Abstract
Key message
The radial growth of Larix sibirica has exhibited divergent responses to temperatures in the growing season due to the temperature-driven drought stress in the eastern Tianshan Mountains of Northwest China.
Abstract
The warming of the continental interior of the Northern Hemisphere in the latter half of the twentieth century has led to divergent responses between trees growth and climate factors at high latitudes. This study explored the variability in the responses of radial growth to climate factors and the temporal stability of growth–climate relationships under climate change for Siberia larch (Larix sibirica Ledeb.) in the mid-latitude area of the eastern Tianshan Mountains. The results indicated as follows: (1) Analysis of the relationships between tree-ring width chronology and climate factors before and after the abrupt increases in temperature demonstrated that divergent responses of radial growth occurred in the eastern Tianshan Mountains, suggesting that correlation coefficients with temperature varied significantly in the growing season. (2) Examination of variations in the climate factors limiting radial growth in the two time periods demonstrated that the minimum temperature played a key role from 1989 to 2012. (3) Assessment of the temporal stability of growth–climate relationships by a moving correlation function demonstrated that the positive sensitivity of Siberia larch to temperature during the growing season decreased. The reasons for this ‘divergence problem’ were the increased drought stress and changes in the physiological responses of trees. (4) Simulation of the trend of basal area increment by linear regression showed that the growth rate of Siberia larch first increased and then decreased. The future effects of increasing temperature may cause significant alterations in forest ecosystems, with greater effects on forests in arid and semi-arid areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global climate change is an undeniable fact (IPCC 2013). For average annual Northern Hemisphere temperatures, the period from 1983 to 2012 was likely the warmest 30-year period of the last 800 to 1400 years (IPCC 2013). The forest ecosystem is the most important terrestrial ecosystem for humans, and it is deeply affected by global climate change, with effects on species distribution, forest productivity, biodiversity, plant biotemperature, the global carbon cycle and more (Bonan 2008; Cox et al. 2013). Therefore, it is critical to understand the variations in the global climate and the regularity of the trees’ responses to those variations. This information is likely to provide some theoretical basis for the sustenance, monitoring and assessment of the forest ecosystems.
As natural archives, tree-ring is a critical tool providing high-resolution data on climate change. These data describe the ongoing processes of tree growth and feature benefits including exact dating, high continuity, high sensitivity, long temporal span and easily obtained duplicates (Stoffel and Corona 2014). Meanwhile, dendroclimatology has been used to evaluate tree growth–climate relationships, providing baseline knowledge for climate change researches (Beniston 2002; Zhang et al. 2012). The ‘uniformitarian principle’ generally assumes that the relationship between tree growth and limiting climate factors is stable over time (Fritts 1976). However, as global warming has intensified in the second part of the twentieth century, many recent studies have found evidences violating this principle. Some studies have reported that the relationship between tree growth and temperature weakens as temperature increases, especially at the higher latitudes of the Northern Hemisphere (Briffa et al. 1998; Franceschini et al. 2012). This tendency towards changes in the strength of the responses of tree growth to climate factors has been termed the ‘divergence problem’ (D’Arrigo et al. 2008).
Studies of the ‘divergence problem’ have produced a complex set of results regarding the responses of growth to climate change, with different responses observed for different trees, regions and climate factors (Trindade et al. 2010; Wang et al. 2013). For example, a long-term change was observed in the sensitivity of the tree-ring growth of Larix decidua and Larix sibirica to climate forcing in eastern Italy and north-central Mongolia (De Grandpre et al. 2011). However, no divergence phenomenon was observed for Larix decidua in the European Alps (Büntgen et al. 2008). Diverging growth trends were observed for Pinus tabulaeformis and Sabina przewalskii but not for Picea crassifolia in northwest China (Zhang et al. 2009). While the ‘divergence problem’ has received greater attention and recognition in recent years, the explanation of the ‘divergence problem’ remains a topic of debate, focusing on the response threshold effect of climate change and environmental pollution (Barber et al. 2000; Wilson and Elling 2004), the age effect of biological species heterogeneity (Wu et al. 2013a), the end effect of inappropriate detrending method (Briffa and Melvin 2011) and other concepts. Therefore, it is critical to detect the divergent responses of tree growth to climate change and analyze the potential causes of the ‘divergence problem’.
