Abstract
Key message
The total uptake of 15 NO 3 -N was twofold higher than that of 15 NH 4 -N when supplied with ammonium and/or nitrate in different seasons; the seedlings fertilized with NO 3 -N had good growth with high photosynthetic rate and total biomass.
Abstract
Appropriate fertilization is crucial for maximum plant growth and improving nitrogen use efficiency. Poplar is an important fast-growing tree species for biomass production, however, little is known about fertilizer management of poplar plantations growing on barren soil in different seasons. To understand nitrogen uptake and allocation of Populus simonii supplied with different forms of nitrogen in different seasons, we determined nitrogen uptake and allocation of P. simonii potted seedlings after a 4-day supply of 15NH4-N, 15NO3-N, 15NH4NO3, and NH 154 NO3 in May, July, and September. The total 15N uptake was twofold higher when supplied with sole 15NO3-N compared to sole 15NH4-N in all the investigated seasons. In the presence of ammonium nitrate (15NH4NO3 and NH 154 NO3), the total 15N uptake was two times higher when supplied with NH 154 NO3 compared to 15NH4NO3. Per unit biomass, the 15N-uptake ability of fine roots was higher in May and July compared to that in September. 15N was present mainly in leaves in May and July, and was mainly stored in roots and stems in autumn. The effect of nitrogen on the growth of P. simonii seedlings was studied by fertilizing with NH4-N, NO3-N, and NH4NO3 for 8 weeks. The seedlings fertilized with NO3-N had good growth with high photosynthetic rate and total biomass indicating that NO3-N is crucial for P. simonii growth. These data contribute to understand the nitrogen uptake in different seasons in trees supplied with different forms of nitrogen. This provides important theoretical bases for fertilizer management of poplar plantations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is one of the main macronutrients for plant growth and development. Most crops grow on fertile soils, but forest trees are often planted on marginal lands where N availability is limited (Finzi et al. 2007). Thus, N fertilization is crucial to ensure high productivity and biomass of forest tree plantation. N is available in different chemical forms such as ammonium (NH4-N), nitrate (NO3-N), and organic N forms (McKane et al. 2002). Plants can assimilate both NH4-N and NO3-N, but some species perform better when fertilized with NH4-N (Cruz et al. 1993; Britto and Kronzucker 2002; Bown et al. 2010; Metcalfe et al. 2011), while others show improved performance when grown with NO3-N (Atkin and Cummins 1994) or a mixture of NO3-N and NH4-N (Öhlund and Näsholm 2001; Nicodemus 2007; Domenicano et al. 2011). The choice of nitrogen form for fertilization is very important for plant growth because plants will show best growth when supplied with their “favorite” nitrogen at appropriate times; therefore, appropriate fertilization plays a key role for maximizing plant growth and improving N use efficiency.
NH4-N and NO3-N have highly distinct consequences for plant growth (Haynes and Goh 1978; Britto and Kronzucker 2002; Domenicano et al. 2011). For example, NO3-N treated hybrid poplar (Populus maximowiczii × P. balsamifera) plants show higher ratios of fine roots: coarse roots and higher specific root lengths compared to NH4-N treated poplar plants (Domenicano et al. 2011). Plants might be able to use different forms of nitrogen in different seasons because they have different growth characteristics in spring, summer, and autumn (McKane et al. 2002). In spring, buds and new leaves appear. Plants have rapid growth in summer while they grow slowly in autumn with decreasing temperature and short photoperiod. There is a lot of evidence that available nitrogen compounds vary in relative proportions in different seasons (Cooke and Weih 2005; Contosta et al. 2011; Brereton et al. 2013; Gilson et al. 2014). However, little information is available about NH4-N or NO3-N uptake of fine roots and allocation in different tissues fertilized with NH4-N and/or NO3-N in different seasons (Socci and Templer 2011; Brereton et al. 2013).
