Abstract
Growth and physiological responses of Pinus radiata D. Don seedlings to a combination of N supply regimes (low N = 1.78 mol m−3, high N = 7.14 mol m−3) and ammonium:nitrate ratios (80:20, 50:50 and 20:80; molar basis) were assessed in a hydroponic experiment run over the course of 105 days. Highly significant (P < 0.001) increases in seedling diameter, height, leaf area and dry mass occurred at lower ammonium:nitrate ratios and were two to fourfold greater than the non-significant (for diameter) to marginally significant (P < 0.05 for other dimensions) increases in these dimensions that occurred with greater N supply. Increases in N supply resulted in a highly significant (P < 0.001) reduction in biomass partitioning to roots and highly significant (P < 0.001) increases in allocation to foliage. The ammonium:nitrate ratio was not found to significantly change biomass partitioning to either foliage, stems or roots. Ammonium and nitrate uptake was significantly influenced by N supply and N form and conformed to ammonium and nitrate concentrations in nutrient solution. Uptake rates of ammonium were twice those of nitrate at comparable concentrations suggesting that P. radiata is in the lower end of the ratio of uptake of ammonium to nitrate reported for conifers (range from 2 to 20 mol mol−1). Despite this, plants growing in high ammonium:nitrate ratios were smaller, exhibited luxurious N consumption and lower N use efficiency. Differences in productivity among treatments were partially explained by greater rates of light-saturated photosynthesis associated with nitrate nutrition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) availability is the primary factor limiting productivity in most natural and managed ecosystems (Berendse and Aerts 1987; Aerts and Chapin 2000). Although some plants are reliant on organic forms of N (Ohlund and Nasholm 2004) most N is supplied to plants through ammonification and nitrification (Haynes and Goh 1978; Bloom 1985; Chapin et al. 1987; Marschner 1995). Nitrification plays a minor role in climax communities whereas in most disturbed and cultivated soils, where early successional species dominate, it may assume a major role (Haynes and Goh 1978). Consequently, plants exhibit great differences in their ability to take up and use ammonium and nitrate as sources of N (Haynes and Goh 1978), which reflects the environment to which the species are adapted (Kronzucker et al. 1997, 2003; Min et al. 1999).
Conifers are usually reported to grow faster under ammonium than nitrate (McFee and Stone 1968; van Den Driessche 1971; Kronzucker et al. 1997). However, this generalisation could be biased as most research has been undertaken in temperate and boreal ecosystems in the Northern Hemisphere (e.g. Lavoie et al. 1992; Downs et al. 1993) or mature plantation forests in the Southern Hemisphere where nitrification is minimal (e.g. Adams and Attiwill 1982a, b). Exceptions to this generalisation have been noted. For instance Pinus radiata D. Don, the most widely planted conifer species in the Southern Hemisphere (Lewis and Ferguson 1993), shows enhanced growth on disturbed sites, such as old-fields and pastures (Skinner and Attiwill 1981) that are high in nitrate (Haynes and Goh 1978; Vitousek et al. 1989; Parfitt et al. 2003). Despite this, previous work with P. radiata has shown poor growth for this species when nitrate is the sole N source (i.e., McFee and Stone 1968). This apparent paradox may be due to the often noted increase in plant growth performance under a mixture of ammonium and nitrate rather than either source alone (Haynes and Goh 1978; Chapin et al. 1987; Marschner 1995; Warren and Adams 2002a; Rothstein and Cregg 2005). Further research is required to clarify how varying ratios of ammonium:nitrate influence growth of P. radiata.
The influence of N supply and N form on biomass partitioning is also of considerable interest and controversy. Afforestation with fast growing species such as P. radiata represents an effective means of offsetting carbon dioxide emissions to meet national commitments under the Kyoto protocol (Dixon et al. 1994). Despite the importance of root biomass as a contributing component to the carbon budget, below ground biomass remains one of the most poorly quantified components in terrestrial ecosystems (Clark et al. 2001; Gower et al. 2001; Litton et al. 2007). As this component accounts for a significant proportion of the total plantation biomass it is imperative that our understanding of how soil and environmental factors influence this component is improved (Cairns et al. 1997; Kurz et al. 1996; Peichl and Arain 2006). A large body of research has investigated the effects of soil N availability on allocation to roots, and this research generally shows a reduction in biomass allocation to roots as N supply increases (Beets and Whitehead 1996; Santantonio and Santantonio 1987), although exceptions have been noted (King et al. 1999; Nadelhoffer et al. 1985). In contrast, little research has investigated how N form affects allocation to roots and the few studies that have been undertaken are contradictory suggesting that N form may (Kruse et al. 2003, 2010) or may not (Zerihun et al. 1998) have an effect on C allocation to roots.
The aim of the study was to examine the influence of a factorial combination of N form and N supply on growth, nitrogen uptake, nitrogen use efficiency (NUE) and photosynthetic capacity of P. radiata seedlings. We also assessed whether N-level and N-form treatments brought about changes in C partitioning between shoots and roots.
Materials and methods
Plant material
Pinus radiata D. Don seedlings were hydroponically grown under a factorial combination of N supply regimes (low N = 1.78 mol m−3 and high N = 7.14 mol m−3) and ammonium to nitrate ratios (80:20, 50:50 and 20:80). The high N regime was defined as 100 ppm (7.14 mM) as this concentration has been shown to provide optimal growth in Pinus pinaster Ait (Ingestad 1979). The low-N regime was chosen as one-fourth of the high-N concentration. The ammonium to nitrate ratios are expressed on a molar basis, e.g. a ratio 80:20 contains 4 mol of NH4+ for each mol of NO3− in nutrient solution. Following Ingestad (1979), nutrients other than N were provided at 0.420 mol m−3 P, 0.512 mol m−3 K, 0.250 mol m−3 Ca, 0.411 mol m−3 Mg, 0.281 mol m−3 S, 12.535 mmol m−3 Fe, 0.459 mmol m−3 Zn, 0.472 mmol m−3 Cu, 7.281 mmol m−3 Mn, 0.072 mmol m−3 Mo, 18.501 mmol m−3 B, 0.846 mmol m−3 Cl and 0.130 mmol m−3 Na (Table 1).
