Abstract
Key message
Endo - 1,4 - β - glucanase gene was isolated from Phyllostachys heterocycla var. pubescens internodes. It is engaged in the culm or stem development by regulation of the biosynthesis of cellulose.
Abstract
The cellulase protein endo-1,4-β-glucanase is a member in the large glycosyl hydrolase gene family 9 (GH 9). It is widely distributed in plants, animals, microorganisms and plays roles in cell wall metabolism, including cellulose biosynthesis and degradation, modification of cell wall polysaccharides and cell wall loosening during cell elongation. Our previous studies have identified a gene homologous to endo-1,4-β-glucanase and found its expression pattern changed significantly during the rapid elongation of Phyllostachys heterocycla var. pubescens internodes. In this work, we isolated the full length endo-1,4-β-glucanase gene from bamboo shoot and named as Phbeta-1,4-glu. Further characterization showed that Phbeta-1,4-glu belongs to GH 9 family, a conserved family in bamboo with slight variation in intron numbers and positions. Phylogenetic analysis revealed that P. heterocycle GH 9 genes exhibit a diverse phylogeny with rice, maize, Brachypodium, poplar and Arabidopsis. Among them, bamboo GH 9 genes show a closer relationship to ones of Brachypodium. RT-PCR analysis demonstrated that the expression pattern of Phbeta-1,4-glu varied among different tissues of bamboo shoot with a lowest expression level in the tender parts. The function of Phbeta-1,4-glu in bamboo’s height growth and cellulose content was confirmed via its transformation into the model plant-Arabidopsis. Functions of Phbeta-1,4-glu in bamboo shoot have been analyzed through a combinational methods of gene structure and phylogeny. Its gene expression pattern has been investigated and its function verified. The results confirmed that Phbeta-1,4-glu engages in the rapid elongation of bamboo culm from cellulose biosynthesis to cellulose degradation. Current investigations provide valuable information for its functional studies and potential utilization in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As being one of the most significant non-timber forest products in the world, bamboo not only can be used as building materials, it can also be used as food materials. Bamboo fibers possess versatile advantages in biodegradability, fast renewability, high annual biomass yield, high aspect ratio, and high strength to weight ratio, which make them ideal substitutions for man-made fibers. Currently, the latter is dominantly used in the production of fiber-reinforced composites and biorefinery bioethanol, such as bio-ethanol, bio-methane, flavonoids, and functional xylo-oligosaccharides (He et al. 2014; Yu et al. 2014). In China, there are 48 genera and nearly 500 species reported in bamboo subfamily (Bambusoideae), among them Phyllostachys heterocycla var. pubescens accounts for over two thirds of commercially planted bamboo. Bamboo leads a rather striking life history, characterized by a prolonged vegetative phase lasting for decades before heading to the flowering stage (Peng et al. 2013a). What’s more, it grows quite quickly during culm elongation, with a maximum rate reaching to 100 cm per day (Ueda 1960).
Anatomical observations indicated that the development of culms is dominated by cell division in the initial stages while by cell elongation in the middle and late stages. The continuously elongated cell length and gradually decreasing numbers of cell nuclei imply that cell division and elongation occur simultaneously, and this finally affects internode elongation (He et al. 2013; Cui et al. 2012). Physiological and biochemical research revealed that the rapid elongation of culms was mainly attributed to intercalary meristem initiation and proliferation, high-level protein synthesis, cellular respiration, cell wall synthesis and hormone regulation (Peng et al. 2013b; Zhou et al. 2011). He et al. (2013) reported that phytohormones such as indole-3-acetic acid (IAA), gibberellic acid (GA3), abscisic acid (ABA) and cytokinin zeatin riboside (ZR) appeared to actively promote culm development. Application of variety of molecular studies, such as cDNA library (Das et al. 2005; Gui et al. 2010; Liu et al. 2012; Sharma et al. 2008; Sungkaew et al. 2009; Zhang et al. 2011), Expressed Sequence Tags (EST) (Wang et al. 2009; Wei et al. 2011), proteomics (Logacheva et al. 2011), draft genome (Li et al. 2011), RNA-seq (Scurlock et al. 2000), transcriptome sequencing (Peng et al. 2013b), have uncovered that the plant hormones, cell cycle regulation, cell wall metabolism and cell morphogenesis genes might cooperate together to regulate the fast growth of bamboo shoots (He et al. 2013). A homologous fragment of endo-1,4-β-glucanase gene was reported in Ph. heterocycla var. pubescens via suppressive subtractive hybridization, the gene expression was found remarkably changed during the rapid elongation of P. heterocycle internodes (Zhou et al. 2011).
Endo-1,4-β-glucanases belong to glycosyl hydrolase gene family 9 (GH 9), and are kinds of cellulose proteins widely distributed in animals and plants (Maloney et al. 2012; Sami and Shakoori 2008). Glycoside hydrolases could hydrolyse glycosidic bonds between carbohydrates or between carbohydrates and non-carbohydrate moieties, and collectively exhibiting a wide range of substrate specificities (Urbanowicz et al. 2007). Endo-1,4-β-glucanases have been implicated in several aspects of cell wall metabolism in higher plants, including cellulose biosynthesis and degradation, modification of other wall polysaccharides that contain contiguous (1,4)-β-glucosyl residues, and wall loosening during cell elongation (Buchanan et al. 2012). Endo-1,4-β-glucanase gene plays vital role for proper plant height growth. For example, OsGLU1, a putative membrane-bound endo-1,4-D-glucanase from rice, affected plant internode elongation (Zhou et al. 2006); d2003, a novel allele of Viviparous 8 (VP8), was required for maize internode elongation (Lv et al. 2014); PtrCel9A6 from Populus promoted the cell wall formation (Yu et al 2013). In Arabidopsis (Arabidopsis thaliana), tomato (Solanum lycopersicon) and poplar (Populus tremuloides), expression of endo-1,4-β-glucanase genes impacted cellulose content of the cell wall (Bhandari et al. 2006; Brummell et al. 1997; Buchanan et al. 2012; Shani et al. 2004, 2006; Zuo et al. 2000). While in light-grown plants, such as in Brassica napus, expression of a membrane-anchored endo-1,4-β-glucanase was inversely correlated to elongation (Mølhøj et al. 2001).
Although functions of several members of endo-1,4-β-glucanases have been studied, phylogenetic relationship among them is still unclear. Its clarification will simplify our understanding towards assigning the functions for other related genes. In the regularly updated database, Carbohydrate-Active Enzymes (CAZY; http://www.cazy.org), 133 distinct GH families are listed. They correspond to 14 clans. Here, a clan is illustrated as a group of families sharing a common ancestry and is recognized by significant similarities in tertiary structures together with a conservation for the catalytic residues and catalytic mechanism (Henrissat and Bairoch 1996). It has been reported that GH 9 family can be further divided into three sub-families (GH 9A, GH 9B and GH 9C sub-families) (Henrissat and Bairoch 1993; Urbanowicz et al. 2007). The members of the GH 9A sub-family are membrane bound proteins with a short NH2-terminal cytosolic domain, a putative single spanning transmembrane helix and a characteristic DAGD motif (Brummell et al. 1997). The putative transmembrane domain was identified by a hydrophobic region of 39 amino acids flanked on each side with four or five charged amino acids (Boyd and Beckwith 1990; Brummell et al. 1997). The COOH-terminus is a proline-rich region, suggesting the possibility for its involving in protein-protein interactions (Kay et al. 2000). The GH 9B sub-family contains proteins with a catalytic domain and a signal peptide at the NH2-terminus.This peptide may target the protein to the cell wall through the Golgi apparatus or endoplasmic reticulum. While for the GH 9C sub-family, apart from a catalytic domain and an NH2-terminal signal peptide, the COOH-terminal end has a carbohydrate binding module (CBM) (Henrissat and Bairoch 1993; Buchanan et al. 2012; Urbanowicz et al. 2007).
