table 7).
Chlorophyll, anthocyanin, flavonol, and NBI indices in mature leaves were significantly affected by soil P availability and species (Supplementary Figs. 4, 5, 6 and 7 and Table 1). Overall, chlorophyll indices were significantly higher in leaves of plants grown under low P (Supplementary Fig. 5), whereas flavonol and anthocyanin indices were lower under low P conditions (Supplementary Figs. 6 and 7). The studied eucalypt species presented significant variation in the analysed leaf pigment indices (Table 1).
Pearson correlation and parameter integration via network analysis
To verify the level of association between the studied parameters, the Pearson correlation coefficients (r) between variable pairs were calculated (Supplementary Figs. 8A and 8B) and included in a correlation network (Fig. 5). A higher number of significant correlations were observed for the treatment with low P (230 significant correlations) than for the treatment with sufficient P (112 significant correlations), demonstrating that P deficiency changed the variable-variable relationships and highlighting the importance of P availability for the determination of relationships between nutritional performance and biomass in eucalypt species (Supplementary Figs. 8A and 8B).
Correlation network based on Pearson coefficients derived from the average data for 22 eucalypt species cultivated in soil with sufficient (a) and low (b) availability of P. Trait associated with nutrient in the shoot are shown as red dots. Parameters related to growth and biomass are displayed as grey and blue dots, respectively. Phosphorus efficiency parameters are showed as beige. Gas exchange and chlorophyll a fluorescence are shown as orange dots and pigments as green dots. Green and red connecting lines represent positive and negative correlations, respectively. Line width is proportional to the strength of the correlation. Abbreviations: CO2 assimilation rate (A), stomatal conductance (gs), intercellular CO2 concentration (Ci), non-photochemical quenching (NPQ), instantaneous carboxylation efficiency (A/Ci), effective quantum yield of PSII (ɸPSII), maximum quantum efficiency of PSII (Fv/fm), fluorescence quantum efficiency (Qy), stem diameter (Diamet), plant height (Height), root:shoot ratio (R:S), relative P-use efficiency (REP), agronomic P-use efficiency (APE), P uptake efficiency (PUpE), anthocyanin (Anth), total chlorophyll (Chl), flavonoids (Flav), nitrogen balance index (NBI), macro (N, P, K, S, Ca and Mg) and micronutrient (Fe-Shoot, Cu-Shoot, Zn-Shoot, Mn-Shoot, and B-Shoot) contents in shoots
In the detailed evaluation of the effect of P availability on the studied variables, all biomass parameters were observed to be positively correlated for both treatments (Supplementary Figs. 8A and 8B). Interestingly, a high positive correlation between shoot biomass and root dry matter was observed at low P (r = 0.85), indicating no changes in carbon partitioning (R:S), even at low P availability (Supplementary Fig. 8B). Also, height and diameter were positively correlated with all biomass parameters for this treatment (Fig. 5b and Supplementary Fig. 8B). Moreover, a strong positive correlation between P and Cu content was only observed for the sufficient soil P treatment (r = 0.80), exhibiting higher uptake of these two nutrients at sufficient P levels (Fig. 5a and Supplementary Fig. 8A).
Although P demand is high during photosynthetic carbon assimilation, in the present study, no significant correlations were observed between these parameters, independent of P availability (Fig. 5a and b). However, when the relationship between the gas exchange and chlorophyll a fluorescence data and the remaining parameters was analysed, a clear network of negative correlations between these variables and all dry matter data was observed, especially for the low P treatment (Fig. 5b), indicating that the observed differences in biomass cannot be explained by variations in photosynthetic performance. However, the dense network of positive correlations between nutrient concentration and biomass parameters (Fig. 5b) indicated that the uptake of some macro- and micronutrients, such as N, Ca, Mg and Mn, best explained the variations in total biomass observed for the studied eucalyptus species. In addition, the strong correlations observed between most nutrients and P uptake and use parameters (REP, APE and PUpE) strongly indicated that the mechanisms of P uptake and assimilation also change other macro- and micronutrient contents at a global scale in eucalypt plants grown at low P availability (Fig. 5b and Supplementary Fig. 8B).
Principal component analysis (PCA)
The PCA for all studied parameters is presented in Fig. 6. For the sufficient P treatment, the first (PC1) and second (PC2) components explained 30.4% and 12% of the total variation, respectively (Fig. 6a and b). For low P, the first two components explained more than 60% of data variability, with PC1 and PC2 explaining 47.4% and 13.7% of variability, respectively (Fig. 6c and d).
