Abstract
The declines in soil fertility and productivity in continuously cropped poplar plantations are related to phenolic acid accumulation in the soil. Nitrogen is a vital life element for poplar and whether the accumulation of phenolic acid could influence nitrogen metabolism in poplar and thereby hinder continuous cropping is not clear. In this study, poplar cuttings of Populus × euramericana ‘Neva’ were potted in vermiculite, and phenolic acids at three concentrations (0X, 0.5X and 1.0X) were added according to the actual content (1.0X) in the soil of a second-generation poplar plantation. Each treatment had eight replicates. We measured gas exchange parameters and the activities of key enzymes related to nitrogen metabolism in the leaves. Leaf photosynthetic parameters varied with the concentration of phenolic acids. The net photosynthetic rate (PN) significantly decreased with increasing phenolic acid concentration, and non-stomatal factors might have been the primary limitation for PN. The activities of nitrate reductase (NR), glutamine synthetase (GS) and glutamate synthase (GOGAT), as well as the contents of nitrate nitrogen, ammonium nitrogen, and total nitrogen in the leaves decreased with increasing phenolic acid concentration. This was significantly and positively related to PN (P < 0.05). The low concentration of phenolic acids mainly affected the transformation process of NO3− to NO2−, while the high concentration of phenolic acids affected both processes, where NO3− was transferred to NO2− and NH4+ was transferred to glutamine (Gln). Overall, phenolic acid had significant inhibitory effects on the photosynthetic productivity of Populus × euramericana ‘Neva’. This was probably due to its influence on the activities of nitrogen assimilation enzymes, which reduced the amount of amino acids that were translated into protein and enzymes. Improving the absorption and utilization of nitrogen by plants could help to overcome the problems caused by continuous cropping.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
China’s demand for timber is large and exceeds the domestic supply capacity. Due to the shortage of soil resources, continuous cropping management has typically been used in most forest cultivation (Li et al. 2014, 2016). However, this has resulted in problems such as reductions in productivity and stand volume growth (Zhang and Li 2005; Wu et al. 2014). Poplar is widely planted in the Yangtze-Huaihe river basin to help supply China’s demand for timber. However, due to limited suitable land for poplar plantations, continuous cropping is practiced and has caused the problems cited above (Liu et al. 2005; Wang et al. 2010a, 2011). Rlum and Gric (2005) reported that the reductions observed in continuously cropped plantations were related to allelochemical accumulation in the soils. Phenolic acid is an allelochemical with strong allelopathic effects that have been studied in tree plantations (Schutter et al. 2001; Ye et al. 2004; Jeffrey and John 2004; Li et al. 2005; Cao et al. 2005; Wang et al. 2006). Tan et al. (2008) and Wang et al. (2010a) detected phenolic substances in the soils of continuously cropped poplar plantations and reported that allelochemical accumulation increased with each successive generation of trees. Wang et al. (2010a, b) and Yang et al. (2010) studied the mechanisms of plantation productivity decline by quantifying the accumulation patterns of soil chemicals and their effects on rhizosphere soil and the root physiology of poplars. They reported that phenolic acid was the primary cause of reduced productivity in continuously cropped poplar plantations.
The soil concentrations of phenolic acids affect the nitrogen uptake of plants. For example, phenolic acids restrain the NO3 uptake by Cucumis sativus and lower its productivity (Lü et al. 2002). After generations of continuous cropping, the nitrogen content in continuously cropped poplar trees has declined significantly with increasing soil concentrations of phenolic acids (Liu et al. 2005). Phenolic acids impair the activity of poplar nitrate reductase (NR) and affect the uptake and use of nitrate nitrogen (Wang et al. 2011). The allelopathic effects of phenolic acids are compounded in the field because poplar trees can produce greater quantities of phenolic substances under nitrogen deficient conditions (Wang et al. 2011). To date, the effects of phenolic acids on the photosynthetic physiological and biochemical parameters of poplar and on nitrogen metabolism in poplar have not been reported. Similarly, the relationships between phenolic substances and nitrogen and ammonia assimilation enzymes have not been reported. Thus, our objectives in this study were to investigate the effects of phenolic acids on poplar photosynthetic physiology and the nitrogen cycle of Populus × euramericana in an effort to find solutions to the problems caused by the continuous cropping of Populus × euramericana. We studied the effects of phenolic acids on photosynthetic physiology parameters, nitrate nitrogen, ammonium nitrogen, and nitrogen and ammonia assimilation enzymes in the leaves of Populus × euramericana ‘Neva’.