In this work, we isolated a putative endo-1,4-β-glucanase gene from bamboo shoot, deciphered its structural and physicochemical properties, as well as its phylogenetic relationships with three plants from the grass family (rice, maize, and Brachypodium) and two from dicots (Arabidopsis, and Populus). By transforming the Arabidopsis model plants, we further analysed how much the gene contribute in plant height, cellulose content and cell wall composition.
Results
Structural and physicochemical properties of Phbeta-1,4-glu
To analyse the structural and physicochemical properties of endo-1,4-β-glucanase in bamboo, the putative gene, named as Phbeta-1,4-glu, was cloned from the cDNA library isolated from bamboo shoot. Sequencing results revealed that it has a total length of 1934 bp, with a complete open reading frame from 196 to 1854 bp, encoding a protein with 617 amino acids. The calculated molecular weight for the protein is 68.9 kDa with an isoelectric point of 9.04. Moreover, 21 α-helices (14.09 %), 47 β-sheets (31.54 %), 47 corners (31.54 %) and 34 random coils (22.83 %) were detected from the predicted secondary structure of the protein (Fig. S1). Besides, analysis of the protein sequence revealed that the amino acid residues from 72 to 94 form an N-terminal transmembrane region with 1–71 towards the inner side of the membrane, and 95–617 towards the outside (Fig. S2).
Urbanowicz et al. (2007) proposed that TomCel3 (GenBank ID: U78526), TomCel1 (GenBank ID: U13054) and TomCel8 (GenBank ID: AF098292) were the classic GH 9 sub-family members responding to GH 9A, GH 9B and GH 9C sub-families, respectively, with an unambiguous domain structure. After multiple alignment of Phbeta-1,4-glu against these three GH 9 representatives, it revealed that Phbeta-1,4-glu shared sequence similarity with TomCel3, a member in GH 9A sub-family (Fig. 1). Domain architecture comparison further claimed Phbeta-1,4-glu’s position in GH 9A sub-family (Table 1).
Homology alignment and phylogeny analysis
The whole GH 9 family members of bamboo were retrieved by blasting the cloned Phbeta-1,4-glu to the moso bamboo genome. The identified gene numbers are listed as: Ph01001209G0550, Ph01001459G0050, Ph01000332G1080, Ph01000780G0630, Ph01001005G0230, Ph01001262G0150, Ph01000900G0690, Ph01000003G1660, Ph01000609G0140, Ph01000342G0910, Ph01001590G0100, Ph01000060G1680, Ph01003005G0220, Ph01004196G0020, Ph01000060G0530, Ph01001311G0450, Ph01000114G1210, Ph01000092G0890, Ph01000836G0270, Ph01003078G0310, Ph01000058G1610, Ph01001311G0460, Ph01001406G0160, Ph01001406G0170.
Examination of the intron numbers and positions of GH 9 genes in bamboo manifested that although the number of introns varies in general, the intron positions and exon sizes do not (Fig. 2 middle): five introns and six exons are often found in most of the GH 9 genes. Nevertheless, there are exceptions. Ph01003078G0310 and Ph01000058G1610 contain seven introns and eight exons; Ph01001311G0460 and Ph01001406G0170 have only one intron and two exons. A phylogenetic tree of bamboo GH 9 members was built based on the 28 amino acid sequences, including Phbeta-1,4-glu (Fig. 2 left). According to the tree characteristics, the GH 9 family members in bamboo could be divided into three groups, nominated as PhGH9-1, PhGH9-2 and PhGH9-3. Genes with similar number of introns and exons clustered together, with 6/5, 2/1, 5/4 (intron/exon), respectively, indicating that genetic relationships within these three clades are closer than with others. Furthermore, TomCel3, TomCel1 and TomCel8 were all embodied in PhGH9-2. Consequently, domain architectures of these 28 species were speculated on the NCBI website (Fig. 2 right). The results suggested that genes showing closer relationship in phylogenetic tree exhibited the most identical domain structures. The domain architectures of Ph01001209G0550, Phbeta-1,4-glu, TomCel3, Ph01001459G0050 and Ph01000332G1080 displayed close relationship and contained an N-terminal cytosolic domain, a transmembrane region and a characteristic DAGD motif. The gene structures are also fairly similar too.
Phylogenetic tree, gene structures and domain architectures in bamboo and tomato GH 9 members. Left Phylogenetic tree built on endo-1,4-β-glucanase amino acid sequence of bamboo and tomato. Clades with red lines are grouped into PhGH9-1; clades with green lines grouped into PhGH9-2; clades with blue lines grouped into PhGH9-3. Middle Gene structures of bamboo and tomato GH 9 members. Introns: black boxes; exons: white boxes. The red shades refer to the conserved exons/intron. Right Domain architectures of bamboo and tomato GH 9 members speculated from the NCBI website
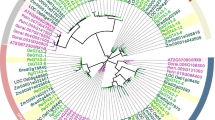
An unrooted parsimonious phylogenetic tree was generated based on GH 9 amino acid sequences of the four species (maize, rice Brachypodium, poplar) obtained from PlantGDB website (http://www.plantgdb.org/PtGDB/), and the 24 moso bamboo sequences (Fig. 3). According to the tree’s dendron topology, four groups were separated, namely GH 9-I, GH 9-II, GH 9-III, GH 9-IV. Interestingly, rice, Brachypodium, poplar and Arabidopsis occurred in every group. However, what’s worth noting was that, in GH 9-I, GH 9-II, GH 9-III, bamboo GH 9 genes were always clustered with rice, maize and Brachypodium, but kept a relatively far relationship with poplar and Arabidopsis. While in GH 9-IV, there were only one rice (Os05g03840.1), one Brachypodium (Bradi2g38360.1), one poplar (PtPOPTR0014s04390.1) and four Arabidopsis (AtGH9B12, AtGH9B11, AtGH9B10, AtGH9B9) involved, except bamboo and maize, suggesting mutation or genome duplication event may take place during the evolution history (Paterson et al. 2004, 2009; Salse et al. 2008).
Unrooted phylogenetic tree of four grasses (bamboo, maize, rice and Brachypodium) and two dicots (poplar and Arabidopsis) based on GH 9 gene amino acid sequences. GH 9-I: mazarine lines; GH 9-II: green lines; GH 9-III: pink lines; GH 9-IV: yellow lines. Bamboo: black square; Brachypodium: blue prism; rice: yellow triangle; maize: pink circle; poplar: green empty prism; Arabidopsis: blue empty circle
Expression pattern of Phbeta-1,4-glu in different parts of bamboo
In order to investigate the role of Phbeta-1,4-glu in the rapid elongation of P. heterocycla culm, the expression pattern of Phbeta-1,4-glu gene were measured by real-time fluorescent quantitative PCR (RT-PCR). Previous research uncovered that bamboo with culm height below 1 meter (m) is in the initial or ascending stage, which mainly conducts cell differentiation. Whereas, when the plant reaches to 6 m or higher, it is in the boosting or terminal stage primarily dominated by cell elongation and fibrocyte addition (He et al. 2013). Samples collected in this paper were in the initial and promoting phage, with height reaching 0.6 and 3.2 m, respectively. Various expression levels of Phbeta-1,4-glu among different tissues of bamboo shoot were detected (Fig. 4a, b). For bamboo 1 which was unearthed for seven days (Fig. 4a), it was in the initial stage of bamboo culm development and dominated by cell division. The gene expression level from tender parts to mature parts increased progressively with the lowest expression level in Phin1-1 and the highest in Phin1-11. Within the longest node both above and below ground (node 3), three portions were separated (top, middle and basal nodes). The expression results showed that Phbeta-1,4-glu in middle internode (Phin1-5; Phin1-10) was lowest in expression level while in top (Phin1-3 and Phin1-4; Phin1-8 and Phin1-9) and basal (Phin1-6 and Phin1-7; Phin1-11 and Phin1-12) portions, the expression were a little higher, with the highest in basal node underground. The top and basal nodes of the longest node both above and below ground (node 3) were further divided into two parts: the ring and the internal compartment of a node. They exhibited distinct expression results in above node and underground parts. For the longest node above ground, the node internal compartments possessed higher expression level than rings. On the contrary, the internal compartments of nodes in the underground portions reflected lower expression level than the rings. On the whole, the expression level of Phbeta-1,4-glu increased from top to basal node.