Seeds were germinated in free-draining containers with vermiculite, watered daily with CaSO4 at 10 mol m−3, and then cultured hydroponically for 2 weeks at ¼ and ½ strength Ingestad (1971, 1979) complete nutrient solution before starting the treatments. A total of 192 similar-size seedlings were randomly transplanted, in groups of four, within forty-eight 4.25 dm3 light-tight root boxes. Lids, which were approximately 20 × 20 cm, were perforated in four corners with 4-cm diameter holes, at a square spacing 13 × 13 cm, for the trees to grow through. Foam-plugs were inserted for each plant at the root–shoot junction, and placed hanging from the lids of the root boxes to support the seedlings. Roots were immersed in 4-L treatment solutions that were changed weekly during the experiment. Root boxes were continuously aerated by immersing flexible tubing into treatment solutions through the centre of the lid which was connected to an air-pump (Aqua One, Model SR, Southampton, UK). Boxes were assigned randomly to nutrient treatments so that treatments were replicated eight times (8 boxes × 2 N supply regimes × 3 ammonium to nitrate ratios).
Plants were equally divided, with respect to treatment, into two controlled growth cabinets (Contherm Phytotron Climate Simulator, Petone, New Zealand). The environment was set to a 22/18°C day/night temperature, 16/8 h day/night regime, with irradiance of 333 ± 49 μmol m−2 s−1 photosynthetically active radiation and a constant relative humidity of 75%.
The experiment was run from December 19, 2004 to April 2, 2005. Plants were destructively harvested at four dates at 32, 57, 77 and 105 days after seedling establishment in the root boxes. At each date one plant per root box was randomly selected and harvested without replacement. A total of 48 plants, comprising 8 plants from each treatment combination, were harvested during each of these times. Dry mass of roots, stems and foliage were determined after oven-drying at 70°C to constant mass. Tissue nitrogen concentration at day 105 was determined based on Kjeldahl digestion and colorimetric methods using a Segmented Flow Analyser (SKALAR Analytical BV, Breda, The Netherlands) at Veritec Laboratories, Rotorua. Plant net N uptake was calculated as the difference between tissue N content at day 105 and day 0.
Ammonium and nitrate uptake and water use measurements
Nutrient solution was sampled (50 cm3) at the beginning and at the end of each of 7 weeks (n = 168 observations) from day 54 to the end of the experiment on day 105, on 24 root boxes (4 boxes per nutrient treatment per week). Uptake rates were determined over a 7-day period from ammonium and nitrate depletion in nutrient solution. Uptake rates were expressed in micromoles of N per gram of dry root tissue to account for differences in root mass between root boxes. Ammonium and nitrate concentration in sample solutions were determined by steam-distillation methods (Bremner 1965). Values of pH were determined in all sample solutions with a pH and conductivity meter. By comparison of initial and final hydroponic solution in three root boxes without plants over a week, ammonium volatilisation was found to be negligible. Water depletion by evapo-transpiration was measured weekly in all root boxes using a graduated cylinder and used to determine long-term water use efficiency (WUE) as plant dry matter/water consumption.
Stable isotope analysis
Oven-dried samples from harvest at day 105 were ball-milled, and the carbon and nitrogen composition determined using a mass spectrometer at the Stable Isotope Laboratory at the University of Waikato, New Zealand. Isotopic composition (δ13C and δ15N) was calculated according to the following expression,
where R is the isotope ratio (13C/12C or 15N/14N) of the sample and R std is the isotope ratio of the reference material (PeeDee belemnite, PDB, 11237.2 × 10−6 and air, respectively). δ15N for treatment solutions were calculated based on δ15N of salts and their proportions in each treatment solution. For plant tissue, we present N isotope composition as Δδ15N relative to the source treatment solution (i.e., Δδ15N = δ15Nplant − δ15Nsource). Carbon and nitrogen isotope discrimination was determined following the convention of Farquhar and Richards (1984), as,
Gas exchange measurements and calculations
The rate of light-saturated photosynthesis at ambient CO2 concentration (A sat) was measured in six to eight plants per treatment, near the experiment end, from March 21 to March 29, 2005 using a portable photosynthesis system (Model 6400, Li-Cor, Lincoln, NE, USA). For each plant, three fascicles were placed inside a 6 cm2 cuvette avoiding shading between needles. Temperature in the growth chamber and the cuvette was maintained at 20°C while leaf-to-air vapour pressure deficit (D) ranged from 1 to 1.5 kPa. Foliage samples were left to equilibrate for 10 min at ambient external CO2 concentration, C a (360 μmol mol−1), with saturating irradiance, Q (400–700 nm), maintained at 2,000 μmol m−2 s−1. Once values of photosynthesis (A), intercellular CO2 concentration (C i) and stomatal conductance (g s) became stable (coefficient of variation ≤2%) values of A sat were recorded. Following the measurement of A sat, foliage samples were carefully removed from the cuvette and cut to match the leaf area exposed to gas exchange. Total surface area of needles was determined based on water volume displacement as described by Johnson (1984).
