Abstract
Background and aims
Plasticity of plants refers to their ability to produce different phenotypes in different environments. Plants show plasticity aboveground as well as belowground. The influence of the arbuscular mycorrhizal fungal (AMF) symbiosis on root plasticity is poorly known. This study aimed to quantify plasticity of root-system related, morphological, physiological or mycorrhizal traits along a soil phosphorus (P) supply gradient.
Methods
Six varieties of maize (Zea mays L.) were grown in pots with or without AMF at five rates of P supply. Fifteen root traits were measured and calculated after seven weeks of growth.
Results
Root system traits (biomass and length) and physiological traits (phosphatase activity at the root surface and in the rhizosphere) showed high plasticity along the P gradient, whereas morphological traits (specific root length and root diameter) exhibited low plasticity. Mycorrhizal presence reduced root-system plasticity (biomass and length), increased morphological-trait plasticity (specific root length and proportion of fine roots), but had little effects on other traits.
Conclusion
Our results indicate that trait plasticity related to the root system constitutes the most important adaptive strategy for maize to variation in P supply, and that the mycorrhizal symbiosis reduces root-system plasticity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is an essential nutrient for plant growth, development and reproduction. Because most soil P occurs in fractions with slow desorption kinetics, the major limiting factor for crop production is soil P availability rather than the total amount of soil P (Vance et al. 2003). In order to acquire sufficient P, plants can allocate (relatively) more biomass to roots (increasing root/shoot ratio; an architectural or root-system trait), regulate root morphological (root diameter and specific root length) and/or physiological traits (exudation of phosphatase, carboxylates and protons) to enhance P acquisition in P-limiting environments (Lambers et al. 2006, 2015). Furthermore, most plant species form symbioses with arbuscular mycorrhizal fungi (AMF), which improve P capture (Smith and Read 2008). Different plant species have distinct strategies with different combinations of (mycorrhizal) root traits to respond to environmental stress (Li et al. 2017; Zemunik et al. 2015). Different varieties of a species may also exhibit different strategies based on different trait combinations. Still, how belowground traits respond to variations in P supply remains obscure.
Trait plasticity, i.e. species’ adjustment of trait values in response to changing environmental conditions (Callaway et al. 2003), is a vital factor affecting the plant’s ability to withstand environmental stress. Different root and mycorrhizal traits show diverse plastic responses to variation in soil nutrient availability (Iversen et al. 2017; Lambers et al. 2006). Fort et al. (2015) found that at low P availability, members of the Fabaceae increased root/shoot ratio and root surface phosphatase activity. Bayuelo-Jimenez et al. (2011) reported that under P deficiency maize (Zea mays L.) increased root/shoot ratio, nodal rooting, nodal root laterals, nodal root hair density and first-order laterals. These studies indicated that root morphological traits have different variation ranges in response to differences in P availability.
In addition, the presence of AMF also impacts on the plasticity of root traits. Meta-analysis showed that AMF often increase root biomass and root length but decrease root/shoot ratio (Veresoglou et al. 2012), however the mycorrhizal effect on root/shoot ratio was not significant after correction for mycorrhizal effects on plant biomass. Ryan et al. (2016) also reported that AMF significantly decreased root mass ratio (root mass divided by total plant mass) of subterranean clover (Trifolium subterraneum L.). Zhu et al. (2003) found that AMF significantly reduced specific root length of two barley (Hordeum vulgare L.) genotypes with 10–30%. Among Musa genotypes, AMF had positive, but unequal effects on primary (higher-order) and secondary and tertiary (lower-order) roots, resulting in a more branched root system in the mycorrhizal condition (Elsen et al. 2003). Mycorrhizal impacts on physiological root traits have also been described, for example, Ryan et al. (2012) and Gao et al. (2012) reported that AMF downregulated the production of carboxylates.
Interactions between P and AMF on root trait plasticity have also been described. In a study of two inbred lines of maize, Hao et al. (2008) reported a negative effect of both P supply and AMF on specific root length, and the magnitude of both effects was dependent on the specific inbred line studied. Currently, there is only little information how AMF affects plasticity of diverse root traits in relation to P supply, apart from impacts of P supply on mycorrhizal root colonization. Earlier studies have shown that varieties of the same crop species differ in their dependency on, and response to, the mycorrhizal symbiosis (Hetrick et al. 1992; Kaeppler et al. 2000; Zhu et al. 2001, 2003). These studies mainly focused on how P level, AMF and their interaction impacted on shoot growth and P uptake. How AMF and P supply alter root traits, has not yet been explicitly investigated. Plasticity in P transporter expression in roots (with or without induction by AMF) can change with P supply. At low soil P supply, a phosphate transporter located in the periarbuscular membrane of AMF-colonized roots was expressed more strongly than transporters in the root cell plasma membrane (Harrison et al. 2002). Conversely, at high plant P status, the mycorrhizal P uptake pathway was almost completely repressed and the P transporter genes induced by AMF were down-regulated (Nagy et al. 2009).
Crop species may have different plasticity response patterns because crop breeding has often sought to reduce plasticity in order to achieve yield stability over a range of environmental conditions (Semchenko and Zobel 2005). Breeding for yield stability could also have been an underlying cause of modern crop genotypes being less responsive to the mycorrhizal symbiosis than their wild progenitors (Galván et al. 2011; Kaeppler et al. 2000), although exceptions exist as some modern hybrids of maize have even higher mycorrhizal responsiveness than older landraces (Chu et al. 2013). The importance of maintaining plasticity during crop breeding is increasingly recognised (Matesanz and Milla 2018; Sadras and Denison 2016). Understanding the degree of root trait plasticity, including mycorrhiza-dependent root trait plasticity, is therefore crucial for breeding cultivars for sustainable agriculture.
Maize is one of the most widely cultivated crops globally and is used as human and animal food, forage and a source of bio-ethanol. Maize varieties including inbred lines, hybrids, and landraces are always colonized by AMF under field conditions (An et al. 2010). Current agricultural intensive management (e.g. high fertilizer input, monoculture, and high tillage frequency) impacts on the abundance and functional traits of the mycorrhizal symbiosis (Verbruggen and Kiers 2010; Wang et al. 2018b) and therefore modification of root trait plasticity by AMF is likely important. Understanding root trait plasticity in relation to P supply in the presence or absence of AMF may help to improve P acquisition of maize varieties, a prerequisite for breeding for high P uptake efficiency (at a given P level) as a way of saving P in the framework of sustainable agriculture. We therefore selected six maize varieties based on our previous research (Chu 2013; Chu et al. 2013; Wang et al. 2017), including one landrace bred in 1950s, three hybrids bred from 1970 to 2010, and two inbred lines from the beginning of twenty-first century (more information about these six maize varieties in the M&M section). These six varieties were grown at five P supply levels in the absence or presence of AMF to quantify the magnitude of change in root traits and to evaluate the effects of AMF on root trait plasticity in response to variation in P supply.