Two bamboo shoot samples and expression level of Phbeta-1,4-glu gene. a Bamboo 1 unearthed for 7 days. B1-1: the magnified model for bamboo 1. Above the boundary are the parts unearthed, below it the underground parts. Four nodes were collected. The two longest parts were further divided into five segments as showed in B1-2. B1-3: the names of different parts of bamboo nodes and internodes. Phin1-1 the initial node on the morphology top of shoot; Phin1-2 the soft node next to the morphology top; Phin1-3 to Phin1-7 have the longest nodes on the ground and Phin1-8 to Phin1-12 have the longest node below ground. These two longest nodes were further divided into five parts. Phin1-3 and Phin1-8 rings of nodes above the internode; Phin1-4 and Phin1-9: internal compartment of nodes above the internode; Phin1-5 and Phin1-10 the internodes; Phin1-6 and Phin1-11 rings of nodes below the internode; Phin1-7 and Phin1-12 internal compartment of nodes below internode. b Bamboo 2 was unearthed for 20 days. B2-1: the magnified model for bamboo 2 with nodes below the longest internodes omitted. Three nodes were collected and divided into three or five segments as showed in B2-2. B2-3: the names of different parts of bamboo nodes and internodes. Phin2-1: the node above the initial internode; Phin2-2: the initial internode on the morphology top of shoot; Phin2-3: the node below the initial internode; Phin2-4 to Phin2-8 belong to the soft internode/node parts and Phin2-9 to Phin2-13 are the longest internode/node, which has been divided into five parts as described in bamboo 1. Bar charts on the right-side of a and b are the expression of Phbeta-1,4-glu in different parts of the bamboo shoots
While for bamboo 2 unearthed for 20 days (Fig. 4b), it demonstrated the same trend as with bamboo 1. The expression level increased from morphology apex to basal culm and the middle internode possessed lower expression level than the top and basal nodes within an internode. What’s more, Phbeta-1,4-glu in rings exhibited lower expression level than the internal compartment parts. Hence, it was obvious that there were variances in different portions of bamboo culm, which seems to be related with the mature degree of tissues, performing as the more tender the bamboo tissues, the lower the expression level.
Functional verification of Phbeta-1,4-glu in transgenic plants
T3 Arabidopsis plants with Phbeta-1,4-glu transformed were used to study the function of the gene. Plant height was measured and recorded when the flower stalks initiated. The results showed that, transgenic plants exhibited significant difference with an average height of 32.13 cm compared to 25.76 cm in wild type plants, implying that Phbeta-1,4-glu may engage in the growth of stem length (P < 0.01, Table 2). The raw stem materials were collected from transgenic and wild-type plants for cellulose content and cell wall structure analysis. In the transgenic plants, the mean cellulose content was lower than the wild type plant although with low support (the mean cellulose content: (23.86 ± 13.21) % (mean value ± standard deviation) in transgenic plant; (40.74 ± 28.04) % in wild type ones, Table S1). In addition, cells in transgenic plants were noticeably elongated than wild-type ones (Fig. S3), e.g, cells of the transgenic were almost three times longer than WT plants, suggesting that Phbeta-1,4-glu participated in cell prolongation.
Discussion
Phbeta-1,4-glu is a member of GH 9
Base on the sequence available on line (http://www.cazy.org/), a total of 133 classes of glycosyl hydrolase gene family were released (Henrissat 1991; Henrissat and Bairoch 1993, 1996). GH 9 was one among the family members, mainly occupied by endo-1,4-β-glucanases (Cantarel et al. 2009), which had been further classified into three sub-families (GH 9A, GH 9B and GH 9C) based on the variations of protein sequences (Urbanowicz et al. 2007). The predicted secondary structure elements of Phbeta-1,4-glu uncovered 21 α-helices, 47 β-sheets, 47 corners and 34 random coils (Fig. S1). In addition, the deduced Phbeta-1,4-glu protein lacks a typical eukaryotic signal peptide sequence but instead has a highly charged region at the N-terminus (21 of the first 58 amino acid residues being charged), followed by a predominantly hydrophobic region of 39 amino acid residues. This hydrophobic domain is flanked on the N-terminal side by four consecutive, positively charged amino acid residues and on the C-terminal side by five consecutive positively charged residues. This featured charge distribution indicates the existence of a membrane-spanning domain (Boyd and Beckwith 1990). Concurrently, the C terminus of Phbeta-1,4-glu is rich in proline (nine out of the last 16 amino acids), a characteristic of the linker regions between different domains in microbial celluloses (Beguin 1990). Additionally, the assumed protein possesses seven potential N-glycosylation sites (N-X-S/T) (Fig. 1) (Brummell et al. 1997). All of the results suggested that Phbeta-1,4-glu is a member in GH 9A sub-family. Multiple sequence alignment (Fig. 1) and domain architecture (Table 1) analysis further confirmed that Phbeta-1,4-glu has the similar property as members in GH 9A sub-family.
Plant endo-1,4-β-glucanases hydrolyses cell wall polysaccharides that contain contiguous β-1,4-glucosyl residues in the chains, such as xyloglucans and β-(1,3; 1,4)-glucans, and clearly functions in cell wall degradation. Yet, there is a great deal of evidence pointing to an additional and important role for these enzymes in cellulose synthesis during cell growth (Buchanan et al. 2012; Mølhøj et al. 2001; Zhou et al. 2011). An example is that Phbeta-1,4-glu might take part in the rapid elongation of bamboo culms through synthesis of cellulose, as well as cell wall degradation.
Conserved and differentiated phylogeny of endo-1,4-β-glucanase gene
Twenty-four bamboo amino acid sequences were identified to be homology to Phbeta-1,4-glu through Blast. The phylogenetic tree was built with reference to the three tomato sequences (TomCel3, TomCel1 and TomCel8). Clade structure disclosed three clusters with Phbeta-1,4-glu embodied in PhGH9-2. Although there were variations in the number of exons/introns, the retention of exon sizes and intron positions among those orthologues were in general detectable from the phylogenetic tree, suggesting that numbers and positions of the introns among species were relatively conserved, especially for the closely related species (Buchanan et al. 2012; Chen 2010). For instance, Phbeta-1,4-glu and Ph01001209G0550, Ph01001311G0460 and Ph01001406G0170, Ph01000060G1680 and Ph01004196G0020 were clustered together with the same number of introns and exons. Alternatively, the fact that other GH 9 family members in bamboo behaved a differentiation in the ratio of exons/introns might derive from gene variation. Buchanan et al. (2012) found that loss and/or gain of endo-1,4-β-glucanase genes happened during evolutionary process, and the transcription was conserved among orthologous genes across the grass family. On the other hand, when an intron is lost, the number of bases for the resultant single exon is equal to the sum of the two exons in the orthologous or homologous genes if there have been no insertions or deletions within the gene sequence (Buchanan et al. 2012). However, the percentage of total introns in endo-1,4-β-glucanase genes varied from 33 to 77 %, implying that gene gain/loss or gene fragment insertion/deletion has taken place in bamboo GH 9 family members. This may be induced by the inconsistent intron evolution rate (Carmel et al. 2007; Gazave et al. 2007; Igea et al. 2010; Rodova et al. 2003; Soria-Carrasco et al. 2007).