Poplar is an important fast-growing and high-yielding tree species with a short-rotation coppice for bioenergy production or ecological purpose (Schweier and Becker 2013; Manzone et al. 2014). Especially, Populus simonii Carr. is an important ecological and commercial breeding species in northern China; it is tolerant to drought, salinity, cold, and heat with wide distribution from Qinghai to the east coast of China in longitude and from the Heilongjiang River to the Yangtze River in latitude (Weisgerber and Han 2001; Wei et al. 2012). P. simonii is considered as an important afforestation species in China due to its large distribution range, excellent stress tolerance, rapid growth, and regeneration ability. P. simonii often exhibits poor growth with short height in the salty, or water-limited, or barren sandy soil, while P. simonii can reach a height of 30 m and a diameter of 2.5 m in the fertile and moist soil (Lu 2002). The soil in the afforestation areas, particularly the Loess Plateau, typically suffers from nutrient deficiencies, thus, fertilization is needed for afforestation species, such as P. simonii, to improve growth. However, P. simonii plantations lack management; little is known about fertilizer management in different seasons of poplar plantations growing on barren soil.
Populus simonii plants often grow on saline and alkali soil, where most available nitrogen is NO3-N (Weisgerber and Han 2001). We hypothesized that P. simonii seedlings would have higher N uptake when supplied with NO3-N compared to NH4-N irrespective of different N forms and seasons. To test this hypothesis, we investigated short-term nitrogen uptake dynamics in P. simonii supplied with different forms of nitrogen (NH4-N or/and NO3-N) in different seasons. The 15N isotope-labeled NH4-N and NO3-N were applied to 2-year-old P. simonii potted seedlings in three seasons: spring, summer, and autumn. Our specific objectives were to: (1) investigate N uptake of fine roots supplied with different forms of nitrogen, (2) examine the impact of season of application on N uptake, (3) understand the nitrogen allocation to different tissues after a short-time supply of nitrogen at different times in the growing season. Meanwhile, we carried out another independent relative long-term fertilizer experiment to test the effect of nitrogen form on growth of P. simonii seedlings. Based on a previous study (Rennenberg et al. 2010), we hypothesized that NO3-N would be more important compared to NH4-N for the growth of P. simonii seedlings. This study is valuable because it improves our understanding of nitrogen form uptake in different seasons. Meanwhile, the different effects of nitrogen forms on P. simonii growth provide an important theoretical base for fertilizer management of poplar plantations.
Materials and methods
Plant cultivation
Two-year-old seedlings of P. simonii were collected from Wuqi county, Shaanxi province (36°58′57″N, 108°09′34″E) in early April. Each seedling was decapitated to a stem height of about 10 cm with three to five buds and then planted in a 3 l pot filled with a 1:1 ratio of sand and vermiculite. All shoots but one were removed once buds had extended to about 5 cm. The cuttings were cultivated in a glasshouse in the condition of natural light, day/night 25/20 °C, 75 % relative humidity for 5 weeks. After that, the heat in the glasshouse was turned off, a ventilation device was used to exchange air in the greenhouse to the outside, and therefore the temperature was similar to outside before isotope treatment. We watered the cuttings every 3 days and irrigated with 250 ml modified Hoagland nutrient solution once a week (Hoagland and Arnon 1950) containing EDTA·FeNa 10 μM, MnSO4·H2O 5 μM, ZnSO4·7H2O 1 μM, CuSO4·5H2O 1 μM, H3BO3 30 μM, H2MoO4 0.5 μM, KH2PO4 1000 μM, MgSO4·7H2O 1000 μM, CaCl2 1000 μM, Na2SO4 1000 μM, and NH4NO3 100 μM; pH 6.5. To make this study more comparable to the field condition, we cultivated P. simonii seedlings with relatively low N concentration because P. simonii often grows on N-limited sites. To increase nitrogen uptake, plants were irrigated with Hoagland nutrient solution free of nitrogen for 2 weeks before 15N treatment.