In this study, we mainly focused on how P supply, AMF and their interaction affected 15 root traits of six maize varieties, and specifically addressed the following questions: i) do different maize root traits show similar plasticity in response to variation in P levels; ii) how do the mycorrhiza-related traits change in plasticity in response to different P levels; and iii) does the presence of AMF alter root trait plasticity.
Materials and methods
Experimental design
A calcareous loamy soil was collected from field plots at the Changping Long-Term Fertilizer Station of China Agricultural University in Beijing, China (40°05′32″N, 116°20′41″E). Bulk density of the soil was 1.38 g cm−3. The soil contained 17.8 g kg−1 organic matter, 870 mg kg−1 N (C:N ≈ 10), 2.9 mg kg−1 Olsen-P, 156 mg kg−1 exchangeable K, and had pH 7.8 (in 0.01 M CaCl2). The soil was passed through a 2-mm sieve and sterilized by radiation with 60Co γ-rays at 10 kGy.
The experiment was a 2 × 6 × 5 factorial with two mycorrhizal treatments (with and without inoculation with Rhizophagus irregularis), six maize (Zea mays L.) varieties (197, 181, NE15, ND108, ZD2 and HMY; reasons why we chose these varieties were explained below), and five P addition levels (0, 20, 50, 100 or 250 mg of P kg−1 soil as KH2PO4). There were four replicates per treatment. To achieve the same soil K level among all treatments, K2SO4 was supplied at 315, 289, 252, 189 and 0 mg K kg−1 soil in the treatments with increasing P levels. In addition, the following mineral nutrients were added to all treatments (per kg soil): 200 mg of N (as KNO3), 50 mg of Mg (as MgSO4), 5 mg of Zn (as ZnSO4), and 2 mg of Cu (as CuSO4). The nutrients were uniformly mixed in soil before placing the soil in plastic pots (18 cm in height, 16 cm in diameter, 2 kg of soil pot−1).
The AMF Rhizophagus irregularis (Kruger et al. 2012), formerly Glomus intraradices, isolate BGC BJ08, was kindly supplied by Prof. Youshan Wang from the Bank of Glomeromycota of China, Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry Research, China. The fungus was propagated on maize plants growing in a 5:1 mixture (w/w) of zeolite and river sand for 4 months in a greenhouse, and the inoculum consisted of substrate containing spores (150 g−1 potting substrate), mycelium and fine-root segments. The inoculum (20 g kg−1 soil for each pot in the mycorrhizal treatment, and 20 g sterilized inoculum kg−1 soil for each pot in the non-mycorrhizal treatment) was added. To minimize differences in microbial communities of mycorrhizal and non-mycorrhizal treatments, 10 mL of AMF-free filtrate from the inoculum was added to each non-mycorrhizal pot, and 10 mL of deionized water was added to each mycorrhizal pot.
Six Chinese maize varieties were used: 181 and 197 were inbred lines. Variety 181 was defined as a P-efficient, and 197 was defined as a P-inefficient variety (Liu et al. 2004). These two varieties were selected from among 100 lines in a 2-year field experiment in a P-deficient soil (Olsen-P 5.8 mg kg−1) where no P fertilizer had been supplied since 1986; variety 181 showed no symptoms of P deficiency, whereas 197 showed severe symptoms (purple leaves and stem) at the seedling stage. At maturity, 181 showed significantly higher biomass and grain yield than 197. The results by Liu et al. (2004) suggested that, compared with 197, the P-efficient line 181 had a larger root system (including root biomass and length), higher capacity of proton release to acidify the rhizosphere, and increased phosphatase activity at the root surface. NE15 and Nongda108 (ND108), single-cross varieties, were developed in the 1990s and 2000s, respectively; Zhongdan2 (ZD2), a mid-maturity hybrid, was developed in the 1970s; Huangmaya (HMY), an open-pollinated, early-maturity landrace, was developed in the 1950s (Chu et al. 2013). HMY had high biomass and responded strongly to AMF at low P level, whereas ND108 had low biomass and a relatively low mycorrhizal responsiveness; ZD2 produced intermediate values between those for HMY and ND108 (Yang 2009).
Seeds of maize were surface-sterilized in 10% (v/v) hydrogen peroxide for 10 min and rinsed at least five times in deionized water. Three germinated seeds were sown into each pot and were thinned to one seedling per pot after emergence (seedlings with similar size were retained for the four replicates).
The experiment was conducted in a greenhouse at the China Agricultural University (40°01′267″N, 116°16′36″E). The greenhouse temperature range was from 25 °C (night) to 30 °C (day), and average photosynthetically active radiation was 380 μmol m−2 s−1. There was no supplementary lighting.
The greenhouse experiment was set up as a randomized complete block design. Pots within each block were re-randomized weekly, to further decrease effects of plant location within blocks. In the process of re-arranging the relative locations of plants, the possibility of injury to plants was avoided (Hardy and Blumenthal 2008). However, relocation was likely unnecessary. Water was supplied daily, and the pots were weighed every three days to adjust soil moisture content to 20% (w/w); differences in plant weight among treatments were ignored.
Plant harvest and root trait measurements
Plants were harvested seven weeks after sowing. We measured 15 traits belonging to four categories (Bardgett et al. 2014): 1) whole-root system or architectural traits: root biomass, root/shoot ratio and root length; 2) root morphological traits: specific root length, proportion of fine-root length, root tissue density and root diameter; 3) root physiological traits: pH of rhizosphere soil, acid phosphatase at the root surface (RP) and alkaline phosphatase in the rhizosphere (SP); and 4) mycorrhizal traits: mycorrhizal colonization, hyphal length density (HLD), hyphal length per unit root length (HLRL), mycorrhizal root biomass and mycorrhizal root length. A full description of every root trait is provided below in the section on measurements.
At harvest, shoots and roots were separated. Shoots were oven-dried at 70 °C for three days, weighed and ground to fine powder. After digestion in a H2SO4 and H2O2 mixture (Bao 2000), shoot P concentration was quantified by the molybdo-vanado-phosphate method (Kitson and Mellon 1944). In this paper, because we mainly focus on root trait changes with P levels and AMF, we supply shoot biomass, P content and P concentration in the supporting materials (Table S1; Figs. S1, S2, and S3).