The forest ecosystems of arid and semi-arid areas are predicted to experience the most severe effects of global climate change (Ma et al. 2013). Evaluating trees’ responses to climate change under arid environments requires further data analysis at various locations (Lioubimtseva 2004). The Tianshan Mountains of Central Asia are considered to be a sensitive indicator due to their unique natural conditions (Wu et al. 2013b). Studies of tree-ring-based climate reconstructions and climate–growth relationships concentrated on the Tianshan Mountains (Yuan et al. 2003; Wang et al. 2005). However, the divergent responses of radial growth to climate change have not yet been studied in the Tianshan Mountains, and it remains unclear whether the ‘divergence problem’ of Siberia larch occurs in this study region.
Given the background of global climate change, it is essential to investigate the chronological characteristics, the relationships between radial growth and climate factors, and the stability and variance of climate factors limiting radial growth. These studies are able to accurately assess climate change, understand the dynamic responses of tree growth and fill in gaps in our understanding of the ‘divergence problem’ of growth–climate relationships in the mid-latitude region of the Tianshan Mountains. The main aims of this study are (1) to detect whether significant changes have occurred in the growth–climate relationships over time and (2) to analyze the effects of climate change on the tree growth of Siberia larch in this region.
Materials and methods
Study area
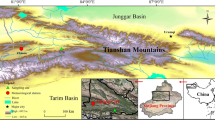
The study area is located on the north side of the eastern Tianshan Mountains, northwest China (Fig. 1), which is dominated by the cold and drought of the temperate semiarid continental climate. From 1957 to 2012, the mean annual temperature was 2.0°, the hottest mean month temperature (July) was 18.1°, the coldest mean month temperature (January) was −17.8°. The total annual precipitation was 219.3 mm, with 73.5 % falling in the spring and summer (Fig. 2).
In this study, we collected samples from the northern slope of the Heixi valley in the Barkol Kazakh Autonomous County. The forested area is distributed on the shady slopes at altitudes of 2100–2700 m, and Siberia larch and Schrenk spruce (Picea schrenkiana Fisch. et Mey.) are the dominant species (Wang et al. 2004). The Siberia larch, a deciduous and cold–drought-resistant tree, is a superior tree species at high elevations from 2300 to 2700 m (Peng et al. 2005).
Meteorological data
Meteorological data of the annual and monthly total precipitation and mean, minimum and maximum temperatures were obtained from the nearest Barkol meteorological station (approximately 12 km from the study site) (Fig. 1). The Mann–Kendall test was applied to detect the abrupt turning point of trends in annual temperature and precipitation (Goossens and Berger 1987). Because radial growth is affected not only by the climatic conditions of the current year but also by those of the previous year (Dang et al. 2013), the correlation analysis between radial growth and the annual climate data (the previous and current year) and the monthly climate data (from June of the previous year to September of the current year) were performed from 1958 to 2012.
Tree-ring materials and statistical analyses
Tree-ring samples may be affected by differences in habitat conditions. To avoid interference from these factors, the tree-ring samples were collected from a consistent north-facing slope with a gradient of 33° in this study region. The elevations ranged from 2590 to 2645 m above sea level and the canopy coverage is approximately 20 % (Table 1). Older trees growing in relatively sparse or isolated conditions without obvious physical damage from fire, disease and insect infestation were selected as samples. Two cores were collected from each tree with a 5.15-mm-diameter increment borer. One core was bored parallel to the contour line and the other core was bored orthogonally to the first at breast height (1.3 m above the ground). In total, 50 cores were extracted from 25 living trees in August 2013.