15N treatment and sampling
Plants were treated with 250 ml 99.09 atom% enriched to 0.1 mM 15N isotope-labeled ammonium (15NH4Cl-N), 99.14 atom% enriched to 0.1 mM 15N isotope-labeled nitrate (K15NO3-N), and 15N isotope-labeled NH4NO3 (either 15NH4NO3 or NH 154 NO3) in three seasons: spring (28 May), summer (28 July), and autumn (28 Sep). Each treatment was applied to four seedlings. The total biomass of plants in May, July, and September was 3.03 ± 0.25, 5.31 ± 0.28, and 7.21 ± 0.44 g, respectively. Four seedlings served as controls. They did not receive any 15N-labeled nitrogen and thus were used to determine the natural abundance of 15N signature of plants (Templer and Dawson 2004). Plants were harvested 4 days following 15N addition and partitioned into fine roots (<2 mm in diameter), roots (coarse root and main root), stems, and leaves. The experimental period was limited to 4 days, which was sufficient to detect enriched 15N in different tissues of plants. The fine roots, roots, stems, and leaves of each seedling were dried at 65 °C for at least 48 h until the dry weight was unchanged, and then ground into powder for further analysis. The total nitrogen (N%) and 15N abundance were determined using a Thermo Electron Flash EA 1112 elemental analyzer coupled to a Thermo Electron Delta V isotope ratio mass spectrometer (EA-IRMS). The 15N abundance was expressed as a delta value (δ 15N, per mil, ‰) relative to atmospheric N2: δ 15N = [(R sample – R standard)/R standard] × 1000, where R = 15N/14N and R standard is atmospheric N2, and atom% of 15N is equal to R × 100/(1 + R). Reproducibility of the δ 15N measurements was better than 0.2 ‰.
We calculated the 15N content of different tissues including fine root, root, stem, and leaf as: the total 15N content = DWtissue × N%tissue × (atom%tissue − atom%control).
The total 15N uptake of the plant was equal to the total 15N content of all tissues.
The 15N uptake of fine roots was calculated as the total 15N uptake of plant/dry weight of fine root reflecting the N-uptake ability of fine root per biomass.
The effect of nitrogen form on P. simonii plant growth
To know the effect of different nitrogen forms (NH4-N, NH4NO3, NO3-N) on plant growth, we carried out another independent experiment with 2-year-old P. simonii seedlings fertilized with NH4Cl, or NH4NO3, or KNO3 for 8 weeks. The P. simonii cuttings were also planted in the same glasshouse described above for 5 weeks and irrigated with 250 ml modified Hoagland nutrient solution once a week (Hoagland and Arnon 1950). The fertilized experiment was started on May 28 with similar height of P. simonii seedlings described above and the temperature was not controlled manually. There was a ventilation device to exchange air in the greenhouse to the outside; therefore, the temperature in the greenhouse was close to outside. To maximize the effect of nitrogen form on plant growth, we added a high concentration of nitrogen (250 ml modified Hoagland nutrient solution including 5 mM NH4-N, NH4NO3, or NO3-N) once a week in the fertilizer experiment compared to that described above. Each fertilizer treatment was applied to ten plants. Near the harvest date, the net photosynthetic rate was measured from 9:00 to 11:00 hours with a portable photosynthesis system (Li-Cor-6400; Li-Cor, Inc, Lincoln, NE) with an attached LED light source (500 μmol photon m−2 s−1). The CO2 concentration in the chambers was 400 μmol min−1, and the air flow was 500 μmol s−1. The chlorophyll content of each plant chosen for gas exchange was measured with a portable meter (Minolta SPAD 502 Meter). Simultaneously, the height of the main shoot of each plant was measured with a ruler. Plants were harvested 8 weeks after fertilization with different forms of nitrogen and partitioned into above-ground and below-ground parts. The above-ground and below-ground samples were dried at 65 °C to constant mass, and then dry weight was recorded.
Statistical analyses
N form and time effect on N uptake were analyzed by two-way analyses of variance (ANOVA). The effect of N form on the plant growth was assessed by one-way ANOVA to test the significant differences between treatments and plant parts. When significant, Fisher’s least significant difference test (LSD) was used to identify differences between treatment means. The significance level was established at p < 0.05.