Materials and methods
Experimental plants
In late March 2014, we selected strong, healthy, and similar 1-year-old Populus × euramericana ‘Neva’ seedlings for sampling. Cutting slips at 1.5 cm diameter and 10 cm length were obtained from these seedlings for experiments. These slips were transferred to flower pots filled with vermiculite for culture (3 treatments with 6 plants per treatment). Each pot (38 cm in inner diameter and 45 cm in depth) was filled with 16 L of vermiculite, and pots were irrigated. In early May, the seedlings were treated with phenolic acids every 10 days by the addition of 1.6 L phenolic acids to each pot. In mid-August we measured photosynthetic gas exchange parameters, activities of nitrate reductase (NR), glutamine synthetase (GS), glutamate synthase (GOGAT), and nitrate reductase (NR), and the amounts of nitrate nitrogen, ammonium nitrogen, and total nitrogen in Populus × euramericana leaves.
According to the amounts of the five types of phenolic acids (X, X = 247 mg L−1 p-hydroxybenzoic acid + 11 mg L−1 vanillin + 7 mg L−1 ferulic acid + 54 mg L−1 benzoic acid + 2 mg L−1 cinnamic acid) in the soil of a second generation poplar plantation and the absorption rates of these acids (Tan et al. 2008; Wang et al. 2010a), three phenolic acid concentrations (CK (0X), T1 (0.5X), and T2 (1.0X)) were used to evaluate the intergenerational cultivation environments during the growth of the poplar seedling root systems (Table 1).
Photosynthetic gas exchange parameter measurements
We used a portable photosynthesis system (CIRAS-2, Amesbury, MA, USA) to measure the photosynthetic light response parameters. All measurements were conducted from 08:30 to 11:30 a.m. on sunny days. During the measurements, the photosynthetically active radiation (PAR = 1500 μmol m−2 s−1) was supplied by cold Light Emitting Diode (LED) (peak emission wavelength: red light (90%) 620–630 nm and white light (10%) 425–625 nm) via the portable photosynthesis system. Three leaves were measured for each treatment, and the measurements were repeated three times for each leaf. For every repetition, PAR was controlled for 120 s to obtain a stable value. The air temperature of the leaf chamber was controlled at approximately 26 ± 1.5 °C, the relative humidity was 60 ± 5%, and the CO2 concentration was 370 ± 6 μmol m−2, as determined by the photosynthesis system. PAR, PN, transpiration rate (Tr), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and the air CO2 concentration (Ca) were recorded automatically through the instrument. The water-use efficiency (WUE) and stomatal limitation (Ls) were calculated using Eqs. (1) and (2) (Berry and Downton 1982; Nijs et al. 1997; Li et al. 2015):
Measurement of the activities of enzymes (NR, GS and GOGAT)
According to the method used by Kong and Yi (2008), we measured NR activity. The activity of GS was calculated using the method described by Wang et al. (2008). The measurement of GOGAT was conducted according to the method introduced in the literature (Lin and Kao 1996; Lin et al. 2000; Zhang et al. 1997) with minor modifications.
Measurement of the contents of nitrate nitrogen (NO3 −–N), ammonium nitrogen (NH4 +–N), and total nitrogen (total N)
Prior to testing, sample leaves were washed with deionized water, and dried at 85 °C to constant weights. The dried leaves were ground and passed through a 60 mesh screen.