What’s noteworthy is that the three reference genes, TomCel3, TomCel1 and TomCel8, were all embeded in PhGH9-2. In the case of TomCel3, it exhibited closer relationship with Phbeta-1,4-glu, Ph01001209G0550, Ph01001459G0050 and Ph01000332G1080. The phylogenetic tree, the gene structure, as well as the domain architecture testified this observation. This illustrated that all the five genes belong to sub-family GH 9A. While for TomCel1, it performed as a sister group with all the other members in PhGH9-2. Previous study revealed a complex phylogenetic relationship in GH 9B sub-family, showing up as that parts of GH 9B members can be grouped with GH 9A sub-family members and some are embodied in GH 9C sub-family (Buchanan et al. 2012). In addition, the structure of endo-1,4-β-glucanase genes from clade B1 indicated that they belong to sub-family GH 9B. However, they were derived from the GH 9A sub-family base on the phylogenetic perspective (Buchanan et al. 2012). Although it is difficult to determine the relationship between GH 9B and other two GH 9 sub-families, TomCel8 demonstrated a closer relationship with two bamboo endo-1,4-β-glucanase genes, Ph01000342G0910 and Ph01003078G0310. Despite the ratio of exons/introns is different from each other for the three genes, they all contain an N-terminal signal peptide, a catalytic domain, as well as a carbohydrate binding domain, confirming that they are members in GH 9C sub-family. Apart from the genes discussed above, other endo-1,4-β-glucanase genes in bamboo GH 9 family exhibit variations in intron/exon or domain structures. Hence, endo-1,4-β-glucanase genes in bamboo were somewhat conserved with clearly identification of GH 9A and GH 9B sub-family members. Moreover, any of the phylogenetic, gene structure or protein architecture analyse alone is insufficient to identify the relationship among the gene sub-family, multi-analysis is necessary, at least in current case.
The number of genes in GH 9 family varies from bamboo, maize, rice, Brachypodium, Arabidopsis and poplar within the range of 23–33. Phylogenetic analysis revealed variation in clade structure between grasses and Arabidopsis, indicating a variation in the level of gene loss and gain during evolution (Buchanan et al. 2012; Peng et al. 2010), as well as the diversity of the endo-1,4-β-glucanase genes in grasses and dicots. In the GH 9-I, GH 9-II and GH 9-III groups, bamboo endo-1,4-β-glucanase genes were always clustered with rice, maize. They are most close to Brachypodium, but relatively far from poplar and Arabidopsis. While GH 9-IV group includes only seven members, one from rice (Os05g03840.1), one from Brachypodium (Bradi2g38360.1), one from poplar (PtPOPTR0014s04390.1) and four from Arabidopsis (AtGH9B12, AtGH9B11, AtGH9B10, AtGH9B9). Previous phylogenetic studies of the chloroplast and nuclear genes in grass family reached a consensus that bamboo and rice were sister groups (Group et al. 2001; Kellogg 2001). However, a recent study conducted by Peng et al. (2010) argued that bamboo and Brachypodium were more closely related (Castresana 2002). Interestingly, the phylogenetic trees generated by Maximum Likelihood or Bayesian Inference in this study were inconsistent with Neighbor Joining method regarding to the relationships between rice, bamboo and Brachypodium based on the putative orthologous genes (Peng et al. 2010). These conflicting might be derived from the rapid diversification of grasses. Buchanan et al. (2012) speculated that the amino acid substitutions of endo-1,4-β-glucanase occurred after the divergence of monocots from dicots, but before the separation of the grasses. In addition, it was estimated that around 50 million years ago (Ma), the antecedents of the cereals, such as Brachypodium and rice, were separated from the maize (Kellogg 1998; Salse et al. 2008). Hence, it becomes clear that endo-1,4-β-glucanase genes in dicots and monocots differentiates distinctly, resulting in a far phylogenetic relationship between them. This was in accord with the phylogeny of GH 9-I, GH 9-II and GH 9-III.
Notwithstanding all the species in Poaceae experienced varying degrees of genomic structure and quantity variation, in silico mapping of endo-1,4-β-glucanase genes in grasses indicated that these genes are broadly distributed across the genomes (Buchanan et al. 2012). For example, the maize genome underwent a doubling of chromosome number by introduction of a foreign genome, or allotetraploid event followed by non-homologous recombination of the genome and subsequent reversion to the diploid state which happened within 10–4.8 Ma (Gaut and Doebley 1997; Paterson et al. 2004; Swigoňová et al. 2004). Nevertheless, endo-1,4-β-glucanase genes were found on every chromosome in maize except chromosome 3 (Buchanan et al. 2012). Consequently, it is relatively easier to analyse the phylogeny of endo-1,4-β-glucanase genes in Arabidopsis, poplar and maize, but challenging to resolve the relationships among bamboo, rice and Brachypodium due to their short divergence time or inadequate resolving power with genetic markers. In addition, as discussed in previous paragraph, Phbeta-1,4-glu, Ph01001209G0550, Ph01001459G0050 and Ph01000332G1080 could be applied as indicators for GH 9A subfamily, Ph01000342G0910 and Ph01003078G0310 for sub-family GH 9C. We found that GH 9-I consists mainly of GH 9A and GH 9B sub-family members, GH 9-II and GH 9-IV are primarily composed of GH 9B sub-family members, while GH 9-III is made up of GH 9C members. Here, we suggested that endo-1,4-β-glucanase gene could be used as genetic marker in resolving phylogeny and evolutionary process on the level of Class, Order or Family.
Expression of Phbeta-1,4-glu in different parts of bamboo shoot
Bamboo is one of the fastest growing lignocellulose-abundant plants on Earth. It can reach a final height of 5–20 m in a single growing season of 2–4 months owing to the rapid elongation of internodes (Magel et al. 2005). The growth of bamboo culms follows the basal-apical order, meanwhile multi-internodes elongate simultaneously though in a different rate (Cui et al. 2012; Xiong et al. 1980; Jiang 2002). Four growth phases in bamboo culms have been pronounced: the initial, ascending, boosting and terminal stages (He et al. 2013). The initial stage was almost dominated by intercalary meristem conducting cell division. The intercalary meristem is derived from the retention of apical meristem or primary meristem (He et al. 2013; Xiong et al. 1980; Jiang 2002). Its cells have been characterized to be rectangular shape, thin walls, large nucleus, abundant of chromatin, dense in cytoplasm, free of accumulated starches. However, they have a higher degree of vacuolization, mainly for horizontal cell division to add cells along the longitudinal direction (Deng et al. 1988). The rapid elongation of bamboo culm was primarily attributed to the activity of intercalary meristem, including cell division, proliferation and elongation (Deng et al. 1988). Bamboos utilized in this study were in the stage of initiation and boosting with their height reaching 0.6 and 3.2 m, respectively. In each bamboo, Phbeta-1,4-glu exhibited distinct expressive abundance in different nodes or even different position within a single node. In bamboo 1, the gene expressive abundance increased sequentially from apical to basal parts with a lowest level in Phin1-1 and a highest in Phin1-11. As Phin1-1 is extremely close to the apical node and tenderer than any other sampling nodes, it was found fully filled with intercalary meristem which conducts cell division rather than gene expression (He et al. 2013; Dong 2007). As a result, Phbeta-1,4-glu in Phin1-1 displays a lowest expression level. However, for the longest nodes on or under the ground (node 3), intercalary meristem in the upper internodes gradually stopped dividing but were still active in the cell differentiation, and gradually the activity was limited to the base internodes. At this time, a little higher expression level of Phbeta-1,4-glu could be detected in the upper internodes (Phin1-3, Phin1-4) where more celluloses have been synthesized, polysaccharides accumulated, and parenchymal cells and fibre cells generated (He et al. 2013; Lao et al. 2013; Xiong et al. 1980). However, as the base internodes (Phin1-6, Phin1-7) are so closely adjacent to the next internode (not sampled) that the expression of Phbeta-1,4-glu may also be influenced and increased by the next internode. This was particularly evident in the underground parts (Phin1-11, Phin1-12) which have a relative expression quantity of 31.930 (Fig. 4a). When the intercalary meristem in the base internode stopped differentiation, cells in this part were mature while cells in the top internode became highly aging. In this case, the gene was more actively expressed in the internodes than in the nodes. Previous studies suggested that the aging of the cells in nodes is more serious than in the internodes, which consolidating bamboo’s mechanical ability (Xiong et al. 1980). Consequently, the expression difference of Phbeta-1,4-glu in top and basal internodes were concealed. The results were consistent with that of He et al. (2013), who also split one internode into three portions, top, middle and basal, respectively.