Roots were carefully lifted out of the soil and shaken to remove loosely adhering soil around roots (considered to be bulk soil), with the tightly adhering soil around roots defined as rhizosphere soil (Veneklaas et al. 2003). The roots with tightly adhering rhizosphere soil were immersed in 100 mL of 0.2 mM CaCl2 solution and shaken repeatedly and carefully for 1 min. Care was taken to minimize root damage. The resulting soil suspension was used to measure the rhizosphere soil pH and alkaline phosphatase activity of the rhizosphere. The pH of the suspension was measured with a microelectrode (Waterproof pHTestr® 10 BNC pH tester, Eutech Instruments, Pte. Ltd., Singapore) (Li et al. 2010). Alkaline phosphatase activity of the rhizosphere [p-nitrophenylphosphate (p-NPP); μmol p-NPP h−1 g−1 dry soil was measured according to Alvey et al. (2001). Two 0.5-mL aliquots of soil suspension were transferred into 2-mL centrifuge tubes for measurement of alkaline phosphatase activity (Alvey et al. 2001; Neumann 2006). The rhizosphere soil in the CaCl2 suspension was separated by centrifugation for 10 min at 12,000×g, dried at 60 °C and then weighed. The concentration of p-NP (derived from p-NPP added before the centrifugation into 2-mL centrifuge tubes) in the supernatant was measured spectrophotometrically at 405 nm. After analysis the dry weight of soil in the tubes was determined to report alkaline phosphatase activity of the rhizosphere per mass unit.
Acid phosphatase activity at the root surface (μmol p-NPP h−1 g−1 root fresh weight) was measured according to the method of Neumann (2006). Excised 1–2 cm maize root segments (a fresh batch of root samples) were washed 3–5 times in acetate buffer (0.2 mol L−1, pH 5.2) in 2-mL centrifuge tubes and immersed in the solution containing 0.5 mL water, 0.4 mL acetate buffer (0.2 mol L−1, pH 5.2) and 0.1 mL 0.15 mol p-NPP substrate. After reaction for 10 min at 25 °C, 0.8 mL of the reaction mixture was transferred to a new tube, and 0.4 mL of 0.5 mol L−1 NaOH was added to terminate the reaction. The concentration of p-NP (derived from p-NPP) was measured spectrophotometrically at 405 nm. After analysis the fresh weight of root in the tubes was determined to report acid phosphatase activity at the root surface per mass unit.
Roots were washed with deionized water, blotted and preserved at −20 °C. Root length and diameter were measured using a WinRHIZO scanning and image-recording system (EPSON 1680, WinRHIZOPro2004b). Specific root length (m g−1) was assessed as the ratio of root length over root weight. The proportion of fine-root length over total root length (roots with a diameter ≤ 0.3 mm were defined as fine roots; Li et al. 2014) was determined by WinRHIZO software automatically. We calculated root tissue density, assuming roots as perfect cylinders (Ostonen et al. 2007).
After scanning, a weighed subsample of the root system was cleared and stained for mycorrhizal colonization. Mycorrhizal colonization was assessed by the method of Trouvelot et al. (1986). Roots were cut into 1-cm segments and thoroughly mixed, and a 0.5-g subsample was cleared with 10% (w/v) KOH at 90 °C for 2 h and stained with trypan blue. The remaining roots were oven-dried at 70 °C for 3 days and weighed. Whole dry root biomass was determined according to the whole fresh root biomass and then root/shoot ratio was calculated. We also calculated mycorrhizal root biomass and mycorrhizal root length, which were determined as the product of fractional mycorrhizal colonization and root biomass and root length. The soil samples for each pot were taken for determination of HLD (meters of hyphae gram-1 dry soil). HLD was determined according to Jakobsen et al. (1992). We also calculated HLRL, hyphal length per unit root length.
Data analysis
We used three-way ANOVA to test for effects of mycorrhiza, P levels, varieties and their interactions on 10 root traits (P ≤ 0.05). When the three-way interaction was significant, it was presented in figures; if the three-way interaction was non-significant, only the significant two-way interactions were presented. In addition, we used two-way ANOVA to test for effects of P levels and varieties and their interaction on five mycorrhizal root traits (P ≤ 0.05). To subsequently address how roots of maize varieties responded to various P supply levels, we calculated the coefficient of variation for each trait across five P treatments. We did this separately for non-mycorrhizal and mycorrhizal treatments, to evaluate how the mycorrhizal symbiosis affected trait plasticity. We assessed the mycorrhizal effect on plasticity using t-test. All analyses were carried out in PASW Statistics 20.0 (IBM Corp., Armonk, NY, USA).
Results
Root traits variation due to AMF and soil P levels
All 15 root traits were significantly affected by varieties and P supply levels. The interaction varieties × P levels was significant for 14 out of 15 root parameters; only for average root diameter was that interaction not a significant source of variation. For the ten roots traits that were assessed under both mycorrhizal and non-mycorrhizal conditions, mycorrhiza was a significant source of variation for root biomass, root length, and rhizosphere pH. Mycorrhiza × varieties interactions were significant sources of variation for root system traits and root physiological traits, but not for root morphological traits. Mycorrhiza × P levels were only significant sources for variation for root system traits, but not for root morphological and physiological traits. There were also several significant three-way interactions (Table 1).
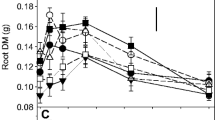
Among root system traits, root biomass and root length increased with increasing P supply (Fig. 1a and c). In the presence of mycorrhiza, root biomass and root length were also higher than in the non-mycorrhizal condition. Root/shoot ratio declined with increasing P-levels but there was no effect of mycorrhiza on this trait (Fig. 1b). Significant P × mycorrhiza interactions were due to the fact that mycorrhizal benefits peaked at intermediate P-supply levels. At the highest P supply, mycorrhiza had no significant effect on root biomass and root length when averaged over the different varieties (Fig. 1a and c). At P20, ND108 and HMY showed a greater difference between mycorrhizal and non-mycorrhizal plants (higher mycorrhizal responsiveness) than the other varieties (Fig. 1a). The root/shoot ratio significantly decreased by mycorrhizal inoculation in varieties 197 and HMY at P50, and increased in variety ZD2 at P0 and P100 (Fig. 1b). While root length increased with an increase in P levels, these increases were smaller in variety 197 than ND108 (Fig. 1c), and were larger at P20 compared with the other four P levels (Fig. 1c inset).