Principal component analysis (PCA) from 22 eucalypt species cultivated in soil with sufficient (a and b) and low (c and d) availability of P. The analysis was performed on the correlation matrix of least square means of averaged accessions. Numbers in parentheses give the percent variation explained by the first and the second principal component. a and (c) show the scores and (b) and (d) show the loading plots obtained for the resulting distribution of the 22 eucalypt species and all parameters analyzed, respectively. Text colors indicate the cluster to which parameters have been assigned (Blue, nutrient contents; red, phosphorus efficiency parameters; orange, gas exchange and chlorophyll a fluorescence; lilac, pigments; black, growth and green, biomass). Abbreviations: CO2 assimilation rate (A), stomatal conductance (gs), intercellular CO2 concentration (Ci), non-photochemical quenching (NPQ), instantaneous carboxylation efficiency (A/Ci), effective quantum yield of PSII (ɸPSII), maximum quantum efficiency of PSII (Fv/fm), fluorescence quantum efficiency (Qy), stem diameter (Diameter), plant height (Height), root:shoot ratio (R:S), relative efficiency of P-use (REP), agronomic P-use efficiency (APE), P-uptake efficiency (PUpE), anthocyanin (Anth), total chlorophyll (Chl), flavonoids (Flav), nitrogen balance index (NBI), macro (N-Shoot, P-Shoot, K-Shoot, S-Shoot, Ca-Shoot and Mg-Shoot) and micronutrient (Fe-Shoot, Cu-Shoot, Zn-Shoot, Mn-Shoot, and B-Shoot) contents in shoots
The species and variable distribution along the first component revealed that, for the treatment with sufficient P (Fig. 6a and b), E. deanei, E. urophylla, E. resinifera, E. saligna and E. botryoides were grouped separately from C. maculata and E. cladocalyx (Fig. 6a). For the first group, this separation was determined by biomass parameters, especially for total biomass, root and shoot dry matter (Fig. 6b). However, C. maculata and E. cladocalyx were grouped primarily due to the high variance in photosynthetic parameters, such as A, gs, A/Ci, ɸPSII and total chlorophyll indices. Given the negative correlations observed between photosynthetic parameters and biomass accumulation (Fig. 6b), it is evident that the eucalypt species with higher total biomass gain (e.g., E. deanei and E. urophylla) did not necessarily present higher photosynthetic efficiency, which supports the antagonistic interactions observed in the previously discussed pairing studies. However, when the nutrient contents were analysed, Mn, Mg, Ca and N contents were observed to be grouped with the biomass data, indicating that these nutrients were more determinant to biomass gain in these species (Fig. 6b).
Regarding PC2, E. globulus and E. urograndis were dispersed in opposite directions to C. henryi (Fig. 6a). Based on the loading plot, the R:S was the variable that most contributed to the displacement of E. globulus and E. urograndis along PC2. However, the high values observed for leaf biomass, Fv/fm and shoot P content, contributed to C. henryi being grouped separately from the remaining species (Fig. 6b). Also, the analysis of PC2 indicated that variations in leaf, not root, dry matter were more determinant to change the R:S. Also, the observed grouping between leaf dry matter and P contents indicates that P directly contributed to this partitioning.
The PCA for low P revealed a clear species separation along PC1 (Fig. 6c). Similar to the sufficient P treatment, biomass parameters presented the highest PC1 score (Fig. 6d). However, different from the sufficient P treatment, analysis of the dispersal of the studied species indicated a positive relationship with dry matter data for E. tereticornis, E. globulus, E. botryoides and E. camaldulensis, and a negative relationship for E. acmenoides (Fig. 6c), indicating that their total biomass decreased due to low P availability. Also, a positive correlation was observed between biomass parameters and Mg, N and Ca contents, indicating that the species with increased dry weight also presented higher contents of these nutrients (Fig. 6d). For PC2, C. maculata, E. amplifolia and E. cladocalyx were grouped based on variations in photosynthetic parameters (Fig. 6c and d). However, similar to sufficient P treatment, the observed biomass variations were not explained by increased photosynthesis. The biomass parameters and part of the nutrient contents were, therefore, more effective in identifying the most P responsive and P-utilization efficient species.
Discussion
Eucalypt species respond differently to P availability
In this study, the growth of young plants of the 24 studied eucalypt species was strongly affected by the low soil P availability, affecting shoot and root biomass allocation. The comparison between plants grown in soil with low and sufficient P presented a wide variation between species in their ability to use P, indicating genetic variability in their responses to P limitation. Characteristics related to growth, nutrient uptake and photosynthetic capacity also indicated species plasticity in response to P availability. In soils with low P availability, uptake at the root surface is primarily limited by the diffusion rate to the root (Clarkson 1981). The size and architecture of the root system, which will determine the plant’s capacity to exploring the soil, become therefore especially important for root P-uptake efficiency (Gamuyao et al. 2012). The conditions studied are different from previous studies using nutrient solutions (Warren 2011; Bahar et al. 2018), where the main pathway of P movement to the roots is by mass flow (Hayes et al. 2004).
Overall, eucalypt species exhibited a positive growth response to sufficient soil P when compared to low soil P treatments (Table 1). Plants under P limitation had lower biomass production, leaf area and stem height and/or diameter (Fig. 1, Supplementary Figs. 2 and 3 and Supplementary Table 3). Thomas et al. (2006) reported increased growth of E. grandis with increasing soil P concentration, resulting in higher biomass production and leaf area. Gotore et al. (2014) also observed a positive effect of P fertilization on growth of E. grandis and other tree species, such as Pinus patula. More recently, Crous et al. (2015) observed that higher P availability increased the growth of mature trees of E. tereticornis in the region of Cumberland Plain Woodland, Australia.
Phosphorus responsiveness and growth efficiency under low P availability
The biomass data showed that most eucalypt species were classified as NE-R and E-NR (Fig. 4). The study of these divergent species will, therefore, allow the identification of the mechanisms involved in PUE, here evaluated as REP, and response capacity (APE). According to this perspective, six species – E. tereticornis, E. cladocalyx, C. maculata, E. urograndis, E. camaldulensis and E. globulus – presented no statistically significant differences in shoot biomass accumulation between the soils with low and sufficient P (Fig. 1a and Supplementary Table 3). Interestingly, all these species, except C. maculata, were classified as E-NR (Fig. 4). This result, together with REP and APE (Table 2), indicates that PUE in these species is strongly associated with lower variation in accumulated biomass between higher and lower soil P availability conditions. Another interesting observation was that, overall, NE-R species presented low growth rates whereas E-NR species presented relatively higher growth rates and the lowest APE (Supplementary Figs. 2 and 3 and Table 2).