The expression levels in the ring and in the internal compartment within a node were compared. The internal compartments (Phin1-4, Phin1-7) appeared higher than the rings (Phin1-3, Phin1-6) regardless of the top node or the basal node when they were on the ground. The observation was reversed when the samples were from underground. During the development of shoot, it firstly differentiated as parts of the screw-shaped structure, and then gradually formed nodes and internodes (Feng 2010). In the node, the pith-ring meristem stretched into the central along the epidermis and then acrossed the pith, finally formed the internal of a node, in other words, the forming of internal node was later than the ring (Feng 2010). For the underground parts, cells in ring were more mature than internals, therefore resulting in a higher gene expression. There is no doubt why the gene in the internal compartments of a node expresses higher than in rings.
It has been reported that different portions of the same plant may be at different stages resulting from the sequential basal-apical elongation of the culm internodes (Jiang 2002). Hence, for plants in the boosting stage, such as the case in bamboo 2, the growth of the basal nodes/internodes might be in the descending stage, while the top nodes/internodes might be in the initial or ascending stage. Interestingly, within an internode, the intercalary meristem in the top section differentiated firstly and then withdrew into basal section, behaving as an opposite order of culm elongation. That’s why the expression of Phbeta-1,4-glu in bamboo 2 demonstrated the same trend as in bamboo 1. In conclusion, our results verified the basal-apical development model for bamboo height elongation. Furthermore, we found that the expressive abundance of Phbeta-1,4-glu was not only concerned with the development stage of bamboo shoot, but also with the mature degree of the tissues.
Phbeta-1,4-glu played a role in transgenic plant’s height and cellulose content
Endo-1,4-β-glucanases are kinds of cellulose that takes part in cell wall metabolism in higher plants (Buchanan et al. 2012). It has been reported that mutation of Korrigan (KOR) in Arabidopsis caused dwarfism, resulting in reduction of cell elongation and cellulose content (Zuo et al. 2000). This is because KOR is a kind of GA synthesis gene, and the mutation of this gene causes a defective GA synthesis leading to dwarf phenotype (Wu et al. 2014). Nicol et al. (1998) noticed that Korrigan mutant cells were enlarged, walls significantly thicker and loosen when compared with the wild-type. Moreover, when the mutant transformed with a complementing genomic fragment, its height was recovered. Using a heat inducible AtKor1 mutant, acw1, Sato et al. reported similar findings (Sato et al. 2001). Cellulose content defects have also been observed in the prc1 (Fagard et al. 2000) and kob1 (Pagant et al. 2002). Both mutants exhibited cell expansion defects and dwarf phenotype, suggesting that cellulose synthesis actively contributes to the control of cell elongation. However, in our study, the height and cell size of T3 transgenic plants elongated notably than the WT plants, while the cellulose content was somewhat decreased. This may owe to the transformation of Phbeta-1,4-glu, which functions not only on cellulose biosynthesis, but also on degradation. That is, on one hand, augment of Phbeta-1,4-glu expression quantity accelerates the hydrolysis of glycosidic bonds between carbohydrates (Delmer and Amor 1995; Du and Jing 2010; Li et al. 2004; Wu et al. 2010; Zhang et al. 2007), resulting in a reduced cellulose content; on the other hand, to maintain a regular metabolism, the decreased cellulose level stimulates Phbeta-1,4-glu to proceed cellulose biosynthesis in turn (Buchanan et al. 2012) and thus facilitates the stem elongation, leading to an ever-increasing plant height. Moreover, different selection pressure may occur because both Arabidopsis and rice are herbs with plant height range from 78 to 170 cm (Kovi et al. 2011; Lee et al. 2014). Whereas bamboo is a kind of special herb with stem highly lignified and the plant height can reach to approximately 20 m. Therefore, Phbeta-1,4-glu is not only involved in the cellulose biosynthesis, but also promotes the fast elongation for the bamboo.
In addition, mechanical changes in the transgenic plants observed in this study might contribute to the stem strength. Although we were unable to provide a direct evidence, it is well known that cellulose content correlates with the mechanical strength. Appenzeller et al. (2004) argued that there was a strong correlation (r 2 = 0.85) between intermodal flexural stem strength and cellulose content of the internode, namely increasing cellulose concentration in the cell wall might improve the mechanical strength of the stem. Moreover, Buchanan (2011) suggested that cellulose is an important determinant of stem strength that increases with the cellulose content. Our continuous efforts may reveal the relationship between the cellulose content and the fibre strength for bamboo, therefore prompt its utilization as an industrial and food material.
Materials and methods
Plant materials and RNA isolation
Two shoots and one culm of P. pubescens were collected in the bamboo gardens located in Zhejiang Agriculture and Forestry University, Zhejiang Province, China. The shoot collected on April 12, 2012, was 0.20 m in height with a ground diameter of 0.07 m. The other shoot (bamboo 1) collected on April 11, 2013, was 0.60 m in height with a ground diameter of 0.73 m (Fig. 4a). There were 27 visible nodes in bamboo 1 counted from the ground boundary to the canopy and six visible nodes on the underground parts. Bamboo 2, also collected on April 11, 2013, was 3.20 m in height with a ground diameter of 0.73 m (Fig. 4b). This one had 35 visible nodes. Internode 18 was the longest one (13 cm) and had lignified, whereas internodes 23 and 28 were shorter (6.5, and 2 cm, respectively) and soft. RNA extraction was performed on all the samples. Tissues that used for real-time fluorescent quantitative PCR (RT-PCR) were further divided into different segments as show in Fig. 4. After division, each sample was frozen in liquid nitrogen and stored at −80 °C. Total RNA was isolated using RNeasy Plant Mini Kit (QIAGEN, German) according to the manufacturer’s instructions. The cDNA was synthesized by Invitrogen SuperScript III™ Kit (Invitrogen, USA). The reaction system consisted of 1 μg total RNA, 1 μl Oliga (dT) (20 μM), 1 μl 10 mM dNTP, 5 μl ddH2O and was kept at 65 °C for 5 min and subsequently in ice-bath for more than 1 min. The 2 μl 10 × RT buffer, 4 μl MgCl2 (25 μM), 2 μl 0.1 M DTT, 1 μl RNase out, 1 μl SS III RT were added, followed by 50 °C extending for 50 min; 85 °C for 5 min. The 1 μl RNase H was added before the final extending in 37 °C for 20 min, the cDNA productions were diluted tenfolds for further experiments.
Clone and sequence analysis of Phyllostachys pubescens endo-1,4-β-glucanases gene
The gene fragment identified from previous research (Zhou et al. 2011) was blasted against the bamboo databank and the full length of endo-1,4-β-glucanases gene was acquired. Primers were designed base on the full length gene sequence (Table 3). PCR was carried out in a volume of 20 μl containing 10 × PCR buffer 2.0 μl, MgCl2 (25 μM) 1.2 μl, dNTP (2.5 mM) 3.2 μl, and primers with each of 0.4 μl (20 μM; Forward primer: GLY-5; Reverse primer: GLY-3). The primer sequences are listed in Table 3. TakaRa rTaq (5 U/μl) 0.2 μl, cDNA 2.0 μl, ddH2O 10.6 μl were included in the reaction. Amplification was performed in a T1 thermocycler (Biometra, Germany) using the following conditions: 1 cycle, 94 °C, 5 min; 23 cycles of 94 °C, 30 s, 55 °C, 30 s, and 72 °C, 1 min; 1 cycle, 72 °C, 4 min. PCR products were visualized by electrophoresis on a 1 % agarose gel and extracted using an SanPrep Gel Extraction Kit (Sangon Biotech, China). Purified PCR products were cloned into plasmids using the pGEM-T vector system (Promega, USA). Five to 20 clones were selected for each amplification product and cultured to isolate the plasmids. Positive clones were confirmed by colony PCR. After successful identification of the endo-1,4-β-glucanases gene through sequencing by Sangon Biotech (Shanghai) Co., Ltd, China, the plasmid with correct sequence was nominated as A1.