Leaf samples were weighed and mixed with deionized water and activated carbon. Next, they were oscillated in boiling water for 30 min. Afterwards, the mixtures were cooled and filtered. The filtrates were analyzed by an automated chemistry analyzer (Smartchem200, Italy) to determine the amounts of nitrate nitrogen and ammonium nitrogen.
Leaf samples were weighed and analyzed using a Costech elemental combustion system (Costech ESC4010/4012, Australia) for total nitrogen amount.
Statistical analysis
The Statistical Program for the Social Sciences (SPSS, Chicago, IL, USA) software was used for one-way analysis of variance (ANOVA), Least Significant Difference method (LSD) and statistical evaluations, and the test measure-ments results of different treatments were examined at the 5% level.
Results and discussion
The effects of phenolic acid concentration on photosynthetic gas exchange parameters
Under fixed light intensity (PAR = 1500 μmol m−2 s−1) and at different concentrations of phenolic acids, the PN, E, gs, Ci, Ls, and WUE of the leaves of poplar changed significantly but with various trends (Table 2).
PN of poplar leaves was affected by phenolic acid concentration (Table 2). Compared with the control group, PN decreased by 11.4 and 22.9% for T1 and T2, respectively, and the differences between treatments T1 and T2 were significant. The photosynthetic rate of leaves decreased with increase in Ci (Table 2). Farquhar and Sharkey (1982) reported that a non-stomatal factor led to the decrease in PN, indicating that phenolic acid affected the internal stomatal photosynthesis of Populus × euramericana. For Gs and Tr, the same fixed models were found with the decrease in PN, and both Gs and Tr had similar trends (Table 2). Therefore, Tr was obtained through stomatal adjustment, indicating that no plasmolysis occurred in the cells after phenolic acid treatments, and the leaves had normal moisture evaporation function. WUE is affected by both PN and Tr (Nijs et al. 1997); therefore, any factors affecting PN and Tr could also affect WUE. In this study, PN was restrained by non-stomatal factors, while Tr was affected by stomatal factors; therefore, WUE was affected by phenolic acids via both stomatal and non-stomatal factors. Moreover, WUE was negatively related to Gs; therefore, we expected that the stomatal factor might have a major effect on WUE.
Non-stomatal factors resulted in a decline of photosynthetic intensity, possibly due to reduced photosynthetic activities in the mesophyll cells. The might have been due to fact that the fixed photosynthetic enzymes were affected. For example, the photosynthetic rates of leaves at different nitrogen nutrition statuses are closely related to the activity of ribulose diphosphate carboxylase (Xu 2002). Nitrogen is a component substance of all enzymes in plants. Therefore, study of the effects of phenolic acids on nitrogen is important in solving the problems discussed above with the continuous cropping of Populus × euramericana.
The effects of phenolic acid stress on assimilation enzymes
The effects of phenolic acid stress on NR activities
Plants absorb ammonium salts from the soil, and these are used for composing amino acids. However, if nitrates are absorbed, they can only be used through nitrogen metabolism (Liu et al. 2007; Brandao and Sodek 2009). In general, under field conditions, NO3− is the primary form absorbed by plants. When NO3− enters plant cells, it is first reduced to nitrite (NO2−) by NR and then to NH4+ by NiR. The activity level of NiR is much higher than that of NR in plants (Nigel et al. 1992; Wei et al. 2008). Our results confirmed these patterns. Therefore, NiR was not the rate-limiting enzyme that influenced the reduction of nitrate in the leaves. NR is a type of inducible enzyme, which is also known as an adaptive enzyme. Inducible enzymes are not from plants but are produced by the inducement from some specific foreign substances, such as the substrate (Meng and Gao 2010). NR is a rate-limiting enzyme that controls the inorganic assimilation of NO3− (Campbell 1988). Chaffei et al. (2004) reported that Cd could reduce the activity of NR in tomato (Lycopersicon esculentum). Gouia et al. (2003) reported that Cd could reduce NR activity in broad bean (Phaseolus vulgaris L. cv. Morgane) seedlings. We found that phenolic acids inhibited the activity of NR, and the inhibiting effect increased with the concentration of phenolic acid. Compared to the control group, the activity of NR in T1 was reduced by 24.2%, while that of NR in T2 was reduced by 71.8% (Table 3). The differences between treatments were significant. Therefore, phenolic acids affected the conversion of NO3− to NH4+. NR is a kind of enzyme containing a mercapto group, which can combine with heavy metals to inhibit its activity (Chen et al. 1998; Chang et al. 2005; Chang and Ma 2007). However, further study is needed to evaluate the mechanisms that enable phenolic acids to reduce NR activity.