Nitrogen foliage concentrations were expressed on an leaf area basis (N a) while photosynthetic nitrogen use efficiencies (E N) were defined as A sat/N a. Water use efficiency (E W) was determined as the ratio of A sat to transpiration (E), while long-term NUE was determined as the ratio of total plant dry mass and N content at the experiment end (day 105).
Data analysis
All analyses were undertaken at the plant level using SAS (SAS Institute Inc., Cary, NC, USA). Variables were tested for normality and homogeneity of variance and transformations were made as necessary to meet the underlying statistical assumptions of the models used. The main and interactive effects of N supply and N form on growth and physiological responses at day 105 were examined by analysis of variance and covariance. Adjusted means were presented in the results when analysis showed the covariate initial plant mass (for the variable plant growth) and N m (for the variable Δ15N) to be significant. Tukey’s least significant difference test was used to distinguish among individual means where applicable at a significance level of P ≤ 0.05.
Plant size (mass) may confound the effect of treatments on dry matter partitioning among roots, stems and foliage, and therefore allometric analysis was carried out. Allometric analysis was used to remove the influence of growth on allometry, so that the direct influence of N supply and N form on allocation could be determined independent of plant size. Using data from all harvests at days 32, 57, 77 and 105 (192 plants), the relationship between a particular component, y (root, stem and foliage dry mass), and total plant mass, x, was modelled using the following log linear model; ln y = b 0 + b 1 ln x. Analysis of covariance was used to determine if slopes or intercepts of fitted equations significantly differed between treatment. Using these models predictions of allocation to foliage, stems and roots were made as a function of plant biomass and N supply. Analysis of covariance was also used to determine how treatment influenced fractional root, stem and foliage masses, using total plant mass as the covariate, at days 32, 57, 77 and 105 (192 plants). These analyses also investigated the effect of treatment on root:shoot ratio, defined as the ratio of below ground biomass and above-ground biomass.
The main and interactive effects of N supply and N form on weekly measurements of ammonium and nitrate uptake and pH over 7 weeks were modelled using a mixed effects model that accounted for the repeated measurements made within the experiment. Using averages determined over the 7-week period this data were also analysed using analysis of variance. As both analyses resulted in equivalent results findings from only the simpler analysis of variance are presented.
Results
Treatment influences on growth
There were highly significant (F 2,41 > 9.51, P < 0.001) increases in seedling diameter, height, leaf area and dry mass as the ammonium:nitrate ratios decreased from 80:20 to 20:80 (Table 2). These increases were two to fourfold greater than the non-significant (for diameter) to marginally significant (F 1,41 > 4.57, P < 0.05 for other dimensions) increases in these metrics that occurred with greater N supply (Table 2). None of these plant characteristics were significantly influenced by the interaction of N form and N supply (F 2,41 > 1.95, P > 0.16).
Stem, foliage and root N concentrations were significantly higher in plants grown in ammonium than nitrate-dominated solutions. However, plant N uptake was greater in nitrate-dominated solutions as the plants were larger (Table 2). The leaf area to mass ratio (M) was not significantly influenced by the main or interactive effects of N supply and N form and was on average (± SE) 18.5 ± 0.4 m2 kg−1.
Treatment influences on photosynthetic characteristics
The rate of photosynthesis at saturating irradiance and ambient CO2 (A sat) was significantly influenced by N form (F 2,36 = 7.64, P = 0.002) but not by N supply (F 1,36 = 0.96, P = 0.33) or their interaction (F 2,36 = 0.43, P = 0.65). Values of A sat were significantly greater by 53% at 20:80 (9.5 ± 0.7 μmol m−2 s−1) than at 80:20 (6.2 ± 0.5 μmol m−2 s−1). Values of A sat did exhibit an insignificant increase with nutrient supply from 7.3 ± 0.5 μmol m−2 s−1 at low N to 8.3 ± 0.6 μmol m−2 s−1 at high-N supply regimes (Table 2). Stomatal conductance to CO2 diffusion (g s) was not significantly influenced by the main or interactive effects of N supply and N form (F 5,36 = 1.17, P = 0.34). However, despite this insignificance g s did increase with N supply (from 61 mmol m−2 s−1 at low N to 67 mmol m−2 s−1 at high N) and as the ammonium:nitrate ratio decreased (from 50 mmol m−2 s−1 at 80:20 to 78 mmol m−2 s−1 at 20:80) (Table 2).
Values of E N, NUE, and leaf 15N discrimination (Δ15N) were strongly influenced by N form (F 2,36–41 > 4.7, P < 0.02), while all these characteristics apart from E N (F 1,36 = 2.41, P = 0.13), were significantly influenced by N supply (F 1,39–41 > 34, P < 0.001). The interaction between N form and N supply on all these characteristics was not significant (Table 2). Values of E N and NUE significantly increased from high N to low N and as the proportion of ammonium in nutrient solution decreased (or nitrate increased) (Table 2). Values of Δ15N followed the opposite pattern to E N and NUE significantly increasing from low N to high N and as the proportion of ammonium in nutrient solution increased (or nitrate decreased) (Table 2). The effect of N supply was almost twice than that of N form on Δ15N values (Table 2).
Values of photosynthetic water use efficiency (E W), long-term WUE, foliage C isotope discrimination (Δ13C) and long-term C i/C a values were not significantly influenced by main or interactive effects of N supply and N form (F 5,35–41 = 0.18–0.84, P > 0.52) (Table 2).