Variation in the whole-root-system traits (root biomass, a; root/shoot ratio, b; root length, c) as influenced by P rates (0, 20, 50, 100 and 250 mg kg−1 soil), AMF and maize varieties (197, 181, NE15, ND108, ZD2 and HMY). If the three-way interaction varieties (V) × mycorrhiza (M) × P rates (P) was significant, the complete data set was presented; if not, the significant two-way interactions were presented. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. Significant t-test analyses for effects of mycorrhizal treatment within a variety at a given P rate were displayed as asterisks above the bars. Means ± SE
Among root morphological traits, only specific root length was not significantly affected by the interaction of varieties × mycorrhiza × P levels. Specific root length and the proportion of fine-root length decreased with increasing P supply, whereas root diameter increased with increasing P supply, and root tissue density hardly changed with increasing P supply (Fig. 2). Variety 181 tended to have higher specific root length than the other varieties at P0, P20 and P250, but not at the other two P levels (Fig. 2a). Variety NE15 had a smaller proportion of fine-root length in mycorrhizal than in non-mycorrhizal plants at P20, P50 and P100 (Fig. 2b). Only in three cases did AMF significantly increase the proportion of fine-root length, and two of these (variety 197 and HMY) were at P250 (Fig. 2b). Average root diameter increased by mycorrhization only in some varieties in the range of P levels of P50-P250, particularly in ZD2 at P100 and P250 (Fig. 2c), whereas root diameter decreased only in two cases (variety 197 and HMY) by mycorrhization at P250 (Fig. 2c). Only in four cases did AMF significantly affect root tissue density, and these were at P0 (variety ND108 and HMY), P100 (variety ZD2) and P250 (variety 197) (Fig. 2d).
Variation in the morphological traits (specific root length, a; proportion of fine-root length, b; root diameter, c; root tissue density, d) as influenced by P rates (0, 20, 50, 100 and 250 mg kg−1 soil), AMF and maize varieties (197, 181, NE15, ND108, ZD2 and HMY). If the three-way interaction varieties (V) × mycorrhiza (M) × P rates (P) was significant, the complete data set was presented; if not, the significant two-way interactions were presented. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. Significant t-test analyses for effects of mycorrhizal treatment within a variety at a given P rate were displayed as asterisks above the bars. Means ± SE
Among the three root physiological traits, only rhizosphere pH was significantly affected by the three-way interaction (Table 1). Across mycorrhizal treatments and varieties, rhizosphere pH increased with increasing P. Importantly, ND108 had the lowest rhizosphere pH among all varieties, with mycorrhiza decreasing pH of the ND108 rhizosphere significantly at all P levels except P0 (Fig. 3a). AMF significantly increased the HMY rhizosphere pH at P20, P50 and P100 (Fig. 3a). Both acid phosphatase activity at the root surface and alkaline phosphatase activity in the rhizosphere were influenced significantly by the varieties × mycorrhiza and varieties × P levels interactions (Table 1). Both phosphatase activities significantly decreased with increasing P (Fig. 3d and e). Compared with the other varieties, NE15 and ZD2 had a relatively high phosphatase activity at the root surface (Fig. 3b), particularly at P0 and P20 (Fig. 3d). Varieties 197 and ZD2 had a relatively high phosphatase activity in the rhizosphere soil (Fig. 3c), again particularly at P0 and P20 (Fig. 3e).
Variation in the physiological traits (pH of rhizosphere soil, a; acid phosphatase at the root surface, b and d; alkaline phosphatase in the rhizosphere, c and e) as influenced by P rates (0, 20, 50, 100 and 250 mg kg−1 soil), AMF and maize varieties (197, 181, NE15, ND108, ZD2 and HMY). If the three-way interaction varieties (V) × mycorrhiza (M) × P rates (P) was significant, the complete data set was presented; if not, the significant two-way interactions were presented. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. Significant t-test analyses for effects of mycorrhizal treatment within a variety at a given P rate were displayed as asterisks above the bars. Means ± SE
Non-mycorrhizal maize roots, inoculated with sterilized AMF inoculum, remained free of mycorrhizal colonization. All five mycorrhiza-related traits were significantly affected by the varieties × P levels interaction (Table 1). Mycorrhizal colonization tended to be lower at the extreme P levels (P0 and P250) than at the other three P rates (Fig. 4a). Maize varieties 181 and ZD2 had relatively high colonization at P0, but the order of varieties varied at other P levels. HLD was significantly higher at P20, P50 and P100 than that at P0 and P250. HLD was higher in varieties ND108 and HMY at P0 (and in ZD2 at P250) compared with the other varieties (Fig. 4b). Mycorrhizal root biomass of six varieties varied at five P levels (Fig. 4c). At P20, ND108 and HMY had higher mycorrhizal root biomass than other varieties, and ZD2 had the highest mycorrhizal root biomass at P250 (Fig. 4c). At P100, mycorrhizal root mass was highest and at P0 lowest (Fig. 4c). Generally, mycorrhizal root length showed similar trends as mycorrhizal root biomass (Fig. 4d). There was a significant positive correlation between mycorrhizal root biomass and length (r = 0.96; P < 0.001). HLDL significantly decreased with increasing P (Fig. 4e). Compared with the other varieties, 197 tended to have a relatively high HLRL at all P levels.
Variation in the mycorrhizal traits (mycorrhizal colonization, a; hyphal length density, b; mycorrhizal root biomass, c; mycorrhizal root length, d; hyphal length per unit root length (HLRL), e) as influenced by P rates (0, 20, 50,100 and 250 mg kg−1 soil) and maize varieties (197, 181, NE15, ND108, ZD2 and HMY) in the mycorrhizal treatment. The significance of the two-way interaction varieties (V) × P rates (P) is presented. ANOVA results as follows: *** P ≤ 0.001. Means ± SE
Root trait syndromes
Syndromes here are defined as sets of traits that occur together. Averaged over P supply levels, variety ND108 exhibited a much lower rhizosphere pH than the other varieties. At the same time ND108 exhibited higher root/shoot ratio, thinner root diameter, lower root tissue density, lower proportion of fine-root length, lower specific root length and lower phosphatase levels at both root surface and rhizosphere compared to the other varieties (Figs. 1, 2, 3, and 4). Also mycorrhizal colonization in ND108 tended to be lower than in the other varieties. Variety 197 generally had lowest root biomass, root length and root/shoot ratio (Fig. 1). At the same time it showed the highest root tissue density in most cases (Fig. 2d). Moreover, 197 had the lowest mycorrhizal root biomass and length, and the highest HLRL compared with the other varieties (Fig. 4). Compared with variety 197, variety 181 had a much lower RSR plasticity under both non- and mycorrhizal conditions as well as higher plasticity of root tissue density, phosphatase activity and four mycorrhiza-related traits at the root surface when plants were colonized by AMF (Table 2).
Root trait plasticity
Data on root trait plasticity are shown in Table 2. There was large variation in trait plasticity both between and within trait categories. Rhizosphere pH was the least plastic trait. Low trait plasticity was also observed for root/shoot ratio, specific root length, the proportion of fine-root length, and root diameter. The other traits showed a much higher variability. Mycorrhizal root traits were only assessed for the inoculated plants. In that trait category, HLD showed less plasticity than mycorrhizal root colonization. Different varieties varied in their plasticity for individual traits, however, there was little evidence that some varieties were more plastic than others as a general varietal characteristic. Plasticity in root/shoot ratio was much higher for 197 than for the other varieties, and much lower for NE15 (particularly when plants were non-mycorrhizal). ZD2 showed high plasticity of physiological traits both in the absence and presence of AMF, but showed lower plasticity of all five mycorrhiza-related traits than the other varieties (Table 2). HMY, on the other hand, showed higher plasticity of mycorrhiza-related traits than other varieties.