The lower responsiveness to P can be attributed to the fact that maximum growth potential was attained already with lower P availability, or because the species simply did not possess a mechanism of P acquisition and use at higher P availability able to result in growth. Thus, whilst P-efficiency is related to the capacity to grow under low P inputs, responsiveness is associated with increased growth potential with additional P-inputs. Therefore, non-responsiveness to P addition can be indicative of high PUE under low P, which is a wanted trait as long as yields are maintained (Hammond et al., 2009).
Some forest species are better adapted to low P and can grow faster at lower soil P availability (Turner et al. 2018). Species with this behaviour decrease their overall need of leaf P, decreasing allocation into the metabolic fraction so that the plants adapt to low P conditions (Hidaka and Kitayama 2011). Moreover, the possibility cannot be excluded that differences in species P-demand exist, leading to the variations in biomass accumulation observed for plants grown in low soil P conditions; i.e., if the species differ in P critical demand due to differences in uptake efficiency, they may also differ in the degree of stress when there is no addition of external P. The differences in growth potential at low P between species, therefore, explain the variations in REP. It has been suggested that species with higher P-uptake capacity and lower demand for external P may achieve higher growth rates at lower internal P (Turner et al. 2018). In the present study, overall, species with higher PUpE (Table 2) also presented higher growth at low P (Supplementary Figs. 2 and 3), but not necessarily with lower tissue P (leaf and/or stem) Fig. 2a and b). Interestingly, low P availability resulted in higher biomass accumulation and increased stem diameter and height in E. tereticornis, indicating that this species is well adapted to low soil P availability. In this species, this higher capacity may also be explained by higher root mass, which may have promoted the uptake of P and other nutrients. On the other hand, E. tereticornis, which showed the highest REP (Fig. 4), had little variation regarding growth (Fig. 1a, b, Supplementary Figs. 2 and 3), nutritional (Supplementary Tables 4 and 5) and photosynthesis-related data (Supplementary Table 6) in response to soil P availability, indicating that in this species the adaptations to P limitation may be related to cell metabolic changes in P utilization, resulting in increased P-efficiency.
Root growth is not necessarily related with low P availability
Root biomass production was one of the allometric characteristics that exhibited higher variability than those related to shoot growth, both between genotypes and different soil P availability (Table 1 and Fig. 1b). However, the response to P availability was not always consistent for all species, which may reflect the different strategies of P uptake and use among eucalypt species. As an overall strategy to increase the soil volume explored and uptake capacity, plants subjected to nutrient limitations allocate more carbon to the roots, increasing root growth and altering their architecture (Wrage et al. 2010; Kramer-Walter and Laughlin 2017). This change in photoassimilate carbon partition leads to an increased R:S in plants under P limitation (Ryser and Lambers 1995). Here, the R:S of most studied species did not change significantly in response to P, or, in some species – E. amplifolia, E. deanei, E. microcorys and E. urophilla – decreased (Fig. 1c), due to lower relative root growth under P limitation conditions. This probably indicates that biomass allocation to the root may be relatively insensitive to soil P availability in eucalypt plants (Fig. 1c). Low R:S ratios under P-limitation can be because eucalypts may in part rely P-uptake on mycorrhizal symbionts (Adams et al. 2006). Similarly, Santiago et al. (2012) observed no changes in the R:S in tropical forest species in response to P addition to the soil, when compared to plants that received no P. In the tropical tree Ficus insipida, biomass allocation to the roots responded positively to N but not to P availability, resulting in no changes to the R:S under these conditions (Garrish et al. 2010). Some authors have suggested that in tree species, the investment in wood may affect carbon allocation between roots and shoot under nutrient limitation (Poorter et al. 2012). This could explain the weak relationship between the R:S and low soil P in eucalypts plants. This investment in wood may be related to an increased lignification rate induced by P deficiency (Uhde-Stone et al., 2003).
Investing in root growth is not a guarantee of high P uptake
When the interspecific response for each soil P availability level was isolated, E. robusta and E. botryoides, classified as E-NR (Fig. 4), were observed to present low leaf P concentration under P limitation (Fig. 2a) and the highest R:S (Fig. 1c), indicating that higher investment in roots did not necessarily result in higher P uptake efficiency. E. exerta and E. globulus presented the highest PUpE under P limitation (Table 2), indicating that under limiting conditions they must have efficient P uptake mechanisms, such as induction of high P affinity transporters, ability to form efficient mycorrhizal symbioses, and changes in root morphology, which do not necessarily alter root biomass. Also, overall, the R:S was not significantly correlated with higher uptake of other nutrients from the soil, as species with a higher R:S did not present higher nutrient concentrations (Fig. 1c, Supplementary Tables 3 and 4). However, PUpE was positively correlated with root and shoot biomass (Supplementary Fig. 8B), indicating that species with higher root production were more efficient in P uptake, which may have led to higher shoot biomass, without changing R:S ratio.