Treatment influences on dry matter partitioning
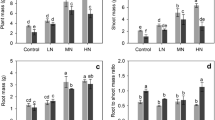
After correcting for the effects of plant biomass, allocation to roots was significantly higher, while allocation to foliage was significantly lower in the low N compared to the high-N supply regime. The N form had no significant influence on allocation (Fig. 1). Slopes (F 1,184 > 4.3, P < 0.04), but not intercepts (F 1,184 < 0.85, P > 0.35), of the linear relationships between log-transformed foliage and root mass to log-transformed total plant mass were significantly different between N supply regimes (Fig. 1a, e). Stem mass fraction was not significantly influenced by N supply (Fig. 1c). Using these models predicted variation in fractional masses of roots, stems and foliage as a function of seedling size (mass), and N supply, are shown in Fig. 1b, d, f, respectively. These figures show divergence in treatment allocation to roots and foliage with increasing seedling biomass.
On the left side, relationships between plant mass (W T) and a root (W R), c stem (W S) and e foliage (W F) mass as influenced by N supply regimes (n = 182). Open symbols and dashed lines represent low N (1.78 mol m−3) and closed symbols and solid lines high N (7.14 mol m−3), that differ between N supply for a and e, but not c. a W R = 0.3277 W 0.9667T , r 2 = 0.97, P < 0.001 (LN); W R = 0.3176 W 0.9234T , r 2 = 0.98, P < 0.001 (HN). c W S = 0.0637 W 1.2866T , r 2 = 0.98, P < 0.001. e W F = 0.6187 W 0.9622T , r 2 = 0.99, P < 0.001 (LN); W F = 0.6668 W 0.9612T , r 2 = 0.99, P < 0.001 (HN). All these relationships were not influenced by N form (P > 0.05) and additionally, the relationship between W S and W T was not influenced by N supply regime and therefore a single equation was fitted. On the right side, relationships between plant mass and b root fraction, d stem fraction and f foliage fraction as influenced by N supply regimes. These values were calculated based on equations fitted in a, c and e
Analyses undertaken using allometric analysis agree with results from the analysis of covariance undertaken on fractional biomasses. Least square means from these models show a significant decrease in root mass fraction with N supply that was on average (±1 SE) 0.294 ± 0.005 at LN and 0.266 ± 0.005 at HN supply (Table 2). In contrast, foliage mass fraction significantly increased with N supply, and was 0.567 ± 0.005 at LN and 0.595 ± 0.004 at HN supply (Table 2). These changes were reflected in the highly significant variation in the root:shoot ratio, that decreased from 0.425 ± 0.011 at LN supply to 0.367 ± 0.009 at HN supply (F 1,181 = 15.07, P < 0.001). Stem mass fraction was not significantly influenced by main or interactive effects of N supply and N form (F 6,181 = 1.63, P = 0.14) being on average 0.138 ± 0.002 (Table 2).
Treatment influences on ammonium and nitrate uptake
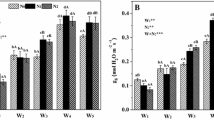
Ammonium, nitrate and ammonium plus nitrate uptake (over 24 h) were significantly influenced by N supply (F 1,18 > 11, P < 0.004) and N form (F 2,18 > 11, P < 0.001), but not their interaction (F 2,18 < 2.4, P > 0.12) with the exception of nitrate uptake (F 2,18 = 7.8, P = 0.004). The relative rates of ammonium and nitrate uptake conformed to ammonium and nitrate concentrations in nutrient solutions (Fig. 2a, b). However, the rate of ammonium uptake was on average 2.0 ± 0.2 times greater than that of nitrate at comparable concentrations, and the difference tended to increase with N supply from 1.65 ± 0.15 at LN (50:50) to 2.31 ± 0.30 at HN (50:50). This preferential uptake of ammonium was also reflected in the ratio of ammonium to nitrate uptake across the range in N form solutions, from 0.74 ± 0.18 at a ammonium:nitrate ratio of 20:80 to 6.04 ± 0.82 at a ammonium:nitrate ratio of 80:20. Analysis of covariance showed that nitrate uptake did not influence ammonium uptake and vice versa (F 1,17 = 2.11, P = 0.16).
Influence of NH4 +:NO3 − ratio under low (open bars) and high (filled bars) N supply on a ammonium, b nitrate, c ammonium plus nitrate uptake (24 h) and d pH of nutrient solution (at the end of a week). Values are presented as means (±1 SE; n = 4) for each treatment. Different letters indicate significant differences between NH4 +:NO3 − ratios at P < 0.05. Differences between N supply regimes within the same NH4 +:NO3 − ratio are shown as: ns, non-significant; * significant at P < 0.05; ** significant at P < 0.01; *** significant at P < 0.001. Interactive effects between N supply regime and NH4 +:NO3 − ratio were not significant (P > 0.05) for ammonium and ammonium plus nitrate uptake, but were significant (P < 0.05) for nitrate uptake and pH
Ammonium plus nitrate uptake (24 h) significantly increased by 72% with N supply from 187 ± 12 μmol g−1 at LN to about 322 ± 19 μmol g−1 at HN. Seedlings grown at a greater proportion of ammonium exhibited luxurious consumption of nitrogen (Fig. 2c), that translated into greater tissue N concentration (Table 1). Total N uptake increased by 56% from 202 ± 18 μmol g−1 for plants grown at 20:80 to 315 ± 41 μmol g −1 at 80:20.
The pH of nutrient solution measured at the end of each week was significantly influenced by N supply (F 1,18 = 257, P < 0.001), N form (F 2,18 = 126, P < 0.001) and their interaction (F 2,18 = 40, P < 0.001) (Fig. 2d). Values of pH were adjusted to lie between 5.0 and 5.5 at the beginning of each week. We observed a decrease in ending pH with N supply being on average 5.3 ± 0.4 at LN compared to 3.5 ± 0.1 at HN. Ending pH also increased with [NO3 −] and decreased with [NH4 +] in nutrient treatments being on average 3.5 ± 0.1 at 80:20 compared to 5.5 ± 0.5 at 20:80, respectively.