The effects of phenolic acid stress on the activities of GS and GOGAT
GS and GOGAT are the key enzymes for nitrogen metabolism (Zhao et al. 1998). Their activities levels directly influence the nitrogen assimilation of plants (Wang et al. 2005; Xiong et al. 2011). The cyclic reaction in which GS and GOGAT are incorporated into the coupling is the main pathway of ammonia assimilation in advanced plants (Singaram and Kamalakumari 1999). In the assimilation of NH4+, it first combines with glutamic acid to form glutamine (Gln) under the catalysis of GS. Gln then combines with α-ketoglutaric acid to form glutamic acid under the catalysis of GS (Mo et al. 2001; Meng and Gao 2010).
The activities of GOGAT and GS significantly declined with increasing phenolic acid concentration. The activities of GS in T1 and T2 were reduced by 11.9 and 42.5%, respectively, compared with the control group. The activities of GOGAT in T1 and T2 were reduced by 20.0 and 41.6%, respectively, and the values of T2 (GOGAT and GS) were almost half of those in the control group. Therefore, GOGAT was more susceptible to phenolic acids. Lin et al. (2000) found that the activities of GS and GOGAT declined with increasing NaCl concentration. Our results were similar to those reported by Lin et al. (ibid.) However, whether the mechanisms of salt stress and phenolic acid stress on these two kinds of enzymes were similar is not clear and needs further study.
Overall, the nitrogen cycle was affected by phenolic acids. Phenolic acids affected both processes in which NO3− is converted to NO2− and NH4+ is converted to Gln. However, a low concentration of phenolic acids mainly affected the transformation process of NO3− to NO2−, while a high concentration affected both processes in which NO3− was converted to NO2− and NH4+ was converted to Gln.
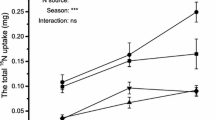
The effects of phenolic acid stress on the contents of NO3 −–N, NH4 +–N, and total N
With increasing concentration of phenolic acid, the contents of total nitrogen, ammonium nitrogen and nitrate nitrogen significantly decreased (Table 4). The content of nitrate nitrogen significantly correlated with the activity of NR. The results were consistent with other findings; for example, Lu and Chen (2011) found that the activity of NR in the leaves of Chinese chive increased with increasing NO3−–N content. Chen et al. (2004) reported that NO3−–N induction significantly increased NR activity in soybean cultivars. Gao et al. (1989) found that nitrate content of spinach and cabbage increased with increasing NR activity in the leaves. Therefore, we hypothesized that the NR activity of Populus × euramericana leaves was influenced by NO3−–N content. For NH4+–N, it was influenced by both the activity of the enzyme and NH4+ absorption capacity. The main reason for the decrease in NH4+–N content in the T1 treatment was the decrease in NH4+ absorption, while the activity of NiR was much higher than that of NR (Nigel et al. 1992). The reason for the decrease in NH4+–N content in the T2 treatment was the activity of NR and the NH4+ absorptive capacity. Although glutamate dehydrogenase (GDH) can degrade NH4+, it has a lower affinity for NH3, and Km of GDH was at a 10−3 mol L−1 level; therefore, it could be negligible, as reported by Wei et al. (2008) and Meng and Gao (2010). However, whether the decline of absorbability of NH4+ by Populus × euramericana was due to the effect of phenolic acids on the NH4+ carrier protein needs further study.