Results
Nitrogen uptake of P. simonii in different seasons
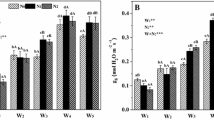
To understand the total N uptake by the whole P. simonii plants within 4 days, we determined the total 15N uptake by summing the 15N content of all the tissues. P. simonii plants had different total 15N uptake when fertilized with different forms of nitrogen across the three investigated seasons (Fig. 1). The total 15N uptake of plants was significantly affected by nitrogen forms (15NO3-N or 15NH4-N) and the seasons (p < 0.05). The total N supplied by 15NO3-N or 15NH4-N was almost the same (0.375 mg/plant), yet different total 15N uptake was observed in May, July, and September. The total uptake of 15N when supplied with sole 15NO3-N was two times higher than that of sole 15NH4-N in all three investigated seasons. In the ammonium nitrate treatments, the total 15N uptake was also two times higher when supplied with NH 154 NO3 compared to 15NH4NO3. Of the different nitrogen forms, the total 15N uptake was the highest when supplied with NH 154 NO3. It was the lowest when supplied with sole NH4 +. Across the three investigated seasons, the total 15N uptake was highest in September and lowest in May.
Fine root is the most important organ for nitrogen acquisition in plants; therefore, we investigated N-uptake ability of fine roots defined as the total 15N uptake of plant/dry weight of fine root. 15N uptake of fine roots was significantly affected by the nitrogen forms and season of application (Fig. 2, p < 0.05). Per unit biomass, the 15N-uptake ability of fine roots was higher in May and July compared to that in September supplied with a given nitrogen form (Fig. 2). Of the different nitrogen forms, the fine roots had the highest 15N uptake when supplied with 15NO3-N and the lowest 15N uptake when supplied with 15NH4-N. Per unit fine root biomass, the uptake of 15N was significantly greater when supplied with NO3-N compared to 15NH4-N in P. simonii in the three investigated seasons, especially in May.
N allocation to different tissues
The relative 15N distribution in different tissues is defined as 15N content of a given tissue relative to the total uptake of 15N by P. simonii plant in this study. The relative 15N distribution varied in different tissues from May to September when supplied with ammonium and/or nitrate (Fig. 3). In May and July, most of the supplied 15N was distributed in leaves, and the percent of 15N was significantly influenced by the nitrogen forms (p < 0.01). In September, the proportion of 15N allocation increased in stems or roots, while it largely decreased in leaves irrespective of the supplied nitrogen form suggesting that nitrogen assimilation was slowed in autumn.
The 15N content of different tissues was significantly affected by N form (Fig. 4, p < 0.05). Generally, different tissues of plants (fine roots, main roots, stems, and leaves) had a higher 15N content when supplied with 15NO3-N or NH 154 NO3 compared to 15NH4-N or 15NH4NO3 (Fig. 4). The supply of extra non-labeled NH4-N (NH 154 NO3) increased the 15N content compared to sole nitrate (15NO3–N) in all tissues except for the main roots. Leaves generally had the largest total 15N content followed by fine roots and stems. The main roots had the lowest N content. The 15N content of plants supplied with 15NO3-N or 15NH4-N varied from May to September in different tissues including fine roots, main roots, stems, and leaves (Fig. 4). All tissues generally had the lowest 15N content in May among the three investigated seasons (May, July, and September) (Fig. 4). 15N content of leaves decreased significantly from July to September in sole N-source treatments indicating that the translocation of N slowed down in September.
The percent of total N was also significantly affected by nitrogen forms in different tissues after a 4-day supply of different forms of nitrogen (Fig. 5). Of different nitrogen forms, nitrate could increase the total N concentration of the roots and stems compared to the other nitrogen forms (NH4Cl or NH4NO3) in a given season. Leaves could have more total N concentration when supplied with sole ammonium compared to the other nitrogen forms (KNO3 or NH4NO3). The total N concentration varied in different tissues in a given season. Generally, leaves had high total N concentration followed by fine roots and stems. The main roots had the lowest total N concentration. Across different seasons, the total N concentration in most of the tissues increased from May to September which was consistent with the total 15N uptake indicating that plants could accumulate more nitrogen as they increased in size.
The effect of nitrogen forms on the growth of Populus simonii
The P. simonii seedlings showed slightly different growth characteristics when supplied with different forms of nitrogen (NH4-N, NO3-N, and NH4NO3). The height increment and chlorophyll content were not significantly affected by the supplied nitrogen forms (Table 1). Plants supplied with NO3-N or NH4NO3 had significantly higher net photosynthetic rate than those supplied with NH4-N.