The effect of AMF on the plasticity of different root traits
The effect of AMF on root trait plasticity is shown in Fig. 5. AMF reduced root system trait plasticity for both root biomass and root length and had a marginally significantly positive effect on root/shoot ratio. Root morphological traits were significantly affected by AMF, with proportion of fine-root length showing a significantly higher plasticity, and specific root length showing a marginally significantly higher trait plasticity. Mycorrhization did not affect the plasticity of physiological traits.
Coefficient of variation (%) for six maize varieties grown at five P rates for: root biomass (RB), root/shoot ratio (RSR), root length (RL), specific root length (SRL), proportion of fine-root length (FRLP), root diameter (RD), root tissue density (RTD), pH of rhizosphere soil (pH), phosphatase at root surface (RP), and phosphatase in rhizosphere soil (SP) in the presence or absence of AMF. Mycorrhizal colonization (MC), hyphal length density (HLD), mycorrhizal root mass (MRB), mycorrhizal root length (MRL), mycorrhizal root biomass (MRB), mycorrhizal root length (MRL) and hyphal length per unit root length (HLRL) were determined only in the presence of AMF. †, * and ** indicate significance at P ≤ 0.1, P ≤ 0.05 and P ≤ 0.01 between non-mycorrhizal (-AMF) and mycorrhizal (+AMF) plants (t-test)
Discussion
Our six maize varieties showed significant plasticity in their responses to variation in P supply. However, the degree of plasticity was different in the absence and in the presence of AMF for key functional traits such as root biomass, root length and root diameter, but not for three root physiological traits. Our study provides evidence for changes in plasticity of P uptake-related root traits among maize varieties. Next to varietal differences in root traits, and in the degree of plasticity, the effect of AMF on plasticity was substantial across these six varieties, implying that breeding directly and indirectly (through symbiosis with AMF) impacts on root trait plasticity (Table 2). Furthermore, our results showed that the plasticity of different categories of functional traits (root system, morphology and physiology) was affected by AMF in different ways (positively, negatively or neutral; Fig. 5), which may have implications for breeding efficient maize varieties under environmentally variable conditions.
Variation in plasticity of root functional traits among varieties in response to different P levels and AMF
Maize varieties showed different plasticity pattern in four categories of functional traits, and the presence of AMF generally changed the plasticity of each root trait for each variety (Table 2). These results indicate that different varieties have different strategies when responding to variation in P supply. For example, ND108 has the strongest rhizosphere effect (Fig. 3a) and the largest root diameter (Fig. 2c), but at the same time the lowest root tissue density, proportion of fine root length (Fig. 2b and d), and low enzymatic activity. Trade-offs among different functional traits were also evident for ZD2, which showed higher plasticity of physiological traits both in the presence and absence of AMF (Table 2), but lower mycorrhizal trait plasticity than the other varieties (Table 2). So there is a possible trade-off among root traits, consistent with the viewpoint of Denison (2012). Thus, it may be impossible that breeding programs can stack different plant traits that increase P nutrient uptake in one variety (Bayuelo-Jimenez et al. 2011).
As described elsewhere (Liu et al. 2004), variety 181 was more P-efficient than variety 197. Our study confirmed this report because, compared with variety 197, variety 181 had higher root biomass and root length, higher shoot biomass and shoot P content, and also higher phosphatase activity at the root surface (but not in the rhizosphere soil). Our results also demonstrated varietal differences in root plasticity: mycorrhizal traits were more plastic in variety 181 than variety 197, whereas root/shoot ratio exhibited higher plasticity in variety 197. Our data also confirmed the higher mycorrhizal responsiveness (at low P supply) of variety HMY than ND108, as described by Yang (2009).
Root and mycorrhizal traits response to variation in P supply
In contrast to the four root morphological traits (specific root length, proportion of fine-root length, average root diameter and root tissue density; Table 2) that showed low plasticity, root biomass, root length and phosphatase activity in the rhizosphere showed high plasticity at different P supply, especially when plants were non-mycorrhizal. Regarding root biomass, these results agree with a previous study that reported increased relative allocation of carbohydrates to roots of nutrient-deficient plants (Poorter et al. 2012), supporting the recent suggestion that increased relative carbohydrate allocation to root biomass rather than morphological trait plasticity is key how plants acclimate and adapt to nutrient limitation (Freschet et al. 2015; Kramer-Walter and Laughlin 2017).
Average root diameter and specific root length showed low plasticity, somewhat more so when plants were non-mycorrhizal (Table 2, Fig. 5). This result could be due to strong evolutionary constraints (Valverde-Barrantes et al. 2017; Wang et al. 2018a) which allows only limited variation between diverse soil environments (Zhang et al. 2016). In contrast, Lynch (2013) claimed that under low P supply, maize roots increased cortical aerenchyma, which may lead to a decrease in root tissue density and, without changes in root diameter, to an increase in specific root length. Such morphological responses would result in higher morphological trait plasticity, which was not found by us. Plasticity in root tissue density in our study suggests that the effect of changes in cell structure is relatively weak (Fig. 2d), and plasticity of specific root length (and from the underlying determinants root diameter and root tissue density) therefore relatively low as well (Table 2). In our study, proportion of fine-root length did not vary much across variable environments. Fine roots form mainly at the end of lateral roots and have a strong absorptive function (McCormack et al. 2015). Hence, lateral-root density would be expected to strongly influence the proportion of fine-root length; unfortunately, we were unable to assess lateral-root branching density, and this needs to be tested in future research.
The activity of phosphatase either at the root surface or in the rhizosphere showed high plasticity, but pH of rhizosphere soil did not (Table 2). Phosphatase activity was higher in low-P than high-P environments, consistent with earlier studies on the importance of plant adaptation to low P supply (Lambers et al. 2006). However, Wen et al. (2017) found increasing maize shoot P concentration induced a decrease in root morphological responses and an enhancement in root exudation including acid phosphatase and carboxylates. We cannot explain this difference but note that different varieties may respond differently. In addition, we observed low plasticity of the rhizosphere soil pH in response to P limitation. A low plasticity of rhizosphere pH may be partly due to our metric of plasticity (pH being the logarithm of proton concentration), and is likely additionally due to a large extent to the fact that our soil was well buffered. Rhizosphere pH was 8.4 and apparently proton exudation was too limited to have an impact on that pH. Only ND108 (Fig. 3a) decreased rhizosphere pH substantially. The decrease was significantly larger in mycorrhizal maize than in non-mycorrhizal maize, a result that contrasts with earlier studies by Gao et al. (2012) and Ryan et al. (2012). The ability to modify rhizosphere pH in well-buffered soils and the mycorrhizal role therein demands further study.