P uptake and accumulation was not related with P responsiveness
Twelve of the studied species presented no differences in leaf P concentrations between plants grown in low and sufficient soil P availability (Fig. 2a). This could also be explained by the association of eucalyptus plants with mycorrhizal fungi. Several studies have revealed strong colonization of eucalypts by these soil microorganisms (Warcup 1980). Also, plants in symbiosis with mycorrhizal fungi may present a lower R:S and root biomass, because extra-radicular hyphae act as extensions of the root system, increasing the soil volume explored by plants and plant nutrient uptake (Smith and Read 2010). P availability also affected the soil microbiota associated with tree species (Zavišić et al. 2016), inducing the release to the rhizosphere of enzymes that solubilize P retained on soil particles (Richardson and Simpson 2011).
Plants and microorganisms may release organic acids and protons, which may dissolve P retained on soil particles into the soil solution allowing their uptake by roots. Some eucalyptus species, such as E. gummifera, can access insoluble inorganic P forms, such as Al and Fe phosphates, indicating organic acid exudation (Mullette et al. 1974). It has been suggested that plants able to mobilize soil P through organic acid exudation tend to have higher leaf Mn concentrations (Lambers et al. 2015) due to Mn mobilization from soil particles. However, this strategy does not seem to occur in eucalyptus, because most species present lower leaf Mn concentrations in plants at sufficient-P than at low-P, what might indicate the release of carboxylates (Table 1 and Supplementary Table 4). In E. camaldulensis and the hybrid E. grandis × E. urophylla, a relationship between root organic acid exudation and Al tolerance was previously observed (Ikka et al. 2013; Teng et al. 2018).
Leaf P was between 0.28 and 1.83 g kg−1 (Supplementary Table 4), indicating high variability in P uptake capacity and accumulation in leaves. However, shoot P content (Fig. 3) and, leaves and stem P concentrations (Fig. 2a and b) in young eucalypt plants were not necessarily associated with gains in biomass production (Fig. 1a and Supplementary Table 3), or vice-versa; i.e., higher biomass production was not always positively correlated with higher tissue P concentration. Species with high responsiveness to P, such as E. dunii, E. microcorys and C. citriodora (Fig. 4), therefore presented similar shoot P contents at low and sufficient soil P availability levels (Fig. 3), and species without significant biomass gain resulting from higher soil P availability, i.e., non-responsive species, such as E. tereticornis and E. camaldulensis (Fig. 4), had higher shoot P contents at higher P availability. This may be explained by the fact that gains in biomass production are not always of the same magnitude as P accumulation in a plant (Marschner 1995), because Pi can be stored in vacuoles, where it does not contribute to physiological processes that lead to increased biomass (Bieleski 1973; Martinez et al. 2005). In the present study, because there was no significant correlation between leaf P content and biomass production (Supplementary Figs. 8A and 8B), leaf P concentration or even shoot P content can not be considered an indication of responsiveness to P in young eucalypt plants.
Relationships between P and other nutrients
The multivariate analysis revealed group formation between elements such as K and Ca, and P, in the low P treatment (Supplementary Fig. 8 and Fig. 6a). Complementarily, the positive correlation of leaf P with other nutrients, such as B, Mn, Zn and Mg (Fig. 5b), indicates that in species with higher P uptake capacity, such as C. henryi, E. camaldulensis, E. tereticornis and E. globulus, the adopted P-acquisition strategies may contribute to mobilize (Lynch 2011) and promote the accumulation of other nutrients (Suriyagoda et al. 2010; He et al. 2017), thus allowing plants to maintain metabolic functions. Such P-acquisition strategies may include the association with root symbionts, altered morphology of the roots or root exudation to mobilize nutrients in the rhizosphere (Lambers et al. 2011). This hypothesis is also supported by the fact that the same was not observed at higher soil P availability (Fig. 5a).
In the present study, other nutrients were not limiting, and during the period of plant growth, N was added in the same amounts for the treatments with low and sufficient P, as top-dressing fertilization by fertirrigation. The plant N:P ratio may indicate which of the two nutrients is more limiting at a given condition; a shoot N:P lower than 14 may indicate N limitation, between 14 and 16 co-limitation and higher than 16 P limitation (Koerselman and Meuleman 1996; Elser et al. 2007; Lambers et al. 2011). Here, most eucalypt species presented a higher leaf N:P under P limitation than with sufficient soil P (Fig. 2e). The N:P was negatively correlated to soil P availability and very high under low P (up to 34), indicating P limitation under these conditions (Supplementary Table 4). In addition, when analyzed the relationships between N and P concentrations and N:P ratios in leaves and stems, it was observed that N:P ratio and N concentrations were largely unrelated, while N:P ratios correlated negatively with P concentration (Fig. 2e, f). This suggests that interspecific variation in N:P ratios was therefore primarily determined by variation in P concentration as observed by Güeswell (2004) (Supplementary Fig. 9). However, the possibility that some species under P limitation have mechanisms that increase the N uptake effectiveness relative to P cannot be excluded, which, together with the very low P concentrations, may have contributed to the high N:P observed.
Photosynthetic traits were not related with P concentration in leaves
In the present study, the leaf chlorophyll indices of several eucalypt species were higher with low soil P than with sufficient soil P (Supplementary Fig. 5). Thomas et al. (2006) observed that low soil P concentration did not cause chlorophyll decrease in E. grandis. Because we observed a high N:P in most species studied, the higher amount of chlorophyll may reflect an excess of N in plants grown with low soil P availability; i.e., the N applied was possibly not fully used by the plant’s metabolism because of P limitation but was used in chlorophyll biosynthesis.