Discussion
This study suggests that P. radiata seedlings are well adapted to use both NH4 + and NO3 − as sources of N, which may partly explain the success of this species over a wide range of ecological conditions. This is consistent with a large body of evidence that shows most species grow better with a mixture of ammonium and nitrate rather than either source alone (e.g. van Den Driessche 1971; Cox and Reisenauer 1973; Bigg and Daniel 1978; Haynes and Goh 1978; Chapin et al. 1987; Marschner 1995; Warren and Adams 2002a; Rothstein and Cregg 2005). In a previous study, McFee and Stone (1968) showed that P. radiata performed poorly when supplied with nitrate as the sole source of N. Although we did not include a nitrate-only control, previous research on other species suggests that sole use of nitrate results in lower productivity. When nitrate is supplied as the sole N source for P. pinaster growth is reduced to a greater extent than that explained by slower uptake or lower photosynthetic rates (Warren and Adams 2002a). Among the likely reasons for this are nutrient imbalances (Haynes and Goh 1978; Marschner 1995), greater synthesis of organic acids to restore imbalance of hydroxyl ions (Raven and Smith 1976; Warren and Adams 2002a) and excessive carbon loss to the growing media (Vuorinen et al. 1995).
In this study, seedlings of P. radiata developed more rapidly under nitrate- compared to ammonium-dominated N supply. Differences in productivity were at least partially explained by greater photosynthetic rates in plants grown in nitrate-dominated solutions. Similarly, Rothstein and Cregg (2005) found that photosynthesis rates of Abies fraseri declined markedly under ammonium compared to nitrate-dominated N supply. Bloom et al. (1989) suggested that nitrate assimilation was not competitive with carbon fixation in barley plants grown at high irradiance suggesting that chloroplast electron transport has a capacity beyond that immediately required for carbon fixation. Similarly, Zerihun et al. (1998) in a study of energy costs of N form acquisition showed that nitrate assimilation in well-illuminated leaves might not be much more expensive than ammonium acquisition. Thus, additional energetic costs associated with nitrate nutrition might be offset by excess reductants supplied from a surplus in electron transport (Bloom et al. 1989) or by up-regulation of photosynthesis as suggested by Rothstein and Cregg (2005) and also by the results of this study.
Few studies have characterised the influence of N form on biomass partitioning in forest species (e.g. Heiskanen 2005; Bauer and Berntson 2001), and we are unaware of any investigation considering N form after accounting for the effect of plant size. Using allometric analysis we found that partitioning to root and stems, and root:shoot ratio was influenced by N supply regime but not by N form. This result is relevant as there is an on-going debate as whether N form may (e.g. Kruse et al. 2003, 2010) or may not (e.g. Zerihun et al. 1998) influence shoot–root biomass partitioning.
Seedlings growing under ammonium-dominated solutions were smaller, exhibited luxurious consumption of N and lower N use efficiency. Several authors have reported luxurious consumption of N associated with ammonium nutrition (McFee and Stone 1968; van Den Driessche 1971; van Den Driessche and Dangerfield 1975; Haynes and Goh 1978; Flaig and Mohr 1991; Lavoie et al. 1992; Malhi et al. 1988; Warren and Adams 2002a). Independent of N form, photosynthesis rates are known to be closely related to foliar nitrogen (Field and Mooney 1986; Walcroft et al. 1997; Grassi et al. 2002; Ripullone et al. 2003), which is explained by the high proportion of total nitrogen partitioned to the carboxylating enzyme Rubisco (Sage and Pearcy 1987; Evans 1989; Warren and Adams 2002b; Takashima et al. 2004). Considering N form, the results of the study show that photosynthetic rates were lower and foliar nitrogen greater in plants growing under ammonium rather than nitrate-dominated solutions, suggesting that N partitioning to active Rubisco decreased while N storage increased with ammonium nutrition. Warren and Adams (2002a) showed that Rubisco concentration remained constant in seedlings of P. pinaster supplied with ammonium, nitrate or a mixture, while foliage N concentration increased with ammonium nutrition, providing further evidence of N storage associated with ammonium nutrition in conifers (e.g. Flaig and Mohr 1991; Lavoie et al. 1992; Kronzucker et al. 1997). Although we found that ammonium-dominated plants showed enhanced N uptake, and nitrogen seems to accumulate in leaves without a concomitant effect on photosynthesis and growth, it is possible that some other factor became growth-limiting. The element that is most likely to become limiting is phosphorus for which uptake is strongly pH-dependent. However, this seems unlikely as plant level foliage phosphorus concentrations at the end of the experiment ranged from 0.255 and 0.429%, which markedly exceed the value of 0.12% thought to be optimal for P. radiata (Turner and Lambert 1986).