Overall, photosynthesis of the plants was positively related to the activities of GOGAT and GS and the contents of NO3−–N, NH4+–N and total N (P < 0.05). The decrease in photosynthesis was affected by non-stomatal factors. The reasons that NR, GS, and GOGAT resulted in decreased photosynthesis needs further investigation because nitrogen is one component of all enzymes in plants, including the enzymes related to the photosynthetic non-stomatal factors. NR, NIR, GS and GOGAT are base enzymes that can transform inorganic nitrogen directly into amino acids (Tischner 2000; Meng and Gao 2010). The activities of these enzymes were directly affected by phenolic acids, which could indirectly affect the nitrogen contents of the plants and thereby impair photosynthesis. Therefore, the absorption and utilization of nitrogen in the continuous cropping of poplar needs to be improved.
Conclusions
Phenolic acids can significantly impair photosynthesis due to limitations exerted by non-stomatal factors. Tr also significantly decreased with the increase in the concentration of phenolic acids, but the concentration had no impact on water evaporation in Populus × euramericana.
The nitrogen metabolism process was influenced by phenolic acids. The activities of NR, GS and GOGAT significantly decreased with the increase in the concentration of phenolic acid, and the inhibition of nitrogen metabolism increased with the concentration of phenolic acid. A low concentration of phenolic acids mainly affected the transformation process of NO3− to NO2−, while a high concentration of phenolic acids affected both processes in which NO3− was converted to NO2− and NH4+ was converted to Gln.
The content of NO3−–N was significantly positively correlated with the activity of NR. The contents of NH4+–N and total nitrogen were reduced with increasing concentration of phenolic acids.
References
Berry JA, Downton WJS (1982) Environmental regulation of photosynthesis. In: Govindjee (ed) photosynth, vol 2. Acad Press, New York, pp 263–342
Brandao AD, Sodek L (2009) Nitrate uptake and metabolism by roots of soybean plants under oxygen deficiency. Braz J Plant Physiol 21(1):13–23
Campbell WH (1988) Nitrate reductase and its role in nitrate assimilation in plants. Plant Physiol 74(1):214–219
Cao GQ, Lin SZ, Wang AP, Peng YR (2005) Bioassay and identification of allelochemicals in pinusmassoniana root. Chin J Appl Environ Biol 11(6):686–689
Chaffei C, Pageau K, Suzuki A, Gouia G, Ghorbel MH, Masclaux-Daubresse C (2004) Cadmium toxicity induced changes in nitrogen management in Lycopersicon esculentum leading to a metabolic safeguard through an amino acid storage strategy. Plant Cell Physiol 45(11):1681–1693
Chang SM, Ma XM (2007) Effects of arsenic on nitrogen metabolism of flue-cured tobacco (Nicotiana tabacum L.). Acta Agron Sin 33(1):132–136
Chang SM, Ma XM, Jiang YY, He DX, Zhang GL (2005) Research progress on arsenic contamination in soils and arsenic toxicity in crops. J Henan Agric Univ 39(2):161–166
Chen Y, Ren JC, Cai XM (1998) Effects of cadmium on nitrate reductase and speroxide dismutase of submerged macrophytes. Acta Sci Circumst 18(3):313–317
Chen Y, Zhu BG, Zhang J, Liang ZS (2004) Effects of different nitrogens on activities of nitrate reductase, glutamine synthetase and seed protein vontents in soybean vultivars. Soybran Sci 2(2):143–146
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Ann Rev Plant Physiol 33:317–345
Gao ZM, Zhang YD, Zhang DY, Shi RH (1989) Effects of N, P, K treatments on the accumulation of nitrate and the activities of nitrate reductase and superoxidase in two leafy vegetables. Acta Hortic Sin 16(4):293–297