Interestingly, in all species leaf N concentration was higher under low P than under sufficient P availability (Fig. 2c), but only E. globulus, E. tereticornis, E. robusta, E. botryoides and C. maculata presented a significant decrease in photosynthetic nitrogen use efficiency (PNUE) (approximately 50%) compared with plants grown with sufficient P (data not shown).
Bahar et al. (2018) studied photosynthesis in 17 eucalypt species grown in sand and a low P nutrient solution. Unfortunately, only two species coincided with those in our study, E. grandis and E. camaldulensis. Bahar et al. (2018) observed decreased photosynthesis and respiration, but increased leaf mass area, under P deficiency conditions. They also observed decreased photosynthetic nitrogen use efficiency (PNUE) in these plants and proposed that this could be related to the higher leaf mass area. This indicates that higher N allocation to leaf growth would have occurred at the expense of N allocation to the photosynthetic machinery.
Although low soil P availability frequently decreases the photosynthetic capacity of tree species (Bloomfield et al. 2014), in the present study, overall, the photosynthesis rate (A) of plants under P limitation was not affected by low P availability, with higher variability being observed for the genotype (Table 1). In this sense, it has been reported that whereas P-limitation is primarily reflected in growth reduction, with decreased leaf number and area (Fredeen et al. 1989; Turnbull et al. 2007; Bahar et al. 2018), processes such as photosynthesis may or may not be affected by P-limitation (Chandler and Dale 1993; Bahar et al. 2018). Some studies have suggested that P deficiency firstly restricts leaf expansion, which directly affects biomass production, and that reduction of photosynthesis may only occur at very low P concentrations (0.05 mM) (Waddell et al. 2016, Fredeen et al., 1989). Complementarily, some studies have shown that low inorganic P concentrations in the cytosol, resulting from inorganic P sequestration by mannose, strongly limit photosynthesis in E. globulus (Turnbull et al. 2007). In 2.5-month-old seedlings of E. globulus, P limitation decreased intracellular Pi concentrations and sugar phosphates (glucose-6-P and fructose-6-P) to a greater degree than photosynthesis, indicating higher PUE in photosynthesis under P deficiency. The authors concluded that the plants remained under continuous low P stress long enough to establish functional homeostasis between photosynthesis, respiration and growth (Warren 2011). Our experiment also exposed eucalypts to low P stress for approximately six months (23 weeks), enough time to establish a balance between growth and the photosynthesis rate (Warren 2011). In addition, the low photosynthetic response to P limitation (Supplementary Table 6) may be related to the positive effect of high N availability for these plants, which accumulate chlorophyll and leaf N (Supplementary Fig. 5 and Fig. 2c, respectively), which may favour plant investment in photosynthetic machinery components, such as chlorophyll molecules.
In our study, the highest leaf N was observed for C. maculata (Fig. 2c), which presented the highest photosynthetic rate (A) among the 22 species evaluated for this characteristic (Supplementary Table 6). We believe that this may be related to higher investment in photosynthesis, per leaf area, because CO2 assimilation is strongly correlated with leaf N levels, either expressed per leaf area or weight (Wright et al. 2005; Lloyd et al. 2013). It has been reported that higher N allocation to components such as chlorophyll may not be associated with higher photosynthetic N use efficiency under P limitation, indicating that P availability may change leaf N allocation to processes unrelated with photosynthesis or that less leaf N is metabolically active in chloroplasts (Bloomfield et al. 2014; Bahar et al. 2018).
Conclusions
Under the experimental conditions, young plants of the studied eucalypt species presented clear differences in biomass production efficiency under low P availability and responsiveness to increased soil P concentration. Eucalypts had two contrasting responses: i) efficient P-use under low soil P concentrations, without responding to P addition, or ii) inefficient P-use, with high growth restrictions under P limitation, but a highly positive growth response to increasing soil P availability. The most efficient species were therefore also the least responsive, whereas growth was inhibited among the most responsive species under limited P availability. These responses were not directly related to photosynthetic capacity, with efficient P-allocation to the leaves, but were more related to P-uptake capacity under P limitation and P-use efficiency for biomass production. Consequently, there should be significant variability between species regarding the physiological mechanisms that increase P uptake efficiency in soils with low P availability. An understanding of the physiological and molecular mechanisms involved in the responses of species with contrasting P utilization efficiencies and responsiveness to P addition may contribute to improve the management of eucalypt plantations and decrease P utilization. Measures taken towards meeting this objective would increase the sustainability of eucalypt plantations, which occupy large areas in several countries.