NH4-N and NO3-N also had different effects on the biomass of P. simonii seedlings. The P. simonii seedlings fertilized with NO3-N had significantly higher total biomass and aboveground biomass (dry weight) compared to those fertilized with NH4-N (Table 2). No significant difference was observed in the biomass of P. simonii seedlings supplied with NO3-N and NH4NO3. Of the different nitrogen forms, NH4NO3 could slightly enhance the belowground biomass of plants. The ratio of belowground biomass to aboveground biomass in P. simonii seedlings was the highest when supplied with NH4-N.
Discussion
N is one of the main macronutrients for plant growth, appropriate N addition is crucial to maximize the plant growth. Excess N fertilization is both an unnecessary cost to growers and a pollution risk to groundwater, while N deficiency often results in poor growth. Thus, appropriate N fertilization is necessary to improve plant growth and avoid N pollution in the soil. N is available in different forms, such as NH4 +, NO3 −, and organic N, and plants often show maximum growth with their preferred N form. It is crucial to determine the effect of different nitrogen forms on plant growth for a given species. Meanwhile, plants may need different forms of nitrogen in different seasons due to variable growth characteristics (Brockley 1995). It is therefore necessary for forest managers to understand nitrogen uptake of forest trees supplied with different forms of nitrogen in different seasons (Lea and Azevedo 2006).
N uptake of P. simonii with different N forms in different seasons
Fast-growing tree species such as Populus spp. are thought to be adapted to a high N supply, mainly NO3 −, in the moving water table of the floodplain forests they inhabit (Rewald et al. 2014); Rennenberg et al. (2010) proposed that nitrate is much more important for nutrition of poplar species than for many other tree species. In the present study, the studied species P. simonii often grows on barren and N-deficient soils, thus we supplied low N content to make this study more close to the field conditions. Actually, under low nutrient supply net uptake of NO3 − has been shown to be higher in Populus tremuloides than in Pinus contorta or Pseudotsuga menziesii (Min et al. 1999). In the present study, higher 15N uptake was observed in P. simonii plants when supplied with 15NO3-N compared to 15NH4-N in different seasons. This result supported our initial hypothesis that P. simonii seedlings had higher N uptake when supplied with NO3-N compared to NH4-N irrespective of seasons. This might be due to higher concentration of nitrate when supplied with ammonium nitrate compared to sole ammonium/nitrate. A previous study reported that the nitrate reductase enzyme was active in P. tremuloides roots (Min et al. 1998). Additionally, nitrate is much more mobile in soil than ammonium, so the greater 15N uptake of plant supplied with NO3-N could be due to its greater mobility (Vitousek et al. 1982). The high NO3-N uptake observed in this study may be an adaptation of P. simonii to its native habitat, which is on saline and alkali soil, where, the most available nitrogen is NO3-N (Weisgerber and Han 2001).
The different amounts of nitrogen taken up in spring, summer, and autumn suggested that plants might require different amounts of nitrogen during different seasons. The different amounts of N taken up in different seasons could relate to the size of the root system. As the plants grew, their root systems grew, and therefore, could take up more nitrogen. The amount of N might be affected by the biomass of P. simonii seedlings which were different in spring, summer, and autumn. The uptake of 15N increased with increasing plant biomass from May to September which suggests that the requirement of nitrogen increased as the plants increased in size (Li et al. 2009). The P. simonii seedlings still require nitrogen to maintain growth in autumn. Therefore, the seasonal rates of N addition should be considered to avoid N deficiency or N pollution in different seasons.
N allocation of P. simonii with different N forms during different seasons
For trees, N is variable in roots, stems, and leaves in different seasons (Weatherall et al. 2006; Brereton et al. 2013). In summer, most of the N was distributed in leaves because leaves constitute the dominant N sink during the summer. However, in autumn, N concentration is reduced in leaves and increased in roots and stems. This suggests that roots or stems are the main storage organs for N in autumn. High N in stems by autumn was also observed in willow (Brereton et al. 2013). Different N allocation in different tissues reflected N recycling across different seasons (Dohleman et al. 2012). This suggests that the stem is the major N storing tissue during winter (von Fircks et al. 2001).