There is an on-going discussion whether foliage sucrose, foliage protein concentration or plant/shoot N controls the biomass partitioned between roots and shoots (Andrews et al. 2007; Hermans et al. 2007). Hermans et al. (2007) argues that plant root:shoot increases with N deficiency as shoot N concentration decreases, and also noted that nitrate content in leaves is directly related to N supply and negatively correlated to the carbon partitioned to the root. Hermans et al. (2007) propose that an increase in shoot sucrose leads to an increase in plant root:shoot when sucrose can be translocated to the root. Andrews et al. (2007), on the other hand, proposes that leaf soluble protein concentration drives the root:shoot ratio regardless of N form (NO3 −, NH4 +, glutamine, among others). Hermans et al. (2007) and Andrews et al. (2007) positions are reconciled by recognising that carbon partitioning between shoots and roots are co-limited by the availability of C and N substrates, and that the shoot C:N ratio, determines the relative partitioning of C for shoot metabolism or phloem export. Plants growing under ammonium-dominated solutions in our study were smaller, exhibited luxurious consumption of N, had a greater N concentration and did not exhibit changes in C partitioning to roots or shoots compared to other mixtures of ammonium and nitrate. Only the N supply regime changed the proportion of carbon partitioned to roots. It seems likely that not all C assimilated through photosynthesis and not all N content at the leaf is involved as a signal for carbon allocation. Plants growing in the ammonium-dominated solutions exhibited high foliage N concentration and they did not exhibit smaller root:shoot ratios, possibly as leaf soluble proteins were uniform across N-form treatments (Andrews’s hypothesis). On the other hand, plants growing in nitrate-dominated solutions exhibited greater photosynthesis rates compared to other mixtures of ammonium and nitrate and did not exhibit differences in C partitioned to roots, indicating that probably not all C assimilated, maybe shoot sucrose only, is involved in signalling root:shoot partitioning (Herman’s hypothesis). The carbon fraction partitioned to roots in this study was independent of N form suggesting that unravelling drivers behind carbon allocation require further experiments involving shoot and root C and N fractionation of plants growing under different N sources.
Ammonium uptake was about twofold greater than that of nitrate (on a molar basis) at comparable concentrations in nutrient solutions. Kronzucker et al. (1997) proposed that the ability to use ammonium and nitrate depends on the species successional stage, with early successional species growing better on nitrate (more disturbed soils) and late successional species growing better on ammonium (less disturbed soils). The empirical evidence strongly supports this theory (e.g. Krajina et al. 1973; Hangs et al. 2003; Chen et al. 2005). Thus, the ratio of ammonium to nitrate uptake on a molar basis might be used as an index of this successional ability, with values close to 1 mol mol−1 (equal uptake of ammonium and nitrate) occurring in agricultural species and values in the range between 2.9 and 20 mol mol−1 for conifers (Kronzucker et al. 1997, 2003). This would suggest that P. radiata occurs at the lower end of the range given for conifers from Kronzucker’s work, partly explaining its enhanced early growth on disturbed sites such as old-fields and pastures (Skinner and Attiwill 1981) where light is not usually as limiting as it is in mature undisturbed forests. Therefore, conifer species could be ordered using the ammonium:nitrate uptake ratio (Φ, mol mol−1) as a surrogate for their capacity to use nitrate, e.g. P. radiata (Φ = 2, this study) < Pseudotsuga menziesii (Φ = 3, Kronzucker et al. 2003) = Pinus sylvestris (Φ = 3, Flaig and Mohr 1992) < Picea abies (Φ = 4, Buchman et al. 1995) < Pinus contorta (Φ = 6, Min et al. 2000) < Pinus strobus (Φ = 12, Bauer and Berntson 2001) < Picea glauca (Φ = 20, Kronzucker et al. 1997), among others.
Nitrate and ammonium uptake were independent of each other within the range of concentrations used in this study. Some studies suggest that high ammonium concentration will reduce nitrate reductase activity in shoots and roots and therefore inhibit nitrate uptake (Haynes and Goh 1978; Downs et al. 1993; Sagi and Lips 1998). Others, like Flaig and Mohr (1992), found that ammonium and nitrate were taken up at the same rate in seedlings of P. sylvestris as if they were applied separately suggesting that ammonium does not inhibit nitrate uptake (the same as this study) at least in longer-term studies than those involved in uptake kinetics.
Although pH was set to a value of between 5.0 and 5.5 on a weekly basis by changing the solutions, the pH did systematically vary in different treatments over the course of the week. We observed a decrease in ending pH with increasing N supply. In response to N form there was increase in pH with increasing [NO3 −] and a decrease with increasing [NH4 +]. Previous research has shown the pH of the nutrient solution changes the rate of plant uptake of ammonium versus nitrate (van Den Driessche 1978). However, results presented here clearly show that the relative rates of ammonium and nitrate uptake largely conformed to the ammonium and nitrate concentrations in nutrient solution. It is therefore likely that treatment variation in results was attributable to variation in N form and supply and not confounded by treatment variation in pH.
In conclusion, we examined the effects of N supply and N form on growth, uptake and photosynthetic characteristics of P. radiata seedlings grown at high irradiance. Plants grown in ammonium-dominated solutions were smaller, had lower photosynthetic rates and exhibited luxurious consumption of N. Uptake rates of ammonium were about twofold greater on a molar basis than those of nitrate at comparable concentrations, suggesting that P. radiata occurs at the lower end of the ammonium:nitrate uptake ratio expected for conifers (2.9–20 mol mol−1). This may help to explain success of this species in fertile disturbed sites such as ex-pastures where nitrate may represent a high proportion of available N.