Compared with plasticity of root traits, mycorrhizal colonization, mycorrhizal root biomass/length and HLRL exhibited high variation in different P environments (Table 2), which is consistent with the recent results of some thin-root grass species by Li et al. (2017) in drought-stressed environments. Similarly, earlier studies showed strong variation in mycorrhizal colonization of maize roots (Hao et al. 2008), as well as in HLD in one maize variety (Zheng et al. 2013), along a P supply gradient. HLD values in this study were not particularly high (ranging from 2 to 4 m g−1), whereas Nagy et al. (2009) reported values of 8–13 m g−1 and also showed a much stronger effect of P application on mycorrhizal colonization than on HLD, suggesting a stronger reduction (and hence higher plasticity) in colonization than in HLD might occur commonly. Although HLD exhibited much lower plasticity than mycorrhizal colonization (Table 2 and Fig. 5), HLRL showed higher plasticity than mycorrhizal colonization. This result implies that there is a compensatory reaction between hyphae and roots in P capture (Smith et al. 2011), indirectly explaining why HLD was less plastic than colonization in our study. Our results hint that an exclusive focus on mycorrhiza-related traits (fractional colonization, HLD) as the traits to describe the plant adaptive strategy may be limited and potentially misleading, and that root traits need to be considered as well in future studies.
The influence of AMF on plasticity of root traits
Our results on AMF symbiosis altering the coefficient of variation of some traits of maize plants; for example, increasing the coefficient of variation of root/shoot ratio, specific root length and proportion of fine-root length, and decreasing the coefficient of variation of root biomass and root length (Fig. 5), were consistent with earlier studies (Farrar and Gunn 1998; Ryan et al. 2012; Veresoglou et al. 2012). The reduced coefficients of variation of root biomass and root length by AMF could be explained by the strong P-acquisition capacity of AMF (Smith et al. 2011), whereby the mycorrhizal pathway of P uptake could be dominant over the direct root pathway (Liu et al. 2015). Because mycorrhizal benefit (expressed as a biomass ratio of mycorrhizal and non-mycorrhizal plants) is larger at lower P supply, this decline in responsiveness mathematically translates into a lower coefficient of variation.
The literature on mycorrhizal effects on root/shoot ratio is inconsistent. Veresoglou et al. (2012) noted after a meta-analysis that mycorrhizal plants showed on average a lower root/shoot ratio, and attributed that to alleviation of nutrient limitation due to the mycorrhizal symbiosis. However changes in root/shoot ratio can be caused both by differential partitioning of carbon between roots and shoots, and by plant biomass itself as the root/shoot ratio changes during plant development. Consistent with the latter explanation we noted a lower root/shoot ratio with increasing P application (hence with larger plants), so part of the mycorrhizal effect on root/shoot ratio should be attributed by changes in plant size. However, averaged over all varieties the relation between plant biomass and root/shoot ratio was neither significant for non-mycorrhizal nor for mycorrhizal plants (data not shown), because of substantial variation and plasticity in root/shoot ratio among varieties. As root/shoot ratio decreased by mycorrhizal inoculation in some varieties (197, HMY, especially at P50) but increased in other varieties (ZD2 at P0 and P100), changes in root/shoot partitioning due to the mycorrhizal symbiosis demand further study to understand the underlying mechanisms. Poorter et al. (2012) noted that for small plants the root/shoot ratio showed only minor changes with plant size, and their observations would support the suggestion that changes in root/shoot ratio are not a simple consequence of changes in plant size.
In the present study, AMF significantly increased the coefficient of variation of proportion of fine-root length across P supply (Table 2, Fig. 5). There may be two causes. First, AMF may partly replace the function of fine roots in taking up P, which increases the plasticity of the proportion of fine-root length along a P gradient. Second, an additional explanation could be that AMF influence plant hormonal status (Gutjahr and Paszkowski 2013), which impacts on branching and hence the levels of fine-root differentiation. Measuring plant hormonal status in mycorrhizal and non-mycorrhizal plants as an additional traits would therefore be important. In contrast, phosphatase activity at the root surface and in the rhizosphere showed high plasticity, but AMF did not lead to significant change (Table 2). This finding suggests that plasticity in these root physiological traits is more dependent on plant identity and/or on other microbiota in the rhizosphere and on roots than on mycorrhizal symbiosis.
In soil and in plant roots, both in natural and in agro-ecosystems, AMF occur as communities of species and not as fungal monocultures. The question is pertinent whether our results would have been different had we executed our experiments with AMF communities. We speculate that this is unlikely and suggest that the major outcomes in this study are of a general nature (Chave et al. 2019; Hazard and Johnson 2018). Lower plasticity of mycorrhizal than of non-mycorrhizal plants along a P supply gradient is a general phenomenon, and higher plasticity of root system traits than of morphological and/or physiological traits is likely a general outcome as well. The result of Shen et al. (2018) supported our speculation, and the root system traits was suppressed by P deficiency more than morphological and/or physiological traits for wheat growth in the non-sterilized soil.
The influence of AMF on shoot P concentration and biomass
Increases in shoot P concentrations after AMF inoculation did not translate into greater shoot biomass compared with non-mycorrhizal plants (Figs. S1 and S3). Increased shoot concentrations are mathematically equivalent to reduced P utilization efficiency (defined as the inverse of P concentration). This phenomenon has been described as luxury uptake, but the term luxury uptake has no explanatory value (Hetrick et al. 1994; Zabinski et al. 2002). Higher P concentrations of mycorrhizal than of non-mycorrhizal plants could be due to the fact that different nutrients are limiting in the mycorrhizal and non-mycorrhizal condition, or alternatively to so-called ‘carbon costs’. The latter explanation is somewhat implausible as Van der Heijden (2002) has shown that plants that show a higher responsiveness to AMF also show a higher increase in P concentration, implying that plants with the highest costs also have the highest benefits. A further and more likely explanation is that P uptake is not perfectly regulated over time (at one point in time, more P may be taken up than can be used at that time), but that ‘excess’ P can be used at later stages, for instance, when the carbon flow to the fungus decreases because developing fruits might form a stronger carbon sink than the fungal mycelium.
Conclusions
Our findings imply that plasticity of root biomass and total root length constitute the key strategy of plants in adapting to variation in P supply. Our result supports a recent study by Zhang et al. (2018), who found that a transgenic line of Brachypodium distachyon (L.) P. Beauv. with increased root hair length did not show increased P uptake because of simultaneous negative pleiotropic effects on plant biomass. Plasticity of root morphological traits has been regarded as the most important P acquisition strategy in many studies (Veneklaas et al. 2003), but only when these morphological traits are combined with root biomass and length can the function of root morphological traits in benefitting P uptake be achieved (Zhang et al. 2018). In the present study, AMF mainly reduced the plasticity of root biomass and length.