The effect of nitrogen form on growth of P. simonii
In the present study, we studied the effect of inorganic N, NH4-N, and/or NO3-N, on P. simonii plant growth. P. simonii seedlings had a higher total biomass and aboveground biomass (dry weight) fertilized with NO3-N or NH4NO3 compared to NH4-N (Table 2). The results of this study support our initial hypothesis that NO3-N would be more important compared to NH4-N for the growth of P. simonii seedlings. This agreed with Rennenberg et al. (2010) who stated that most poplar species prefer NO3-N. It is also consistent with a previous report that 50 % NO3-N fertilization could improve biomass productivity of poplar and willow clones (Domenicano et al. 2011). Similarly, N fertilization with 80 % NO3-N for Populus deltoides used for short-rotation coppice optimized whole-plant growth (Woolfolk and Friend 2003). Nitrogen forms also affect poplar root structure (Domenicano et al. 2011), and hence, the root biomass might also be affected by the nitrogen form.
In conclusion, NO3-N is crucial for P. simonii plant growth. The amount of nitrogen required for P. simonii plants varied in different seasons; thus, the dose of nitrogen should be carefully considered for fertilization management in different seasons.
Author contribution statement
Conceived and designed the experiments: Chunxia Zhang, and Zhong Zhao. Performed the experiments: Chunxia Zhang, Sen Meng, Yiming Li, and Li Su. Analyzed the data: Chunxia Zhang, and Sen Meng. Wrote the paper: Chunxia Zhang, and Zhong Zhao.
References
Atkin OK, Cummins WR (1994) The effect of nitrogen source on growth, nitrogen economy and respiration of two high arctic plant species differing in relative growth rate. Funct Ecol 8:389–399
Bown H, Watt M, Clinton P, Mason E (2010) Influence of ammonium and nitrate supply on growth, dry matter partitioning, N uptake and photosynthetic capacity of Pinus radiata seedlings. Trees 24:1097–1107
Brereton NJ, Pitre FE, Shield I, Hanley SJ, Ray MJ, Murphy RJ, Karp A (2013) Insights into nitrogen allocation and recycling from nitrogen elemental analysis and 15N isotope labelling in 14 genotypes of willow. Tree Physiol 34:1252–1262
Britto DT, Kronzucker HJ (2002) NH4 + toxicity in higher plants: a critical review. J Plant Physiol 159:567–584
Brockley RP (1995) Effects of nitrogen source and season of application on the nutrition and growth of iodgepole pine. Can J For Res 25:516–526
Contosta AR, Frey SD, Cooper AB (2011) Seasonal dynamics of soil respiration and N mineralization in chronically warmed and fertilized soils. Ecosphere 2:art36
Cooke JEK, Weih M (2005) Nitrogen storage and seasonal nitrogen cycling in Populus: bridging molecular physiology and ecophysiology. New Phytol 167:19–30
Cruz C, Lips SH, Martins-Loução MA (1993) Interactions between nitrate and ammonium during uptake by carob seedlings and the effect of the form of earlier nitrogen nutrition. Physiol Plant 89:544–551
Dohleman FG, Heaton EA, Arundale RA, Long SP (2012) Seasonal dynamics of above- and below-ground biomass and nitrogen partitioning in Miscanthus × giganteus and Panicum virgatum across three growing seasons. GCB Bioenergy 4:534–544
Domenicano S, Coll L, Messier C, Berninger F (2011) Nitrogen forms affect root structure and water uptake in the hybrid poplar. New Forest 42:347–362
Finzi AC et al (2007) Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc Natl Acad Sci USA 104:14014–14019
Gilson A, Barthes L, Delpierre N, Dufrêne É, Fresneau C, Bazot S (2014) Seasonal changes in carbon and nitrogen compound concentrations in a Quercus petraea chronosequence. Tree Physiol 34:716–729
Haynes RJ, Goh KM (1978) Ammonium and nitrate nutrition of plants. Biol Rev 53:465–510
Hoagland DR and Arnon DI (1950) The water-culture method for growing plants without soil. Circular California Agricultural Experiment Station 347
Lea PJ, Azevedo RA (2006) Nitrogen use efficiency. 1. Uptake of nitrogen from the soil. Ann Appl Biol 149:243–247