References
Adams MA, Attiwill PM (1982a) Nitrogen mineralization and nitrate reduction in forests. Soil Biol Biochem 14:197–202
Adams MA, Attiwill PM (1982b) Nitrate reductase activity and growth response of forest species to ammonium and nitrate sources of nitrogen. Plant Soil 66:373–381
Aerts R, Chapin FSI (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Andrews M, Raven JA, Sprent JI, Lea PJ (2007) Is shoot growth correlated to leaf protein concentration? Trends Plant Sci 12:531–532
Bauer GA, Berntson GM (2001) Ammonium and nitrate acquisition by plants in response to elevated CO2 concentration: the roles of root physiology and architecture. Tree Physiol 21:137–144
Beets PN, Whitehead D (1996) Carbon partitioning in Pinus radiata stands in relation to foliage nitrogen status. Tree Physiol 16:131–138
Berendse F, Aerts R (1987) Nitrogen-use-efficiency: a biologically meaningful definition? Funct Ecol 1:293–296
Bigg WL, Daniel TW (1978) Effects of nitrate, ammonium and pH on the growth of conifer seedlings and their production of nitrate reductase. Plant Soil 50:371–385
Bloom AJ (1985) Wild and cultivated barley show similar affinities for mineral nitrogen. Oecologia 65:555–557
Bloom AJ, Caldwell RM, Finazzo J, Warner RL, Weissbart J (1989) Oxygen and carbon dioxide fluxes from barley shoots depend on nitrate assimilation. Plant Physiol 91:352–356
Bremner JM (1965) Inorganic forms of nitrogen. In: Black CA, Evans DD, White JL, Ensminger LE, Clark FE (eds) Methods of soil analysis. Part 2: chemical and microbiological properties, sect 84. American Society of Agronomy, New York, pp 1179–1237
Buchman N, Schulze ED, Gebauer G (1995) 15N-ammonium and 15N-nitrate uptake of a 15-year-old Picea abies plantation. Oecologia 102:361–370
Cairns MA, Brown S, Helmer EH, Baumgardner GA (1997) Root biomass allocation in the world’s upland forests. Oecologia 111:1–11
Chapin FS, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. Bioscience 37:49–57
Chen W, Luo JK, Shen QR (2005) Effect of NH4 +–N/NO3 +–N ratios on growth and some physiological parameters of Chinese cabbage cultivars. Pedosphere 15:310–318
Clark DA, Brown S, Kicklighter DW, Chambers JQ, Thomlinson JR, Ni J (2001) Measuring net primary production in forests: concepts and field methods. Ecol Appl 11:356–370
Cox WJ, Reisenauer HM (1973) Growth and ion uptake by wheat supplied nitrogen as nitrate, or ammonium or both. Plant Soil 38:363–380
Dixon RK, Brown S, Houghton RA, Solomon AM, Trexler MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263:185–190
Downs MR, Nadelhoffer KJ, Melillo JM, Aber JD (1993) Foliar and fine root nitrate reductase activity in seedlings of four forest tree species in relation to nitrogen availability. Trees 7:233–236
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11:539–552
Field C, Mooney HA (1986) The photosynthesis–nitrogen relationship in wild plants. In: Givnish TJ (ed) The economy of plant form, function. Cambridge University Press, Cambridge, pp 25–55
Flaig H, Mohr H (1991) Effect of high ammonium supply in Scots pine seedlings (Pinus sylvestris L.). Allg Forst Jagdztg 162:35–42
Flaig H, Mohr H (1992) Assimilation of nitrate and ammonium by the Scots pine (Pinus sylvestris) seedlings under conditions of high nitrogen supply. Physiol Plant 84:568–576
Gower ST, Krankina O, Olson RJ, Apps M, Linder S, Wang C (2001) Net primary production and carbon allocation patterns of boreal forest ecosystems. Ecol Appl 11:1395–1411
Grassi G, Meier P, Cromer R, Tompkins D, Jarvis PG (2002) Photosynthetic parameters in seedlings of Eucalyptus grandis as affected by rate of nitrogen supply. Plant Cell Environ 25:1677–1688
Hangs RD, Knight JD, Van Rees KCJ (2003) Nitrogen uptake characteristics for roots of conifer seedlings and common boreal forest competitor species. Can J For Res 33:156–163
Haynes RJ, Goh KM (1978) Ammonium and nitrate nutrition of plants. Biol Rev 53:465–510
Heiskanen J (2005) Effect of nitrate and ammonium on growth of transplanted Norway spruce seedlings: a greenhouse study. Ann Bot Fenn 42:1–9
Hermans C, Hammond JP, Verbruggen N, White PJ (2007) Response to Andrews et al.: correlations and causality. Trends Plant Sci 12:532–533
Ingestad T (1971) A definition of optimum nutrient requirements in birch seedlings. II. Physiol Plant 24:118–125
Ingestad T (1979) Mineral nutrient requirements of Pinus silvestris and Picea abies seedlings. Physiol Plant 45:373–380
Johnson JD (1984) A rapid technique for estimating total surface area of pine needles. For Sci 30:913–921
King JS, Pregitzer KS, Zak DR (1999) Clonal variation in above- and below-ground growth responses of Populus tremuloides Michaux: influence of soil warming and nutrient availability. Plant Soil 217:119–130
Krajina VJ, Madoc-Jones S, Mellor G (1973) Ammonium and nitrate in the nitrogen economy of some conifers growing in Douglas-fir communities of the Pacific North-West of America. Soil Biol Biochem 5:143–147
Kronzucker HJ, Siddiqi MY, Glass ADM (1997) Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 385:59–61
Kronzucker HJ, Siddiqi MY, Glass ADM, Britto DT (2003) Root ammonium transport efficiency as a determinant in forest colonization patterns: an hypothesis. Physiol Plant 117:164–170
Kruse J, Hetzger I, Mai C, Polle A, Rennenberg H (2003) Elevated pCO2 affects N-metabolism of young poplar plants (Populus tremula × P. alba) differently at deficient and sufficient N-supply. New Phytol 157:65–81
Kruse J, Hänsch R, Mendel R (2010) The role of root nitrate reduction in the systemic control of biomass partitioning between leaves and roots in accordance to the C/N-status of tobacco plants. Plant Soil 332:387–403