Our findings contribute to further insights into plasticity of root traits of maize in response to different P supply and AMF colonization. That knowledge could contribute to targeted breeding for maize adaptive strategies for uptake efficiency under variation in P supply. Whether these adaptive strategies can be generalized across plant species and varieties still needs further study, particularly for plant species mainly depending on root physiological traits to acquire P. On the other hand, improving P uptake efficiency is only one of the aims in plant breeding, in addition to e.g. N uptake efficiency, drought/heat tolerance or yield stability. Breeders therefore should consider the multivariate functions of the plant-soil-climate interactions in breeding new crop varieties for sustainable food-production systems.
References
Alvey S, Bagayoko M, Neumann G, Buerkert A (2001) Cereal/legume rotations affect chemical properties and biological activities in two West African soils. Plant Soil 231:45–54
An GH, Kobayashi S, Enoki H, Sonobe K, Muraki M, Karasawa T, Ezawa T (2010) How does arbuscular mycorrhizal colonization vary with host plant genotype? An example based on maize (Zea mays) germplasms. Plant Soil 327:441–453
Bao SD (2000) Soil agrochemical analysis, 3rd edn. China Agriculture Press, Beijing, pp 1–495
Bardgett RD, Mommer L, De Vries FT (2014) Going underground: root traits as drivers of ecosystem processes. Trends Ecol Evol 29:692–699
Bayuelo-Jimenez JS, Gallardo-Valdez M, Perez-Decelis VA, Magdaleno-Armas L, Ochoa I, Lynch JP (2011) Genotypic variation for root traits of maize (Zea mays L.) from the Purhepecha Plateau under contrasting phosphorus availability. Field Crop Res 121:350–362
Callaway RM, Pennings SC, Richards CL (2003) Phenotypic plasticity and interactions among plants. Ecology 84:1115–1128
Chave M, Angeon V, Paut R, Collombet R, Tchamitchian M (2019) Codesigning biodiversity-based agrosystems promotes alternatives to mycorrhizal inoculants. Agron Sustain Dev 39:48
Chu Q (2013) The contribution of mycorrhizal pathway to p uptake efficiency of maize (Zea mays L.). Dissertation for Doctor Degree, China Agricultural University
Chu Q, Wang XX, Yang Y, Chen FJ, Zhang FS, Feng G (2013) Mycorrhizal responsiveness of maize (Zea mays L.) genotypes as related to releasing date and available P content in soil. Mycorrhiza 23:497–505
Denison RF (2012) Darwinian agriculture: how understanding evolution can improve agriculture. Princeton University Press, Princeton, pp 1–258
Elsen A, Beeterens R, Swennen R, De Waele D (2003) Effects of an arbuscular mycorrhizal fungus and two plant-parasitic nematodes on Musa genotypes differing in root morphology. Biol Fert Soils 38:367–376
Farrar J, Gunn S (1998) Allocation: allometry, acclimation–and alchemy. In: Lambers H, Poorter H, van Vuren MMI (eds) Inherent variation in plant growth. Backhuys Publishers, Leiden, pp 183–197
Fort F, Cruz P, Catrice O, Delbrut A, Luzarreta M, Stroia C, Jouany C (2015) Root functional trait syndromes and plasticity drive the ability of grassland Fabaceae to tolerate water and phosphorus shortage. Environ Exp Bot 110:62–72
Freschet GT, Swart EM, Cornelissen JH (2015) Integrated plant phenotypic responses to contrasting above-and below-ground resources: key roles of specific leaf area and root mass fraction. New Phytol 206:1247–1260
Galván GA, Kuyper TW, Burger K, Keizer LCP, Hoekstra RF, Kik C, Scholten OE (2011) Genetic analysis of the interaction between Allium species and arbuscular mycorrhizal fungi. Theor Appl Genet 122:947–960
Gao XP, Hoffland E, Stomph T, Grant CA, Zou CQ, Zhang FS (2012) Improving zinc bioavailability in transition from flooded to aerobic rice. A review. Agron Sustain Dev 32:465–478
Gutjahr C, Paszkowski U (2013) Multiple control levels of root system remodeling in arbuscular mycorrhizal symbiosis. Front Plant Sci 4:204
Hao L, Zhang J, Christie P, Li X (2008) Response of two maize inbred lines with contrasting phosphorus efficiency and root morphology to mycorrhizal colonization at different soil phosphorus supply levels. J Plant Nutr 31:1059–1073
Hardy E, Blumenthal D (2008) An efficient and inexpensive system for greenhouse pot rotation. HortSci 43:965
Harrison MJ, Dewbre GR, Liu JY (2002) A phosphate transporter from Medicago truncatula involved in the acquisiton of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14:2413–2429
Hazard C, Johnson D (2018) Does genotypic and species diversity of mycorrhizal plants and fungi affect ecosystem function? New Phytol 220:1122–1128
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat-varieties, landraces, and ancestors. Can J Bot 70:2032–2040
Hetrick BAD, Wilson GWT, Schwab AP (1994) Mycorrhizal activity in warm-season and cool-season grasses – variation in nutrient-uptake strategies. Can J Bot 72:1002–1008
Iversen CM, McCormack ML, Powell AS, Blackwood CB, Freschet GT, Kattge J, Roumet C, Stover DB, Soudzilovskaia NA, Valverde-Barrantes OJ (2017) A global fine-root ecology database to address below-ground challenges in plant ecology. New Phytol 215:15–26
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular arbuscular mycorrhizal fungi associated with Trifolium subterraneum l. 2. Hyphal transport of 32P over defined distances. New Phytol 120:509–516
Kaeppler SM, Parke JL, Mueller SM, Senior L, Stuber C, Tracy WF (2000) Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci 40:358–364
Kitson R, Mellon M (1944) Colorimetric determination of phosphorus as molybdivanadophosphoric acid. Ind Eng Chem Anal Ed 16:379–383
Kramer-Walter KR, Laughlin DC (2017) Root nutrient concentration and biomass allocation are more plastic than morphological traits in response to nutrient limitation. Plant Soil 416:539–550
Kruger M, Kruger C, Walker C, Stockinger H, Schüßler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193:970–984
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot-Lond 98:693–713
Lambers H, Martinoia E, Renton M (2015) Plant adaptations to severely phosphorus-impoverished soils. Curr Opin Plant Biol 25:23–31
Li HG, Shen JB, Zhang FS, Marschner P, Cawthray G, Rengel Z (2010) Phosphorus uptake and rhizosphere properties of intercropped and monocropped maize, faba bean, and white lupin in acidic soil. Biol Fert Soils 46:79–91
Li H, Ma Q, Li H, Zhang F, Rengel Z, Shen J (2014) Root morphological responses to localized nutrient supply differ among crop species with contrasting root traits. Plant Soil 376:151–163
Li H, Liu B, McCormack ML, Ma Z, Guo D (2017) Diverse belowground resource strategies underlie plant species coexistence and spatial distribution in three grasslands along a precipitation gradient. New Phytol 216:1140–1150