Li C, Wu C, Duan B, Korpelainen H, Luukkanen O (2009) Age-related nutrient content and carbon isotope composition in the leaves and branches of Quercus aquifolioides along an altitudinal gradient. Trees 23:1109–1121
Lu W (2002) Populus simonii Carr. in North China. Ningxia People’s Press, Yinchuan
Manzone M, Bergante S, Facciotto G (2014) Energy and economic evaluation of a poplar plantation for woodchips production in Italy. Biomass Bioenergy 60:164–170
McKane RB et al (2002) Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415:68–71
Metcalfe RJ, Nault J, Hawkins BJ (2011) Adaptations to nitrogen form: comparing inorganic nitrogen and amino acid availability and uptake by four temperate forest plants. Can J For Res 41:1626–1637
Min X, Siddiqi MY, Guy RD, Glass ADM, Kronzucker HJ (1998) Induction of nitrate uptake and nitrate reductase activity in trembling aspen and lodgepole pine. Plant Cell Environ 21:1039–1046
Min X, Yaeesh Siddiqi M, Guy RD, Glass ADM, Kronzucker HJ (1999) A comparative study of fluxes and compartmentation of nitrate and ammonium in early-successional tree species. Plant Cell Environ 22:821–830
Nicodemus MA (2007) Growth, nutrition, and photosynthetic response of black walnut to varying nitrogen sources and rates. J Plant Nutr 31:1917–1936
Öhlund J, Näsholm T (2001) Growth of conifer seedlings on organic and inorganic nitrogen sources. Tree Physiol 21:1319–1326
Rennenberg H, Wildhagen H, Ehlting B (2010) Nitrogen nutrition of poplar trees. Plant Biol 12:275–291
Rewald B, Kunze ME, Godbold DL (2014) NH4:NO3 nutrition influence on biomass productivity and root respiration of poplar and willow clones. GCB Bioenergy 8:51–58
Schweier J, Becker G (2013) Economics of poplar short rotation coppice plantations on marginal land in Germany. Biomass Bioenergy 59:494–502
Socci AM, Templer PH (2011) Temporal patterns of inorganic nitrogen uptake by mature sugar maple (Acer saccharum Marsh.) and red spruce (Picea rubens Sarg.) trees using two common approaches. Plant Ecol Divers 4:141–152
Templer PH, Dawson TE (2004) Nitrogen uptake by four tree species of the Catskill Mountains, New York: implications for forest N dynamics. Plant Soil 262:251–261
Vitousek PM, Gosz JR, Grier CC, Melillo JM, Reiners WA (1982) A comparative analysis of potential nitrification and nitrate mobility in forest ecosystems. Ecol Monogr 52:155–177
von Fircks Y, Ericsson T, Sennerby-Forsse L (2001) Seasonal variation of macronutrients in leaves, stems and roots of Salix dasyclados Wimm. grown at two nutrient levels. Biomass Bioenergy 21:321–334
Weatherall A, Proe MF, Craig J, Cameron AD, Midwood AJ (2006) Internal cycling of nitrogen, potassium and magnesium in young Sitka spruce. Tree Physiol 26:673–680
Wei Z, Du Q, Zhang J, Li B, Zhang D (2012) Genetic diversity and population structure in Chinese indigenous poplar (Populus simonii) populations using microsatellite markers. Plant Mol Biol Rep 31:620–632
Weisgerber H, Han Y (2001) Diversity and breeding potential of poplar species in China. For Chron 77:227–237
Woolfolk WTM, Friend AL (2003) Growth response of cottonwood roots to varied NH4:NO3 ratios in enriched patches. Tree Physiol 23:427–432
Acknowledgments
This research was sponsored by the State Key Basic Research Development Program (973 Program, Grant No. 2012CB416902), the National Natural Science Foundation of China (Grant No. 31500543), and the Fundamental Research Funds for the Central Universities of China (Grant No. Z109021564).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by H. Rennenberg.
Rights and permissions
About this article
Cite this article
Zhang, C., Meng, S., Li, Y. et al. Nitrogen uptake and allocation in Populus simonii in different seasons supplied with isotopically labeled ammonium or nitrate. Trees 30, 2011–2018 (2016). https://doi.org/10.1007/s00468-016-1428-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1428-z