Kurz WA, Beukema SJ, Apps MJ (1996) Estimation of root biomass and dynamics for the carbon budget model of the Canadian forest sector. Can J For Res 26:1973–1979
Lavoie N, Vezina LP, Margolis HA (1992) Absorption and assimilation of nitrate and ammonium ions by jack pine seedlings. Tree Physiol 11:171–183
Lewis NB, Ferguson IS (1993) Management of radiata pine. Inkata Press, Melbourne, 404 pp
Litton CM, Raich JW, Ryan MG (2007) Carbon allocation in forest ecosystems. Glob Change Biol 13:2089–2109
Malhi SS, Nyborg M, Jahn HG, Penney DC (1988) Yield and nitrogen uptake of rapeseed (Brassica campestris L.) with ammonium and nitrate. Plant Soil 105:231–239
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London, 889 pp
McFee WW, Stone EL (1968) Ammonium and nitrate as nitrogen sources for Pinus radiata and Picea glauca. Soil Sci Soc Am J 32:879–884
Min X, Siddiqi MY, Guy RD, Glass ADM, Kronzucker HJ (1999) A comparative study of fluxes and compartmentation of nitrate and ammonium in early-successional tree species. Plant Cell Environ 22:821–830
Min X, Siddiqi MY, Guy RD, Glass ADM, Kronzucker HJ (2000) A comparative kinetic analysis of nitrate and ammonium in flux in two early-successional tree species of temperate and boreal ecosystems. Plant Cell Environ 23:321–328
Nadelhoffer KJ, Aber JD, Melillo JM (1985) Fine roots, net primary production, and soil-nitrogen availability—a new hypothesis. Ecology 66:1377–1390
Ohlund J, Nasholm T (2004) Regulation of organic and inorganic nitrogen uptake in Scots pine (Pinus sylvestris) seedlings. Tree Physiol 24:1397–1402
Parfitt RL, Scott NA, Ross DJ, Salt GJ, Tate KR (2003) Land-use change effects on soil C and N transformations in soils of high N status: comparisons under indigenous forest, pasture and pine plantation. Biogeochemistry 66:203–221
Peichl M, Arain MA (2006) Above- and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests. Agric For Meteorol 140:51–63
Raven JA, Smith FA (1976) Nitrogen assimilation and transport in vascular land plants in relation to intracellular pH regulation. New Phytol 76:415–431
Ripullone F, Grassi G, Lauteri M, Borghetti M (2003) Photosynthesis-nitrogen relationships: interpretation of different patterns between Pseudotsuga menziesii and Populus × euroamericana in a mini-stand experiment. Tree Physiol 23:137–144
Rothstein DE, Cregg BM (2005) Effects of nitrogen form on nutrient uptake and physiology of Fraser fir (Abies fraseri). For Ecol Manag 219:69–80
Sage RF, Pearcy RW (1987) The nitrogen use efficiency of C3 and C4 plants. Plant Physiol 84:959–963
Sagi M, Lips HS (1998) The levels of nitrate reductase and MoCo in annual ryegrass as affected by nitrate and ammonium nutrition. Plant Sci 135:17–24
Santantonio D, Santantonio E (1987) Seasonal changes in live and dead fine roots during two successive years in a thinned plantation of Pinus radiata in New Zealand. N Z J For Sci 17:315–328
Skinner MF, Attiwill PM (1981) The productivity of pine plantations in relation to previous land use. Plant Soil 60:161–176
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27:1047–1054
Turner J, Lambert MJ (1986) Nutrition and nutritional relationships of Pinus radiata. Annu Rev Ecol Syst 17:325–350
van Den Driessche R (1971) Response of conifer seedlings to nitrate and ammonium sources of nitrogen. Plant Soil 34:421–439
van Den Driessche R (1978) Response of Douglas-fir seedlings to nitrate and ammonium nitrogen sources at different levels of pH and iron supply. Plant Soil 49:607–623
van Den Driessche R, Dangerfield J (1975) Response of Douglas-fir seedlings to nitrate and ammonium nitrogen sources under various environmental conditions. Plant Soil 42:685–702
Vitousek PM, Matson PA, Van Cleve K (1989) Nitrogen availability and nitrification during succession: primary, secondary, and old-field series. Plant Soil 115:229–239
Vuorinen AH, Rossi P, Vapaavuori EM (1995) Combined effect of inorganic carbon and different nitrogen sources in the growth media on biomass production and nitrogen uptake in young willow and birch plants. J Plant Physiol 147:236–242
Walcroft AS, Whitehead D, Silvester WB, Kelliher FM (1997) The response of photosynthetic model parameters to temperature and nitrogen concentration in Pinus radiata D. Don. Plant Cell Environ 20:1338–1348
Warren CR, Adams MA (2002a) Possible causes of slow growth of nitrate-supplied Pinus pinaster. Can J For Res 32:569–580
Warren CR, Adams MA (2002b) Phosphorus affects growth and partitioning of nitrogen to Rubisco in Pinus pinaster. Tree Physiol 22:11–19
Zerihun A, McKenzie BA, Morton JD (1998) Photosynthate costs associated with the utilization of different nitrogen forms: influence on the carbon balance of plants and shoot-root biomass partitioning. New Phytol 1998:1–11
Acknowledgments
During this work the senior author was supported by SCION, the University of Canterbury, the University of Chile and by a Doctoral Scholarship provided by Education New Zealand. We thank Mr. Alan Leckie, Mr. Dave Conder, Mr. Nigel Pink, Mrs. Vicki Wilton and Mr. Lachlan Kirk for their kind advice and valuable technical skills. The experiments and measurements undertaken for this paper comply with the current laws of New Zealand.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Adams.
Rights and permissions
About this article
Cite this article
Bown, H.E., Watt, M.S., Clinton, P.W. et al. Influence of ammonium and nitrate supply on growth, dry matter partitioning, N uptake and photosynthetic capacity of Pinus radiata seedlings. Trees 24, 1097–1107 (2010). https://doi.org/10.1007/s00468-010-0482-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-010-0482-1