Gouia H, Suzuki A, Brulfert J, Ghorbal MH (2003) Effect of cadmium on the co-ordination of nitrogen and carbon metabolism in bean seedlings. J Plant Physiol 160(4):367–375
Jeffrey DW, John TR (2004) Allelochemicals of polygonella myriophylla: chemistry and soil degradation. J Chem Ecol 30(5):1067–1081
Kong YS, Yi XF (2008) Plant physiology experimental techniques. China Agriculture Press, Beijing
Li YM, Hu JC, Zhang J, Wang SL, Wang SJ (2005) Microbial diversity in continuously planted Chinese fir soil. Chin J Appl Ecol 16(7):1275–1278
Li H, Song XH, Liu X, Li CR, Xu JW, Qu MZ, Wu ZP, Cheng XG, Xiao CZ (2014) The human physiological response to volatiles from Juniperus chinensis cv Kaizuka and Pistachia chinensis Bunge. BioResources 9(4):6669–6681
Li H, Zhang GC, Xie HC, Li K, Zhang SY (2015) The effects of the phenol concentrations on photosynthetic parameters of salix babylonica L. Photosynthetica 53(3):430–435
Li H, Li K, Liu HD, Jing T, Song XH, Xue LY, Li CR, Shen WX (2016) The effect of VOCs from the branches and leaves of Pistacia chinensis Bunge and Juniperus Chinensis cv Kaizuka on mouse behavior. BioResources 11(4):10226–10239
Lin CC, Kao CH (1996) Disturbed ammonium assimilation is associated with growth inhibition of roots in rice seedlings caused by NaCl. Plant Growth Regul 18(3):233–238
Lin Q, Li CJ, Peng J, Zhang CF (2000) Effect of NaCl stress on glutamate synthase and glutamate dehydydrogenase of rice plants. J Wuhan Botanical Res 18(3):206–210
Liu FD, Jiang YZ, Wang HT, Kong LG, Wang Y (2005) Effect of continuous cropping on poplar plantation. J Soil Water Conserv 19(2):102–105
Liu SY, Dong ST, Zhao BQ, Li XY, Zhang ZS (2007) Effects of long-term fertilization on activities of key enzymes related to nitrogen metabolism (ENM) of maize leaf. Acta Agron Sin 33(2):278–283
Lu FG, Chen GL (2011) Effect of dry method and extraction pressure on extraction volatile oil in basil. North Hortic 04:41–43
Lü WG, Zhang CL, Yuan F, Peng Y (2002) Mechanism of allelochemicals inhibiting continuous cropping cucumber growth. Sci Agric Sin 35(1):106–109
Meng QW, Gao HU (2010) Plant physiology. China Agriculture Press, Beijing, pp 66–70
Mo LY, Wu LH, Tao QN (2001) Research advances on GS/GOGAT cycle in higher plants. Plant Nutr Fertil Sci 7(2):223–231
Nigel MC, Jack QW, Samuel T (1992) Control of nitrate reduction in plants. Aust J Physiol 19:377–385
Nijs I, Ferris R, Blum H, Hendev G, Impens I (1997) Stomatal regulation in a changing climate: a field study using free air temperature increase (FATI) and free air CO2 enrichment (FACE). Plant Cell Environ 20(8):1041–1050
Rlum U, Gric TM (2005) Relationships between phenolic acid concentrations, transpiration, water utilization, leaf area expansion, and uptake of phenolic acids: nutrient culture studies. J Chem Ecol 31(8):1907–1932
Schutter ME, Sandeno JM, Dick RP (2001) Seasonal, soil type, and alternative management influences on microbial communities of vegetable cropping systems. Biol Fertil Soils 34(6):397–410
Singaram P, Kamalakumari K (1999) Effect of continuous manuring and fertilisation on maize grain quality and nutrient soil enzyme relationship. Madras Agric 86:51–54
Tan XM, Wang HT, Kong LG, Wang YP (2008) Accumulation of phenolic acids in soil of a continuous cropping Poplar plantation and their effects on soil microbes. J Shandong Univ (Nat Sci) 43(1):15–19
Tischner R (2000) Nitrate uptake and reduction in higher and lower plants. Plant Cell Environ 23(10):1005–1024