In the laboratory, the tree cores were mounted on woody supports, air dried and sanded to produce clearly visible boundaries for identification (Stokes and Smiley 1968). Ring widths were measured with 0.001 mm resolution using the LINTAB measurement system (TM5, Rinntech, Heidelberg, Germany). The cross-dating results of the measured tree-ring series were verified using the COFECHA program (Grissino-Mayer 2001). Two series with significant deviations were excluded from the master chronology. The age-related trend in growth from raw series was removed by the negative exponential curve or linear regression method using the ARSTAN program (Cook 1985). The standard chronology (STD) and residual chronology (RES, Fig. 3) were developed. The standard chronology is more suited for the long-term trend analysis, which retains more low-frequency (mainly decade or more) growth variability (Dang et al. 2013). By contrast, the residual chronology by removing the problem of biological persistence in the resulting series using autoregressive modeling, contains more high-frequency signals (mainly annual), which is better in assessing climate-growth relationships (Sánchez-Salguero et al. 2010; Jiang et al. 2014; Gazol et al. 2015). In addition, the residual chronology avoids possible ‘end-effect’ bias due to index inflation after calculating ratios and dose not offset the formerly temperature sensitive with less low-frequency trend, which is suitable for analyzing ‘divergence problem’ (He et al. 2014; Schneider et al. 2014). Given the above, we selected the residual chronology for the analysis.
To assess the reliability of the chronology and compare key properties, several dendrochronological parameters were computed from 1949 to 2012 (common period). The mean sensitivity (MS) indicating the variability of ring width between consecutive years and the standard deviation (SD) estimating the variability of each series can assess the response to considerable high-frequency variation (Fritts 1991). The mean correlation (R) representing the strength of the correlation among all series can express the degree of common signal (Fritts and Shatz 1975). The expressed population signal (EPS) defining the reliable part of the chronology with a threshold of 0.85 is usually accepted as sufficient, and the signal to noise ratio (SNR) reflecting the signal strength of each chronology can quantify the degree of chronological quality (Wigley et al. 1984). The high values of SNR (23.70) and EPS (0.960) for this dataset indicate that the chronology is of high quality and reliable. Meanwhile, we found that the MS (0.254), SD (0.219) and R (0.411) were high, demonstrating that the Siberia larch contain climatic information and are sensitive to climate change.
Analysis of the growth–climate relationship
Growth–climate relationships were investigated by calculating the Pearson correlation coefficients between tree-ring width chronology and climate factors (Fritts 1976). Moreover, to examine the variations in growth–climate relationships over different periods, the value of u was calculated (Lindeman et al. 1980). The value of u is the quantile of a standard normal distribution that shows the correlation discrepancy
where r 1 and r 2 represent the correlation coefficients between tree-ring width chronology and climate factors, and n 1 and n 2 represent the numbers of samples in the two periods. If |u| > 1.96, the correlation coefficients exhibit significant changes at the 0.05 level.
The variations of radial growth over time in response to climate factors were calculated by a moving correlation function with a 30-year time window using the Dendroclim2002 program (Biondi and Waikul 2004). The results of the moving correlation function not only accurately depicted the dynamic growth–climate relationship but also indicated the variation of the physiological responses with mainly climate factors limiting radial growth.
Analysis of the tree radial growth pattern
We calculated the basal area increment (BAI, cm2 year−1) using the non-standardized raw measurement ring width data. Compared with ring-width series, the BAI is a more biologically meaningful variable to quantify the speed and trend of tree growth. This is because the BAI is a highly conservative approach that retains the original data and eliminates any data-transformation biases (Rubino and McCarthy 2000). Meanwhile, the conversion of ring width to BAI overcomes the problem of ring width decline with age (Gómez-Guerrero et al. 2013). Unlike the tree-ring width, age-related trend in unstandardized BAI is generally positive and maintains culminating in a linear level for many decades after trees reach maturity, until senescence is reached or environmental stress is applied (Phipps and Whiton 1988; Schuster and Oberhuber 2013). Therefore, BAI has been considered to be a better tool for assessing tree growth pattern. And the negative trend in BAI is a strong indication of a real decline in tree growth under environmental stress (Xu et al. 2014b). In order to avoid age influence on the growth trend, the mean BAI was calculated using all sampled trees older than 50 years. The BAI is calculated as:
where the BA variable represents consecutive cross-sectional basal areas, R is the core length measured for dated tree-rings formed in years t − 1, and TRW is the measured raw width of the tree-ring in year t.