References
Adams F, Reddell P, Webb MJ, Shipton WA (2006) Arbuscular mycorrhizas and ectomycorrhizas on Eucalyptus grandis (Myrtaceae) trees and seedlings in native forests of tropical North-Eastern Australia. Aust J Bot 54:271–281. https://doi.org/10.1071/BT05028
Bahar NHA, Gauthier PPG, O’Sullivan OS et al (2018) Phosphorus deficiency alters scaling relationships between leaf gas exchange and associated traits in a wide range of contrasting Eucalyptus species. Funct Plant Biol 45:813–826. https://doi.org/10.1071/FP17134
Bhering LL (2017) Rbio: a tool for biometric and statistical analysis using the R platform. Crop Breed Appl Biotechnol 17:187–190. https://doi.org/10.1590/1984-70332017v17n2s29
Bieleski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol 24:225–252. https://doi.org/10.1146/annurev.pp.24.060173.001301
Blair G (1993) Nutrient efficiency — what do we really mean?. In: Randall P.J., Delhaize E., Richards R.A., Munns R. (eds) Genetic Aspects of Plant Mineral Nutrition. Developments in Plant and Soil Sciences, vol 50. Springer, Dordrecht. pp 205–213
Bloomfield KJ, Farquhar GD, Lloyd J (2014) Photosynthesis–nitrogen relationships in tropical forest tree species as affected by soil phosphorus availability: a controlled environment study. Funct Plant Biol 41:820–832. https://doi.org/10.1071/FP13278
Bremner JM (1965) Total nitrogen. In: Black CA (ed) Methods of soil analysis. American Society of Agronomy, Madison, pp 1149–1178
Chandler JW, Dale JE (1993) Photosynthesis and nutrient supply in needles of Sitka spruce [Picea sitchensis (bong.) Carr.]. New Phytol 125:101–111. https://doi.org/10.1111/j.1469-8137.1993.tb03868.x
Cheng L, Bucciarelli B, Liu J et al (2011) White lupin cluster root acclimation to phosphorus deficiency and root hair development involve unique glycerophosphodiester phosphodiesterases. Plant Physiol 156:1131–1148. https://doi.org/10.1104/pp.111.173724
Chowdhury RB, Moore GA, Weatherley AJ, Arora M (2017) Key sustainability challenges for the global phosphorus resource, their implications for global food security, and options for mitigation. J Clean Prod 140:945–963. https://doi.org/10.1016/j.jclepro.2016.07.012
Clarkson DT (1981) Nutrient interception and transport by root systems. In: Johnson, C.B. ed. Physiological processes limiting plant productivity. Butterworths, London, 307–330
Clarkson DT, Hanson JB (1980) The mineral nutrition of higher plants. Annu Rev Plant Physiol 31:239–298. https://doi.org/10.1146/annurev.pp.31.060180.001323
Crous KY, Ósvaldsson A, Ellsworth DS (2015) Is phosphorus limiting in a mature Eucalyptus woodland? Phosphorus fertilisation stimulates stem growth. Plant Soil 391:293–305. https://doi.org/10.1007/s11104-015-2426-4
Del-Saz NF, Romero-Munar A, Cawthray GR et al (2018) Phosphorus concentration coordinates a respiratory bypass, synthesis and exudation of citrate, and the expression of high-affinity phosphorus transporters in Solanum lycopersicum. Plant Cell Environ 41:865–875. https://doi.org/10.1111/pce.13155
Dörmann P, Benning C, Joyard J et al (2002) Galactolipids rule in seed plants. Trends Plant Sci 7:112–118. https://doi.org/10.1016/S1360-1385(01)02216-6
Elser JJ, Bracken MES, Cleland EE et al (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142. https://doi.org/10.1111/j.1461-0248.2007.01113.x
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia 35:1039–1042. https://doi.org/10.1590/S1413-70542011000600001
Fixen PE, Johnston AM (2012) World fertilizer nutrient reserves: a view to the future. J Sci Food Agric 92:1001–1005. https://doi.org/10.1002/jsfa.4532
Fredeen AL, Rao IM, Terry N (1989) Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol 89:225–230. https://doi.org/10.1104/pp.89.1.225
Friesen DK, Rao IM, Thomas RJ et al (1997) Phosphorus acquisition and cycling in crop and pasture systems in low fertility tropical soils. Plant Soil 196:289–294. https://doi.org/10.1023/A:1004226708485
Gamuyao R, Chin JH, Pariasca-Tanaka J et al (2012) The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 488:535–539. https://doi.org/10.1038/nature11346
Garrish V, Cernusak LA, Winter K, Turner BL (2010) Nitrogen to phosphorus ratio of plant biomass versus soil solution in a tropical pioneer tree, Ficus insipida. J Exp Bot 61:3735–3748. https://doi.org/10.1093/jxb/erq183
Gerloff GC (1977) Plant efficiencies in the use of nitrogen, phosphorus and potassium. In: Wright MJ (ed) Plant adaptation to mineral stress in problem soils. Cornell University Press, New York, pp 161–174
Gomez-Roldan V, Fermas S, Brewer PB et al (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194. https://doi.org/10.1038/nature07271
Gotore T, Murepa R, Gapare WJ (2014) Effects of nitrogen, phosphorus and potassium on the early growth of Pinus patula and Eucalyptus grandis. J Trop For Sci 26:22–31. https://doi.org/10.2307/23617010
Grattapaglia D, Kirst M (2008) Eucalyptus applied genomics: from gene sequences to breeding tools. New Phytol 179:911–929. https://doi.org/10.1111/j.1469-8137.2008.02503.x
Güeswell S (2004) N: P ratios in terrestrial plants: Variation and functional significance. New Phytologist 164:243–266
Hammond JP, Broadley MR, White PJ et al (2009) Shoot yield drives phosphorus use efficiency in Brassica oleracea and correlates with root architecture traits. J Exp Bot 60:1953–1968. https://doi.org/10.1093/jxb/erp083