Sequences were edited on Seqman and EditSeq program of DNAStar 5 software. Searching for homologous sequences was conducted by BLAST comparison (at NCBI site). Physicochemical property of the protein was predicted by Protean (http://genome.cbs.dtu.dk/services/TMHMM/). Online tools were used to predict the transmembrane region of the protein. Domain architectures of endo-1,4-β-glucanases protein was conducted on NCBI (http://www.ncbi.nlm.nih.gov/Structure/lexington/lexington.cgi). Excel was used for the analysis of gene intron and exon proportion. ClustalX 1.81 software was utilized to align the amino acid sequence between bamboo and other GH 9 family members (Thompson et al. 1997). Phylogenetic tree was constructed using Maximum Likelihood method by MEGA 5.1 software (Tamura et al. 2011).
Transgene construction and plant transformation
PCR amplification was conducted on A1 with new set of primers GLY-Ba-5 and GLY-Xb-3 applied for the purpose of transgene construction (Table 3): 2.0 μl 10 × PCR buffer, 1.2 μl MgCl2 (25 μM), 1.6 μl dNTP (2.5 mM), 0.4 μl primer (20 μM) for each, 0.2 μl TakaRa rTaq (5 U/μl), 2.0 μl A1 plasmid, 12.2 μl ddH2O. Amplification was performed with the following conditions: 1 cycle, 94 °C, 3 min; 25 cycles, 94 °C, 30 s, 55 °C, 30 s, 72 °C, 1 min; 1 cycle, 72 °C, 10 min; 4 °C for the end. PCR products were visualized by 1 % agarose gel electrophoresis with ethidium bromide staining and UV illumination. Amplified products were purified through SanPrep Gel Extraction Kit (Sangon Biotech China). The purified PCR products and plasmid (pCAMBIA 1301) were digested by restriction enzyme XbaI and BamHI (NEB, USA) and collected using Mini Elute PCR Purification Kit (Sangon Biotech, China). Plasmid segments were purified by SanPrep Gel Extraction Kit (Sangon Biotech, China) through 1 % agarose gel electrophoresis. Both segments were ligated and the mixture was transformed into Escherichia coli. Once succeed, the objective gene was cloned and electro-transformed into Agrobacterium tumefaciens strain EHA105.
The transformed Agrobacterium strain was used to infect the wild type Arabidopsis thaliana plants via the floral dipping method (Clough and Bent 1998). Plants with Phbeta-1,4-glu transformed were selected by 1/2 MS medium containing 100 mg/l hygromycin. After cultivation for two generations, the third generation homozygous transgenic plants (T3) were used for paraffin section, cellulose content and plant height determination (Chen and Sun 2010).
Real-time fluorescent quantitative PCR
Total RNA from different parts of bamboo shoots were extracted according to Fig. 4. After PCR verification, each RNA was reverse-transcripted into cDNA. RT-PCR were conducted on 7500 system using SDS software, with β-actin 3 as the reference gene and GLY-RT as the primers (Table 3). Reaction system (20 μl) contained: 1 μl cDNA, 0.4 μl of each primer (10 μM); 10 μl 2 × comSYBR qPCR Mix; 8.2 μl ddH2O. Amplification conditions were as followed: 1 cycle, 95 °C for 1 min; 40 cycles, 95 °C for 15 s, 60 °C for 33 s. After each cycle, a read was recorded to build the dissociation curve. Following reaction conditions were applied: 95 °C for 15 s; 60 °C for 1 min; 95 °C for 15 s; 60 °C for 15 s. Three repeats were performed for each of the samples.
Paraffin section
WT A. thaliana and T3 transgenic plants after 50 days growth were selected for a traditional paraffin section. Intact leaf and stem sections were stained with safranin-fast green contrast-stain method (Li 1996). Olympus BX 60 fluorescent microscope was used for cell structure analysing and picture capturing.
Measurement of cellulose content and plant height
Plant height was measured and recorded when the flower stalks initiated. Cellulose content was assayed according to Mohr’s method (He 2009). Stem materials of T3 transgenic and WT plants were collected with roots, leaves and buds discarded when the flower stalks initiated. The raw materials were firstly dried at 105 °C for half an hour and then transformed to 65 °C for 2 days. The dried cell wall materials were weighed (n), grinded into powder and then incubated for 25 min in a mixture of ethylic-nitric acid (1:10, v/v) in a boiling-water bath. The samples were centrifuged in 4800 rpm for 10 min and washed with sterile water for three times. The sediments were collected and extracted in 0.5 N vitriol-potassium dichromate mixtures for 10 min in a boiling water bath. The 0.1 N Mohr’s salt was used for titration (b) and the extractant was added with 3–5 drops of phenanthroline as an indicator. The titration was stopped when the solution colour turned into bronzing. A single 10 ml 0.5 N vitriol-potassium dichromate mixture was taken as a titration control (a). For the titer of Mohr’s salt (k), it was acquired by the titration of 25 ml 0.1 N potassium dichromate with Mohr’s salt until the colour become bronzing. Cellulose content (x) were calculated according to the formula: x = 0.675 × k(a − b)/n (detailed information about a, b, n and k were listed above). Six samples were taken from WT and transgenic plants.
Author contribution statement
Ming-Bing Zhou, all laboratory practice, analysis and interpretation of data and drafting the article. Ying Zheng, Phbeta-1,4-glu cloning, characterization, real-time RT-PCR and transgene. Zhi-Gang Liu and Xiang-Wan Xia, phenotype investigation of genetically modified Arabidopsis. Ding-Qin Tang, analysis and interpretation of data.