Wang XC, Xiong SP, Ma XM, Zhang JJ, Wang ZQ (2005) Effects of different nitrogen forms on key enzyme activity involved in nitrogen metabolism and grain protein content in speciality wheat cultivars. Acta Ecol Sin 25(4):802–807
Wang HG, Zhang J, Yang WS, Huang QM, Zou P (2006) A Research on the allelopathic substances in root system and root system soil of eucalyptus grandis. J Sichuan Normal Univ (Nat Sci) 29(3):368–371
Wang L, Zhou QX, Ding LL, Sun YB (2008) Effect of cadmium toxicity on nitrogen metabolism in leaves of Solanum nigrum L. as a newly found cadmium hyperaccumulator. J Heterocycl Chem 154(1–3):818–825
Wang YP, Yang Y, Wang HT, Jiang YZ, Wang ZQ (2010a) Sorption-desorption of two phenolic acids in poplar rhizosphere soil in continuous cropping plantation. Sci Silvae Sin 46(1):48–55
Wang YP, Wang HT, Tan XM, Jiang YZ, Kong LG (2010b) Comparison on rhizosphere effect of cultivar alternation and non-alternation continuous cropping poplar (Populus deltoids) plantation. Acta Ecol Sin 30(5):1379–1389
Wang YP, Wang HT, Jiang YZ, Chen HY, Ni GP (2011) Secretion dynamics of phenolic acids from poplar (Populus × euramericana ‘Neva’) seedling roots under N, P deficiency conditions. Sci Silvae Sin 47(11):73–79
Wei J, Wu J, Zhang Q, Jin S (2008) Determination of loratadine in capsules by NiR. Chinese J Pharm Anal 2811:1896–1899
Wu GX, Teng WC, Liang HP (2014) A Review of the research literature on the site productivity decline of artificial forest. Guizhou For Sci Tech 42(1):55–60
Xiong SP, Wang XC, Ma XM, He JG, Zhao P (2011) Effects of nitrogen forms on the activities of GS, GS isozyme in flag leaf and protein content of kernel in winter Wheat. J Triticeae Crops 31(4):683–688
Xu DQ (2002) Photosynthetic efficiency. Shanghai Science Technology Press, Shanghai, pp 46–53
Yang Y, Wang HT, Wang YP, Jiang YZ, Wang ZQ (2010) Effects of exogenous phenolic acids on root physiologic characteristics and morphologic development of poplar hydroponic cuttings. Sci Silvae Sin 46(11):73–80
Ye SF, Yu JQ, Peng YH, Zheng JH, Zou LY (2004) Incidence of fusarium wilt in cucumis sativus L. is promoted by cinnamic acid, an autotoxin in root exudates. Plant Soil 263(1):143–150
Zhang CS, Li K (2005) Advance in research on soil degradation and Soil Improvement of timber plantations. World For Res 18(1):17–21
Zhang CF, Peng SB, Peng X, Arlene QC, John B (1997) Response of glutamine synthetase isoforms to nitrogen sources in rice (Oryza sativa L.)roots. Plant Sci 125:163–170
Zhao P, Sun GC, Peng SL (1998) Ecophysiological research on nitrogen nutrition of plant. Ecol Sci 17(2):37–42
Author information
Authors and Affiliations
Corresponding authors
Additional information
Project Funding: This work was supported by the Important National Basic Research Program of China (973 Program-2012CB416904), the National Natural Science Foundation of China (Nos. 31700553, 31500511, 31600263, 31370702, 31500371), the research and demonstration on the key technology of vegetation restoration and reconstruction in the open pit of in eastern shandong hilly area (201504406), and the Natural Science Foundation of Shandong Province of China (No. ZR2015CL044).
The online version is available at https://www.springerlink.com
Corresponding Editor: Chai Ruihai.
Rights and permissions
About this article
Cite this article
Li, H., Xie, H., Du, Z. et al. The effects of phenolic acid on nitrogen metabolism in Populus × euramericana ‘Neva’. J. For. Res. 29, 925–931 (2018). https://doi.org/10.1007/s11676-017-0526-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-017-0526-0