Liu Y, Mi GH, Chen FJ, Zhang JH, Zhang FS (2004) Rhizosphere effect and root growth of two maize (Zea mays L.) genotypes with contrasting P efficiency at low P availability. Plant Sci 167:217–223
Liu BT, Li HB, Zhu BA, Koide RT, Eissenstat DM, Guo DL (2015) Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol 208:125–136
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot-Lond 112:347–357
Matesanz S, Milla R (2018) Differential plasticity to water and nutrients between crops and their wild progenitors. Environ Exp Bot 145:54–63
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo D, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207:505–518
Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M (2009) Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol 181:950–959
Neumann G (2006) Quantitative determination of acid phosphatase activity in the rhizosphere and on the root surface. In: Luster J, Finlay R (eds) Handbook of methods used in Rhizosphere research. Swiss Federal Research Institute WSL, Birmensdorf
Ostonen I, Püttsepp Ü, Biel C, Alberton O, Bakker M, Lõhmus K, Majdi H, Metcalfe D, Olsthoorn A, Pronk A (2007) Specific root length as an indicator of environmental change. Plant Biosyst 141:426–442
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50
Ryan M, Tibbett M, Edmonds-Tibbett T, Suriyagoda L, Lambers H, Cawthray G, Pang J (2012) Carbon trading for phosphorus gain: the balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ 35:2170–2180
Ryan MH, Kidd DR, Sandral GA, Yang ZJ, Lambers H, Culvenor RA, Stefanski A, Nichols PGH, Haling RE, Simpson RJ (2016) High variation in the percentage of root length colonised by arbuscular mycorrhizal fungi among 139 lines representing the species subterranean clover (Trifolium subterraneum). Appl Soil Ecol 98:221–232
Sadras VO, Denison RF (2016) Neither crop genetics nor crop management can be optimised. Field Crop Res 189:75–83
Semchenko M, Zobel K (2005) The effect of breeding on allometry and phenotypic plasticity in four varieties of oat (Avena sativa L.). Field Crop Res 93:151–168
Shen Q, Wen ZH, Dong Y, Li HG, Miao YX, Shen JB (2018) The responses of root morphology and phosphorus-mobilizing exudations in wheat to increasing shoot phosphorus concentration. AoB Plants 10:1–11
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis, 3rd edn. Academic, New York, pp 1–769
Smith SE, Jakobsen I, Grønlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057
Trouvelot A, Kough JL, Gianiazzi-Pearson V (1986) Mesure du taux de mycorrhization VA d’un système radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological genetical aspects of Mycorrhizae. INRA Press, Paris, pp 217–221
Valverde-Barrantes OJ, Freschet GT, Roumet C, Blackwood CB (2017) A worldview of root traits: the influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine-root tissues in seed plants. New Phytol 215:1562–1573
Van der Heijden MGA (2002) Arbuscular mycorrhizal fungi as a determinant of plant diversity: in search of underlying mechanisms and general principles. In: Van der Heijden MGA, Sanders I (eds) Mycorrhizal ecology. Ecological studies, vol 157. Springer, Berlin, pp 243–265
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248:187–197
Verbruggen E, Kiers ET (2010) Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evol Appl 3:547–560
Veresoglou SD, Menexes G, Rillig MC (2012) Do arbuscular mycorrhizal fungi affect the allometric partition of host plant biomass to shoots and roots? A meta-analysis of studies from 1990 to 2010. Mycorrhiza 22:227–235
Wang XX, Hoffland E, Feng G, Kuyper TW (2017) Phosphate uptake from phytate due to hyphae-mediated phytase activity by arbuscular mycorrhizal maize. Front Plant Sci 8:1–8
Wang R, Wang Q, Zhao N, Xu Z, Zhu X, Jiao C, Yu G, He N (2018a) Different phylogenetic and environmental controls of first-order root morphological and nutrient traits: evidence of multidimensional root traits. Funct Ecol 32:29–39
Wang XX, Wang XJ, Sun Y, Cheng Y, Liu ST, Chen XP, Feng G, Kuyper TW (2018b) Arbuscular mycorrhizal fungi negatively affect nitrogen acquisition and grain yield of maize in a N deficient soil. Front Microbiol 9:1–10
Wen Z, Li H, Shen J, Rengel Z (2017) Maize responds to low shoot P concentration by altering root morphology rather than increasing root exudation. Plant Soil 416:377–389
Yang Y (2009) Variation of phosphorus (P) efficiencies and mycorrhizal dependence of maize cultivars breeded in different years. Dissertation for Master Degree, China Agricultural University
Zabinski CA, Quinn L, Callaway RM (2002) Phosphorus uptake, not carbon transfer, explains arbuscular mycorrhizal enhancement of Centaurea maculosa in the presence of native grassland species. Funct Ecol 16:758–765
Zemunik G, Turner BL, Lambers H, Laliberté E (2015) Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat Plants 1:1–4
Zhang L, Xu MG, Liu Y, Zhang FS, Hodge A, Feng G (2016) Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol 210:1022–1032
Zhang C, Simpson RJ, Kim CM, Warthmann N, Delhaize E, Dolan L, Byrne ME, Wu Y, Ryan PR (2018) Do longer root hairs improve phosphorus uptake? Testing the hypothesis with transgenic Brachypodium distachyon lines overexpressing endogenous RSL genes. New Phytol 217:1654–1666
Zheng CY, Zhang JL, Li XL (2013) Phosphorus supply level affects the regulation of phosphorus uptake by different arbuscular mycorrhizal fungal species in a highly P-efficient backcross maize line. Crop Pasture Sci 64:881–891
Zhu YG, Smith SE, Barritt AR, Smith FA (2001) Phosphorus (P) efficiencies and mycorrhizal responsiveness of old and modern wheat cultivars. Plant Soil 237:249–255
Zhu YG, Smith FA, Smith SE (2003) Phosphorus efficiencies and responses of barley (Hordeum vulgare L.) to arbuscular mycorrhizal fungi grown in highly calcareous soil. Mycorrhiza 13:93–100
Acknowledgements
This study was financially supported by National Key R&D Program of China (2017YFD0200200) and the National Natural Science Foundation of China (U1703232). We are grateful to four anonymous reviewers for their critical comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Responsible Editor: François Teste.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 385 kb)
Rights and permissions
About this article
Cite this article
Wang, XX., Li, H., Chu, Q. et al. Mycorrhizal impacts on root trait plasticity of six maize varieties along a phosphorus supply gradient. Plant Soil 448, 71–86 (2020). https://doi.org/10.1007/s11104-019-04396-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04396-0