References
Appenzeller L, Doblin M, Barreiro R, Wang HY, Niu XM, Kollipara K, Carrigan L, Tomes D, Chapman M, Dhugga KS (2004) Cellulose synthesis in maize: isolation and expression analysis of the cellulose synthase (CesA) gene family. Cellulose 11:287–299
Beguin P (1990) Molecular biology of cellulose degradation. Annu Rev Microbiol 44:219–248
Bhandari S, Fujino T, Thammanagowda S, Zhang D, Xu F, Joshi CP (2006) Xylem-specific and tension stress-responsive coexpression of KORRIGAN endoglucanase and three secondary wall-associated cellulose synthase genes in aspen trees. Planta 224:828–837
Boyd D, Beckwith J (1990) The role of charged amino acids in the localization of secreted and membrane proteins. Cell 62:1031–1033
Brummell DA, Catala C, Lashbrook CC, Bennett AB (1997) A membrane-anchored E-type endo-1, 4-β-glucanase is localized on Golgi and plasma membranes of higher plants. Proc Natl Acad Sci 94:4794–4799
Buchanan M (2011) Cellulose, stem strength and the Endo-(1, 4)-β-Glucanase gene family in Barley and Maize. Ph. D. thesis, The University of Adelaide, Adelaide, pp 1–405
Buchanan M, Burton RA, Dhugga KS, Rafalski AJ, Tingey SV, Shirley NJ, Fincher GB (2012) Endo-(1, 4)-β-Glucanase gene families in the grasses: temporal and spatial Co-transcription of orthologous genes. BMC Plant Biol 12:235
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Carmel L, Wolf YI, Rogozin IB, Koonin EV (2007) Three distinct modes of intron dynamics in the evolution of eukaryotes. Genome Res 17:1034–1044
Castresana J (2002) Estimation of genetic distances from human and mouse introns. Genome Biol 3:7
Chen B (2010) Application of intron in bioinformatics researches and transgenic engineering. Chem Life 30:59–63
Chen YJ, Sun CW (2010) Transgenic study of chloroplast translocon gene regulation in Arabidopsis thaliana. Bot Stud 51:147–153
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Cui K, He CY, Zhang JG, Duan AG, Zeng YF (2012) Temporal and spatial profiling of internode elongation-associated protein expression in rapidly growing culms of bamboo. J Proteome Res 11:2492–2507
Das M, Bhattacharya S, Pal A (2005) Generation and characterization of SCARs by cloning and sequencing of RAPD products: a strategy for species-specific marker development in bamboo. Ann Bot 95:835–841
Delmer DP, Amor Y (1995) Cellulose biosynthesis. Plant Cell 7:987
Deng G, Zeng G, Wang L (1988) Studies on cell division of intercalary meristem and intercalary growth of Phyllostachys pubescens and Phyllostachys bambusoides, Phyllostachys heteroclada Oliv. Acta Sci Nat Univ Norm Hunan 11:244–250
Dong LN (2007) Studies on developmental anatomy of elongated growth about bamboo culms. Master thesis, Nanjing Forestry University, Nanjing, pp 1–69
Du LZ, Jing AW (2010) cDNA cloning and expression of Phyllostachys praecox Z.d.Chu et C.S.Chao cellulose synthase gene. Acta Agric Univ 32:0535–0540
Fagard M, Desnos T, Desprez T, Goubet F, Refregier G, Mouille G, McCann M, Rayon C, Vernhettes S, Höfte H (2000) PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 12:2409–2423
Feng JY (2010) Studies on primary thickening growth mechanism of Phyllostachys edulis shoot bud. Master thesis, Nanjing Forestry University, Nanjing, pp 1–65
Gaut BS, Doebley JF (1997) DNA sequence evidence for the segmental allotetraploid origin of maize. Proc Natl Acad Sci 94:6809–6814
Gazave E, Marqués-Bonet T, Fernando O, Charlesworth B, Navarro A (2007) Patterns and rates of intron divergence between humans and chimpanzees. Genome Biol 8:R21
Grass Phylogeny Working Group, Barker NP, Clark LG, Davis JI, Duvall MR, Guala GF, Hsiao C, Kellogg EA, Peter Linder H, Mason-Gamer RJ, Mathews SY, Simmons MP, Soreng RJ, Spangler RE (2001) Phylogeny and subfamilial classification of the grasses (Poaceae). Ann Missouri Bot Garden 88: 373–457
Gui YJ, Zhou Y, Wang Y, Wang S, Wang SY, Hu Y, Bo SP, Chen H, Zhou CP, Ma NX, Zhang TZ, Fan LJ (2010) Insights into the bamboo genome: syntenic relationships to rice and sorghum. J Integr Plant Biol 52:1008–1015
He SE (2009) cDNA library construction and molecular cloning of cellulose synthase gene (PeCesA12 and PeCesA11) from Moso bamboo. Master thesis, Zhejiang Forestry University, Zhejiang, pp 1–46
He CY, Cai K, Zhang JG, Duan AG, Zeng YF (2013) Next-generation sequencing-based mRNA and microRNA expression profiling analysis revealed pathways involved in the rapid growth of developing culms in Moso bamboo. BMC Plant Biol 13:119
He MX, Wang JL, Qin H, Shui ZX, Zhu QL, Wu B, Tan FR, Pan K, Hu QC, Dai LC, Wang WG, Tang XY, Hu GQ (2014) Bamboo: a new source of carbohydrate for biorefinery. Carbohydr Polym 111:645–654
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280:309–316
Henrissat B, Bairoch A (1993) New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 293:781–788
Henrissat B, Bairoch A (1996) Updating the sequence-based classification of glycosyl hydrolases. Biochem J 316:695–696
Igea J, Juste J, Castresana J (2010) Novel intron markers to study the phylogeny of closely related mammalian species. BMC Evol Biol 10:369
Jiang ZH (2002) World bamboo and rattan. LiaoNing Science and Technology Publishing House, LiaoNing
Kay BK, Williamson MP, Sudol M (2000) The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J 14:231–241
Kellogg EA (1998) Relationships of cereal crops and other grasses. Proc Natl Acad Sci 95:2005–2010
Kellogg EA (2001) Evolutionary history of the grasses. Plant Physiol 125:1198–1205
Kovi MR, Bai X, Mao D, Xing Y (2011) Impact of seasonal changes on spikelets per panicle, panicle length and plant height in rice (Oryza sativa L.). Euphytica 179:319–331
Lao X, Ji Azuma, Sakamoto M (2013) Two cytosolic aldolases show different expression patterns during shoot elongation in Moso bamboo, Phyllostachys pubescens Mazel. Physiol Plant 149:422–431
Lee S, Jia MH, Jia Y, Liu G (2014) Tagging quantitative trait loci for heading date and plant height in important breeding parents of rice (Oryza sativa). Euphytica 197:191–200
Li ZL (1996) Plant tissue producer. Peking University Press, Beijing
Li C, Qi L, Wang J, Wang Y, Shi S, Zhang S (2004) Cellulose synthase gene and cellulose biosynthesis in plants. Biotechnol Bull 2005:5–11
Li YJ, Fu YR, Huang JG, Wu CA, Zheng CC (2011) Transcript profiling during the early development of the maize brace root via Solexa sequencing. FEBS J 278:156–166
Liu M, Qiao G, Jiang J, Yang H, Xie L, Xie J, Zhuo R (2012) Transcriptome sequencing and de novo analysis for ma bamboo (Dendrocalamus latiflorus Munro) using the Illumina platform. PLoS One 7(10):e46766
Logacheva MD, Kasianov AS, Vinogradov DV, Samigullin TH, Gelfand MS, Makeev VJ, Penin AA (2011) De novo sequencing and characterization of floral transcriptome in two species of buckwheat (Fagopyrum). BMC Genom 12:30
Lv HK, Zheng J, Wang TY, Fu JJ, Huai JL, Min HW, Zhang X, Tian BH, Shi YS, Wang GY (2014) The maize d2003, a novel allele of VP8, is required for maize internode elongation. Plant Mol Biol 84:243–257
Magel E, Kruse S, Lütje G, Liese W (2005) Soluble carbohydrates and acid invertases involved in the rapid growth of developing culms in Sasa palmata (Bean). Camus Bamboo Sci Cult 19:23–29
Maloney VJ, Samuels AL, Mansfield SD (2012) The endo-1, 4-β-glucanase Korrigan exhibits functional conservation between gymnosperms and angiosperms and is required for proper cell wall formation in gymnosperms. New Phytol 193:1076–1087
Mølhøj M, Johansen B, Ulvskov P, Borkhardt B (2001) Expression of a membrane-anchored endo-1, 4-β-glucanase from Brassica napus, orthologous to KOR from Arabidopsis thaliana, is inversely correlated to elongation in light-grown plants. Plant Mol Biol 45:93–105
Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Höfte H (1998) A plasma membrane-bound putative endo-1, 4-β-d-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO J 17:5563–5576