Hayes JE, Zhu YG, Mimura T, Reid RJ (2004) An assessment of the usefulness of solution culture in screening for phosphorus efficiency in wheat. Plant Soil 261:91–97. https://doi.org/10.1023/B:PLSO.0000035561.00460.8b
He H, Peng Q, Wang X et al (2017) Growth, morphological and physiological responses of alfalfa (Medicago sativa) to phosphorus supply in two alkaline soils. Plant Soil 416:565–584. https://doi.org/10.1007/s11104-017-3242-9
Hidaka A, Kitayama K (2011) Allocation of foliar phosphorus fractions and leaf traits of tropical tree species in response to decreased soil phosphorus availability on mount Kinabalu, Borneo. J Ecol 99:849–857. https://doi.org/10.1111/j.1365-2745.2011.01805.x
Huang G, Hayes PE, Ryan MH et al (2017) Peppermint trees shift their phosphorus-acquisition strategy along a strong gradient of plant-available phosphorus by increasing their transpiration at very low phosphorus availability. Oecologia 185:387–400. https://doi.org/10.1007/s00442-017-3961-x
Ikka T, Ogawa T, Li D et al (2013) Effect of aluminum on metabolism of organic acids and chemical forms of aluminum in root tips of Eucalyptus camaldulensis Dehnh. Phytochemistry 94:142–147. https://doi.org/10.1016/j.phytochem.2013.06.016
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps
Kelly AA, Froehlich JE, Dörmann P (2003) Disruption of the two digalactosyldiacylglycerol synthase genes DGD1 and DGD2 in Arabidopsis reveals the existence of an additional enzyme of galactolipid synthesis. Plant Cell 15:2694–2706. https://doi.org/10.1105/tpc016675
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450. https://doi.org/10.2307/2404783
Kramer-Walter KR, Laughlin DC (2017) Root nutrient concentration and biomass allocation are more plastic than morphological traits in response to nutrient limitation. Plant Soil 416:539–550. https://doi.org/10.1007/s11104-017-3234-9
Lambers H, Plaxton WC (2018) Phosphorus: back to the roots. Annu Plant Rev:3–22. https://doi.org/10.1002/9781119312994.apr0516
Lambers H, Brundrett MC, Raven JA, Hopper SD (2011) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 348:7–27. https://doi.org/10.1007/s11104-011-0977-6
Lambers H, Hayes PE, Laliberté E et al (2015) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90
Lloyd J, Bloomfield K, Domingues TF, Farquhar GD (2013) Photosynthetically relevant foliar traits correlating better on a mass vs an area basis: of ecophysiological relevance or just a case of mathematical imperatives and statistical quicksand? New Phytol 199:311–321. https://doi.org/10.1111/nph.12281
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156:1041–1049. https://doi.org/10.1104/pp.111.175414
Marschner H (1995) The mineral nutrition of higher plants. Academic Press, London
Martinez HEP, Novais RF, Rodrigues LA, de Sacramento LVS (2005) Phosphate forms in plant and their internal buffering in five soybean cultivars. Rev Bras Ciência do Solo 29:249–257. https://doi.org/10.1590/S0100-06832005000200010
Morcuende R, Bari R, Gibon Y et al (2007) Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ 30:85–112. https://doi.org/10.1111/j.1365-3040.2006.01608.x
Moura JCMS, Bonine CAV, de Oliveira Fernandes Viana J et al (2010) Abiotic and biotic stresses and changes in the lignin content and composition in plants. J Integr Plant Biol 52:360–376. https://doi.org/10.1111/j.1744-7909.2010.00892.x
Mullette KJ, Hannon NJ, Elliott AGL (1974) Insoluble phosphorus usage by Eucalyptus. Plant Soil 41:199–205. https://doi.org/10.1007/BF00017956
Neto AP, Favarin JL, Hammond JP et al (2016) Analysis of phosphorus use efficiency traits in Coffea genotypes reveals Coffea arabica and Coffea canephora have contrasting phosphorus uptake and utilization efficiencies. Front Plant Sci 7:408. https://doi.org/10.3389/fpls.2016.00408
Neumann G (2016) The role of ethylene in plant adaptations for phosphate acquisition in soils – a review. Front Plant Sci 6:1–9. https://doi.org/10.3389/fpls.2015.01224
Niu YF, Chai RS, Jin GL et al (2013) Responses of root architecture development to low phosphorus availability: a review. Ann Bot 112:391–408. https://doi.org/10.1093/aob/mcs285
Novaes E (2010) Integrating genomics and quantitative genetics for discovery of genes that regulate bioenergy traits in woody species. University of Florida, Gainesville
Ozturk L, Eker S, Torun B, Cakmak I (2005) Variation in phosphorus efficiency among 73 bread and durum wheat genotypes grown in a phosphorus-deficient calcareous soil. Plant Soil 269:69–80. https://doi.org/10.1007/s11104-004-0469-z
Pérez-Cruzado C, Merino A, Rodríguez-Soalleiro R (2011) A management tool for estimating bioenergy production and carbon sequestration in Eucalyptus globulus and Eucalyptus nitens grown as short rotation woody crops in north-West Spain. Biomass Bioenergy 35:2839–2851. https://doi.org/10.1016/j.biombioe.2011.03.020
Poorter H, Niklas KJ, Reich PB et al (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50. https://doi.org/10.1111/j.1469-8137.2011.03952.x
Raij B, van & Quaggio JA (2001) Determinação de fósforo, cálcio, magnésio e potássio extraídos com resina trocadora de íons. In: Raij, B.van; Andrade, J.C.; Cantarella, H. & Quaggio, J.A., eds. Análise química para avaliação da fertilidade de solos tropicais. Campinas, Secretaria de Agricultura e Abastecimento, APTA/IAC, 2001. p.189–199