Results
Climate change characteristics
The results of Mann–Kendall test showed that the year of 1989 was an abrupt transition point in mean annual temperature and that there was no abrupt transition point in total annual precipitation (Fig. 4a, b). The instrumental records exhibit increasing trends in temperature and precipitation from 1957 to 2012 (Fig. 4a, b). The total annual precipitation from 1989 to 2012 increased by 24.4 mm compared with 1957–1988, while the mean annual temperature increased by 2.1°. The rate of rise in mean annual temperature was greater than the rate of rise in total annual precipitation, with temperature increasing by 181.4 % and precipitation increasing by 11.7 %. In addition, the rate of rise in the minimum temperature was greater than those of the mean temperature and maximum temperature. The minimum temperature from 1989 to 2012 was 3.0° higher than in the previous period, whereas the maximum temperature was only 0.9° higher.
Variation of annual total precipitation (A), maximum temperature (B 1), mean temperature (B 2) and minimum temperature (B 3) from 1957 to 2012. The abrupt change was detected by the Mann–Kendall test of annual total precipitation (a) and mean temperature (b). C 1 represents the U values for the normal time series, C 2 represents the U values for the retrograde time series. The dotted lines indicate the value of significance at the 0.01 level. The dotted lines with arrows represent the trends simulated by linear regression
Relationships between tree-ring width chronology and climate factors
The growth–climate relationships were analyzed by Pearson correlations in the two time periods before and after the abrupt increase in temperature (Fig. 5). Overall, the radial growth appeared to have no significant response to precipitation, whereas significant responses to temperature were observed. This finding indicates that temperature exerts the greatest control on the radial growth of Siberia larch in this study region.
The comparison between the growth–temperature relationships in the two time periods was complex. The tree-ring width indices significantly positively correlated with the mean temperature in June of the previous year (r = 0.358, p < 0.05) and significantly negatively correlated with the mean temperature in November of the previous year (r = −0.355, p < 0.05) during the period from 1958 to 1988. For the period from 1989 to 2012, significant negative correlations were observed between the tree-ring width indices and the mean annual temperature of the previous year (r = −0.541, p < 0.01) and the mean temperatures in June of the previous year (r = −0.407, p < 0.05), July of the previous year (r = −0.410, p < 0.05), and August of the current year (r = −0.393, p < 0.05).
The tree-ring width indices were only significantly negatively correlated with the minimum temperature in November of the previous year (r = −0.357, p < 0.05) during the period from 1958 to 1988. However, for the period from 1989 to 2012, significant negative correlations were found between the tree-ring width indices and the minimum annual temperature of the previous year (r = −0.502, p < 0.01) and the minimum temperatures in June of the previous year (r = −0.484, p < 0.05), July of the previous year (r = −0.419, p < 0.05), and August of the current year (r = −0.565, p < 0.01).
A significant positive correlation was observed between tree-ring width indices and the maximum annual temperature of the current year (r = 0.428, p < 0.05) during the period from 1958 to 1988, with significant negative correlations observed for the period from 1989 to 2012 between tree-ring width indices and the maximum annual temperature of the previous year (r = −0.567, p < 0.01) and the maximum temperature in July of the previous year (r = −0.417, p < 0.05).
A moving correlation investigating the dynamic variation of growth–climate relationships confirmed the previous results (Fig. 6). The negative correlation between tree-ring width indices and total precipitation in the current May exhibited a weakening trend over time (y = 0.003x − 0.341, R 2 = 0.216, p = 0.02). The positive correlations have clearly weakened between tree-ring width indices and the mean annual temperature of the current year (y = −0.010x + 0.489, R 2 = 0.525, p < 0.01), mean monthly temperatures in the growing season, such as the previous June (y = −0.017x + 0.583, R 2 = 0.624, p < 0.01), current May (y = −0.005x + 0.293, R 2 = 0.386, p < 0.01) and current June (y = −0.009x + 0.310, R 2 = 0.245, p = 0.01). The same results were observed for the minimum and maximum temperatures.
Temporal variation of radial growth
The inter-annual variation of radial growth was evaluated by the basal area increment (BAI). Overall, the BAI curve increased (R 2 = 0.643, p < 0.01) from 1958 to 2012. However, the BAI was different before and after the abrupt increase in temperature (Fig. 7a). The mean BAI from 1989 to 2012 was about two times (increased by 6.246 cm2 year−1) that from 1958 to 1988 (Fig. 7b). Meanwhile, while the radial growth exhibited a general upward trend (R 2 = 0.317, p < 0.01) in mean BAI from 1958 to 1988, the mean BAI remained quite constant (R 2 = 0.007, p = 0.699) from 1989 to 2012. These results demonstrate that in recent decades, the radial growth has been characterized by a greater BAI and slower trend in growth speed.