Pagant S, Bichet A, Sugimoto K, Lerouxel O, Desprez T, McCann M, Lerouge P, Vernhettes S, Höfte H (2002) KOBITO1 encodes a novel plasma membrane protein necessary for normal synthesis of cellulose during cell expansion in Arabidopsis. Plant Cell 14:2001–2013
Paterson A, Bowers J, Chapman B (2004) Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proc Natl Acad Sci USA 101:9903–9908
Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, Schmutz J, Spannagl M, Tang HB, Wang XY, Wicker T, Bharti AK, Chapman J, Feltus FA, Gowik U, Grigoriev IV, Lyons E, Maher CA, Martis M, Narechania A, Otillar RP, Penning BW, Salamov AA, Wang Y, Zhang LF, Carpita NC, Freeling M, Gingle AR, Hash CT, Keller B, Klein P, Kresovich S, McCann MC, Ming R, Peterson DG, Rahman M, Ware D, Westhoff P, Mayer KFX, Messing J, Rokhsar DS (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457:551–556
Peng ZH, Lu TT, Li LB, Liu XH, Gao ZM, Hu T, Yang XW, Feng Q, Guan JP, Weng QJ, Fan DL, Zhu CR, Lu Y, Han B, Jiang ZH (2010) Genome-wide characterization of the biggest grass, bamboo, based on 10,608 putative full-length cDNA sequences. BMC Plant Biol 10:116
Peng ZH, Lu Y, Li LB, Zhao Q, Feng Q, Gao ZM, Lu HY, Hu T, Yao N, Liu KY, Li Y, Fan DL, Guo YL, Li WJ, Lu YQ, Weng QJ, Zhou CC, Zhang L, Huang T, Zhao Y, Zhu CR, Liu XG, Yang XW, Wang T, Miao K, Zhuang CY, Cao XL, Tang WL, Liu GS, Liu YL, Chen J, Liu ZJ, Yuan LC, Liu ZH, Huang XH, Lu TT, Fei BH, Ning ZM, Han B, Jiang ZH (2013a) The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat Genet 45:456–461
Peng ZH, Zhang CL, Zhang Y, Hu T, Mu SH, Li XP, Gao J (2013b) Transcriptome sequencing and analysis of the fast growing shoots of moso bamboo (Phyllostachys edulis). PLoS One 8(11):e78944
Rodova M, Islam MR, Peterson KR, Calvet JP (2003) Remarkable sequence conservation of the last intron in the PKD1 gene. Mol Biol Evol 20:1669–1674
Salse J, Bolot S, Throude M, Jouffe V, Piegu B, Quraishi UM, Calcagno T, Cooke R, Delseny M, Feuillet C (2008) Identification and characterization of shared duplications between rice and wheat provide new insight into grass genome evolution. Plant Cell 20:11–24
Sami AJ, Shakoori A (2008) Biochemical characterization of endo-1, 4-β-D-glucanase activity of a green insect pest Aulacophora foveicollis (Lucas). Life Sci J 5(2):30–36
Sato S, Kato T, Kakegawa K, Ishii T, Liu YG, Awano T, Takabe K, Nishiyama Y, Kuga S, Sato S, Nakamura Y, Tabata S, Shibata D (2001) Role of the putative membrane-bound endo-1, 4-β-glucanase KORRIGAN in cell elongation and cellulose synthesis in Arabidopsis thaliana. Plant Cell Physiol 42:251–263
Scurlock J, Dayton D, Hames B (2000) Bamboo: an overlooked biomass resource? Biomass Bioenergy 19:229–244
Shani Z, Dekel M, Tsabary G, Goren R, Shoseyov O (2004) Growth enhancement of transgenic poplar plants by overexpression of Arabidopsis thaliana endo-1, 4–β-glucanase (cel1). Mol Breed 14:321–330
Shani Z, Dekel M, Roiz L, Horowitz M, Kolosovski N, Lapidot S, Alkan S, Koltai H, Tsabary G, Goren R, Shoseyov O (2006) Expression of endo-1, 4-β-glucanase (cel1) in Arabidopsis thaliana is associated with plant growth, xylem development and cell wall thickening. Plant Cell Rep 25:1067–1074
Sharma R, Gupta P, Sharma V, Sood A, Mohapatra T, Ahuja PS (2008) Evaluation of rice and sugarcane SSR markers for phylogenetic and genetic diversity analyses in bamboo. Genome 51:91–103
Soria-Carrasco V, Talavera G, Igea J, Castresana J (2007) The K tree score: quantification of differences in the relative branch length and topology of phylogenetic trees. Bioinformatics 23:2954–2956
Sungkaew S, Stapleton CM, Salamin N, Hodkinson TR (2009) Non-monophyly of the woody bamboos (Bambuseae; Poaceae): a multi-gene region phylogenetic analysis of Bambusoideae ss. J Plant Res 122:95–108
Swigoňová Z, Lai J, Ma J, Ramakrishna W, Llaca V, Bennetzen JL, Messing J (2004) Close split of sorghum and maize genome progenitors. Genome Res 14:1916–1923
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Ueda K (1960) Studies on the physiology of Bamboo, with reference to practical application. Reference data No. 34
Urbanowicz BR et al (2007) Structural organization and a standardized nomenclature for plant endo-1, 4-β-glucanases (cellulases) of glycosyl hydrolase family 9. Plant Physiol 144:1693–1696
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63
Wei WL, Qi XQ, Wang LH, Zhang YX, Hua W, Li DH, Lv HX, Zhang XR (2011) Characterization of the sesame (Sesamum indicum L.) global transcriptome using Illumina paired-end sequencing and development of EST-SSR markers. BMC Genom 12:451
Wu LS, Jin XC, Yang YC, Huang HS, Chen ST, Yao WF, Yao FP (2010) cDNA cloning and expression analysis of Phyllostachys edulis beta-1, 4-glycosidase gene. Biotechnol Bull 3:026
Wu T, Shen YY, Zheng M, Yang CY, Chen YL, Feng ZM, Liu X, Liu SJ, Chen ZJ, Lei CL, Wang JL, Jiang L, Wan JM (2014) Gene SGL, encoding a kinesin-like protein with transactivation activity, is involved in grain length and plant height in rice. Plant Cell Rep 33:235–244
Xiong W, Ding Z, Li Y (1980) Intercalary meristem and internodal elongation of bamboo plants. Scientia Silvae Sinicae 16:81–89
Yu Y, Wang H, Lu F, Tian G, Lin J (2014) Bamboo fibers for composite applications: a mechanical and morphological investigation. J Mater Sci 49:2559–2566
Zhang YQ, Liang JH, Li BX (2007) Application of β-glycosidase to cellulose bio-degradation. J Tianjin Univ 3:15
Zhang YJ, Ma PF, Li DZ (2011) High-throughput sequencing of six bamboo chloroplast genomes: phylogenetic implications for temperate woody bamboos (Poaceae: Bambusoideae). PLoS One 6:e20596
Zhou HL, He SJ, Cao YR, Chen T, Du BX, Chu CC, Zhang JS, Chen SY (2006) OsGLU1, a putative membrane-bound endo-1, 4-β-D-glucanase from rice, affects plant internode elongation. Plant Mol Biol 60:137–151
Zhou MB, Yang P, Gao PJ, Tang DQ (2011) Identification of differentially expressed sequence tags in rapidly elongating Phyllostachys pubescens internodes by suppressive subtractive hybridization. Plant Molecular Biology Reporter 29:224–231
Zuo J, Niu QW, Nishizawa N, Wu Y, Kost B, Chua NH (2000) KORRIGAN, an Arabidopsis endo-1, 4-β-glucanase, localizes to the cell plate by polarized targeting and is essential for cytokinesis. Plant Cell 12:1137–1152
Acknowledgments
This work was supported by the grant from the Program of Natural Science Foundation of Zhejiang Province (LR12C16001), and the National Natural Science Foundation of China (31270645, 31500542 and 31470615), and the Opening Foundation of the Key Forestry Disciplines of Zhejiang Province (KF201301).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by J. Carlson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
468_2016_1363_MOESM1_ESM.tif
Fig. S1 Secondary structure of the protein encoded by Phbeta-1,4-glu. α helix: red boxes; β sheet: green boxes; corner: blue boxes; random coil: yellow boxes (TIFF 8247 kb)
468_2016_1363_MOESM2_ESM.tif
Fig. S2 Transmembrane domain of the protein encoded by Phbeta-1,4-glu. Transmembrane: red lines; inside: blue lines; outside: pink lines (TIFF 17860 kb)
468_2016_1363_MOESM3_ESM.tif
Fig. S3 Fluorescent microscopic analysis of the transgenic plant (a and c) and wild-type plant (b and d). Longitudinal sections (a, b) and transverse sections (c, d) along the plant stems. Bars indicate 200 μm for a and b, and 502 μm for c and d. Red circles refer to difference between these two kinds of plants (TIFF 13462 kb)
Rights and permissions
About this article
Cite this article
Zhou, MB., Zheng, Y., Liu, ZG. et al. Endo-1,4-β-glucanase gene involved into the rapid elongation of Phyllostachys heterocycla var. pubescens . Trees 30, 1259–1274 (2016). https://doi.org/10.1007/s00468-016-1363-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1363-z