Raij BV, Cantarella H, Quaggio JA, Furlani AMC (1997) Recomendações de adubação e calagem para o estado de São Paulo. Bol Técnico 100
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol 156:989–996. https://doi.org/10.1104/pp.111.175448
Robinson WD, Park J, Tran HT et al (2012) The secreted purple acid phosphatase isozymes AtPAP12 and AtPAP26 play a pivotal role in extracellular phosphate-scavenging by Arabidopsis thaliana. J Exp Bot 63:6531–6542. https://doi.org/10.1093/jxb/ers309
Ryser P, Lambers H (1995) Root and leaf attributes accounting for the performance of fast- and slow-growing grasses at different nutrient supply. Plant Soil 170:251–265. https://doi.org/10.1007/BF00010478
Salazar-Henao JE, Lin W-D, Schmidt W et al (2016) Discriminative gene co-expression network analysis uncovers novel modules involved in the formation of phosphate deficiency-induced root hairs in Arabidopsis. Sci Rep 6:26820. https://doi.org/10.1038/srep26820
Santana RC, Barros NF, Neves JCL (2002) Eficiência de utilização de nutrientes e sustentabilidade da produção em procedências de Eucalyptus grandis e Eucalyptus saligna em sítios florestais do Estado de São Paulo. Rev Árvore 26:447–457
Santiago LS, Wright SJ, Harms KE et al (2012) Tropical tree seedling growth responses to nitrogen, phosphorus and potassium addition. J Ecol 100:309–316. https://doi.org/10.1111/j.1365-2745.2011.01904.x
Smith S, Read D (2010) Mycorrhizal Symbiosis. Academic Press, London
Suriyagoda LDB, Ryan MH, Renton M, Lambers H (2010) Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus- and moisture-limited environments. Ann Bot 105:755–767. https://doi.org/10.1093/aob/mcq040
Teng W, Kang Y, Hou W et al (2018) Phosphorus application reduces aluminum toxicity in two Eucalyptus clones by increasing its accumulation in roots and decreasing its content in leaves. PLoS One 13:1–20. https://doi.org/10.1371/journal.pone.0190900
Thomas DS, Montagu KD, Conroy JP (2006) Leaf inorganic phosphorus as a potential indicator of phosphorus status, photosynthesis and growth of Eucalyptus grandis seedlings. For Ecol Manag 223:267–274. https://doi.org/10.1016/j.foreco.2005.11.006
Turnbull TL, Adams MA, Warren CR (2007) Increased photosynthesis following partial defoliation of field-grown Eucalyptus globulus seedlings is not caused by increased leaf nitrogen. Tree Physiol 27:1481–1492. https://doi.org/10.1093/treephys/27.10.1481
Turner BL, Brenes-Arguedas T, Condit R (2018) Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 555:367–370. https://doi.org/10.1038/nature25789
Uhde-Stone C, Zinn KE, Ramirez-Yáñez M et al (2003) Nylon filter arrays reveal differential gene expression in proteoid roots of white lupin in response to phosphorus deficiency. Plant Physiol 131:1064–1079. https://doi.org/10.1104/pp.102.016881
van de Wiel CCM, van der Linden CG, Scholten OE (2016) Improving phosphorus use efficiency in agriculture: opportunities for breeding. Euphytica 207:1–22. https://doi.org/10.1007/s10681-015-1572-3
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447. https://doi.org/10.1046/j.1469-8137.2003.00695.x
Veneklaas EJ, Lambers H, Bragg J et al (2012) Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol 195:306–320. https://doi.org/10.1111/j.1469-8137.2012.04190.x
Waddell HA, Simpson RJ, Lambers H et al (2016) Phosphorus-utilisation efficiency and leaf-morphology traits of Rytidosperma species (wallaby grasses) that differ in their growth response to phosphorus fertilisation. Aust J Bot 64:65–76. https://doi.org/10.1071/BT15202
Wang YP, Law RM, Pak B (2010) A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeosciences 7:2261–2282. https://doi.org/10.5194/bg-7-2261-2010
Warcup JH (1980) Ectomycorrhizal associations of Australian indigenous plants. New Phytol 85:531–535. https://doi.org/10.1111/j.1469-8137.1980.tb00768.x
Warren CR (2011) How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiol 31:727–739. https://doi.org/10.1093/treephys/tpr064
Wrage N, Chapuis-Lardy L, Isselstein J (2010) Phosphorus, plant biodiversity and climate change. In: Lichtfouse E (ed) Sociology, organic farming, climate change and soil science. Sustainable agriculture reviews. Springer, New York, pp 147–169
Wright IJ, Reich PB, Cornelissen JHC et al (2005) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496. https://doi.org/10.1111/j.1469-8137.2005.01349.x
Zavišić A, Nassal P, Yang N et al (2016) Phosphorus availabilities in beech (Fagus sylvatica L.) forests impose habitat filtering on ectomycorrhizal communities and impact tree nutrition. Soil Biol Biochem 98:127–137. https://doi.org/10.1016/j.soilbio.2016.04.006
Acknowledgements
This work was supported by the São Paulo Research Foundation (FAPESP – Grant number 2016/25498-0). We thank FAPESP, CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brasil (CNPq) for post-doctoral, doctoral and research fellowships to FMSO (Grant number 2018/09624-0), RGB and PM, respectively, and FAPESP for undergraduate fellowship to SB (Grant Number 2017/21738-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Hans Lambers.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bulgarelli, R.G., de Oliveira Silva, F.M., Bichara, S. et al. Eucalypts and low phosphorus availability: between responsiveness and efficiency. Plant Soil 445, 349–368 (2019). https://doi.org/10.1007/s11104-019-04316-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04316-2