Basal area increment (BAI) curve from 1958 to 2012 (a) and BAI boxplots during the two periods of 1958–1988 and 1989–2012 (b). a The gray dotted lines represent the individual BAI of all tree-rings, the black line represents the mean BAI and the dotted lines with arrows represent piecewise linear regression results of BAI. b The solid lines represent the median and the black squares represent the mean BAI, the differences between two classes were significant (p < 0.01)
Discussion
The divergence response of temperature-dependent radial growth to climate change
This study demonstrated that temperature had a greater impact on the radial growth of Siberia larch than precipitation, with the current and previous growing season temperatures found to be particularly important to control radial growth in the eastern Tianshan Mountains. As the Siberia larch lives at high elevation, where the relatively great amount of precipitation ensures the availability of moisture, the finding that radial growth is limited by temperature is logical (White et al. 2014). Before 1989, the radial growth exhibited an upward trend, with increasing temperature suggested to be the primary driver of growth (Fig. 7). The energy and material required for tree growth may be mainly derived from accumulated carbohydrates. High temperature could increase photosynthesis, and the lengthening growing season due to the warmer climate would delay the cessation of cell differentiation at the cambium level (Fritts 1976; Jiang et al. 2014). However, the stability of the climate signal differed before and after the abrupt increase in temperature (Fig. 5). After 1989, rather than cool temperature limiting radial growth, the warm temperature in the growing season became a limiting factor, apparently by exacerbating moisture limitations. The shift in the climate response of radial growth was interpreted as an excessive response to the ongoing warming speed due to plant respiration and the consumption of available carbohydrates (Schaberg 2000).
The climate factors limiting radial growth revealed some dramatic shifts with climate warming during recent decades as evidence of a divergence response. The discrepancies of correlation coefficients before and after the abrupt increase in temperature also demonstrated the instability of the climate–growth relationships in the eastern Tianshan Mountains. The u test revealed significant changes in the correlation coefficients between tree-ring width indices and the temperature of the previous June (T: u = 2.80, MinT: u = 2.51, p < 0.05), current August (MinT: u = 2.13, p < 0.05) and current annual temperature (MaxT: u = 2.22, p < 0.05) (Table 2). These results were likely associated with the shift from a positive to negative response of radial growth to the temperature of the growing season following the abrupt increase in temperature (Fig. 5). This finding might indicate an ongoing trend of changes in the sensitivity of radial growth to the temperature of the growing season. Based on these results, we posit that recent climate changes might have altered the instability of growth–climate relationships, with the result that temperature-dependent tree species could display more divergent responses to climate factors (Zhang and Wilmking 2010; Filipescue et al. 2014).
In the Tianshan Mountains, the mean temperature in the growing season was shown to have a strong influence on the radial growth of coniferous trees (Peng et al. 2005; Wang et al. 2005). However, our results suggested that minimum temperature was a major climatic factor with a significant negative effect on the radial growth of Siberia larch in recent decades (Fig. 5). The minimum temperature in the late 1980s obviously became abruptly higher, but the increase in maximum temperature was relatively small in this study region, demonstrating that the climate warming and the narrowing of the diurnal temperature range may be mainly caused by the significant increase in the minimum temperature (Figs. 4, 8). In fact, the minimum temperature regulates many terrestrial biogeochemical processes such as plant respiration, plant productivity, plant nutrient uptake and so on (Palombo et al. 2014). Therefore, more nutrients were consumed by dark respiration as the minimum temperature increased, and photosynthesis decreased due to the increasing cloud cover with the decreasing diurnal difference, leading to a negative impact on radial growth (Zha et al. 2004; Franceschini et al. 2013).
Trend of basal area increment (BAI) for Siberia larch, mean temperature and temperature diurnal variation smoothed using a 10-year moving average in the growing season (May to September) from 1957 to 2012. The shadowed area represents the diurnal variation of temperature. The black line represents the mean BAI (older than 50 ages), the grey line represents the BAI of 51–100 ages, the dotted line represents the BAI of 101–150 ages, the dashed line represents the BAI of 151–200 ages, the dashed dotted line represents the BAI of older than 200 ages
The relationships between the minimum temperatures in previous June and current August and tree-ring width were generally stronger than the other factors under climate warming, indicating that the minimum temperatures in previous and current growing season are more likely to be the control factors of radial growth in this region. A similar climatic response has been reported on south-eastern Tibetan Plateau, central Qilian Mountains and eastern Tianshan Mountains (Gao et al. 2013; Li et al. 2013; Xu et al. 2014a). The study of Peng et al. (2013) pointed out that rising the summer minimum temperature was associated with reduced vegetation productivity in our study regions. Additionally, the wood micro-cores analysis revealed that cell growth was assumed to occur mainly during night, the minimum air temperature was dominant factor to constrain the cell production rate and control the length of the growing period by acting on tissue formation (Deslauriers and Morin 2005; Li et al. 2013). The minimum temperature marked increased in June, July and August (June 1.050 ± 0.008 °C/decade, July 1.031 ± 0.009 °C/decade, August 0.975 ± 0.009 °C/decade, p < 0.01) from 1958 to 2012 in our study region. However, the precipitation has not changed significantly in June and August (June −0.113 ± 0.200 mm/decade, August 0.672 ± 0.153 mm/decade, p > 0.05), but increased significantly in July (2.995 ± 0.219 mm/decade, p < 0.05) during the same period. Based on the above results, we concluded that the night moisture stress in June and August caused by high minimum temperature in recent decades. The June is the middle growth season in the Tianshan Mountains (Zhang et al. 2015). The higher June minimum temperatures in the previous year led to enhance trees respiration and reduce accumulation of photosynthetic products, resulting in narrow tree-ring width due to decrease in the number of buds and leaves in the following year (Fritts 1976). Meanwhile, the August is the late growth season in this region. The higher the August minimum temperature in the current year resulted in physiologically dry soil and great dark respiration, which had a negative effect on the tree-ring width (Youngblut and Luckman 2013).
The main reasons for the divergence response
Radial growth of tree could be impaired by heat-induced drought stress (Liang et al. 2013, Jiang et al. 2015). Droughts have been caused not only by low precipitation but also by high temperature because high temperature leads to increased evapotranspiration and reduced available soil moisture (Liang et al. 2006). Although the annual total precipitation increased in the study region from 1958 to 2012, the high solar radiation and soil permeability, the relatively steep slope and the relatively open canopy could lead to a low water-holding capacity, explaining the general negative influence of the drought stress on radial growth in this study region. In addition, the Siberia larch has a rather limited water reserve capacity because its shallow root system is not able to take up deep water.
The divergence responses of radial growth to temperature were induced by the greater water deficit (Oberhuber et al. 2008). Our results showed that the Siberia larch have changes from positive responders (r = 0.358, p < 0.05) to negative responders (r = −0.407, p < 0.05) to the mean temperature of the previous June (Fig. 5). The negative relationship with temperature could indicate a direct stress (Chavardès et al. 2013). Additionally, a moving correlation demonstrated the concurrence between the progressively weakening trend of the positive responses to temperature in the growing season and negative responses to total precipitation of the current May (Fig. 6), confirming that the sensitivity of the Siberia larch to drought stress has been strengthened in the recent exceptionally warm years.
During these decades, the warmer climate conditions that were once favorable to radial growth seemed to become detrimental. A number of forests in Eurasia and western North America also exhibited shifts to negative correlations between summer temperature and radial growth coupled with declining growth rates over the past few decades, with rising temperature induced drought stress invoked as the dominant mechanism (Buermann et al. 2014). The shortage of available soil moisture might lead to the inhibition of root development, cambial activity, nutrient storage, foliage efficiency and the proportion of early wood (Andreu-Hayles et al. 2011; Xu et al. 2012). Therefore, we believed that the intensification of drought stress might be a main reason for the ‘divergence problem’ in the eastern Tianshan Mountains.
Climate warming is by now unequivocal, as it is evident from observations of increases in global average temperatures (IPCC 2013). The climate warming poses a risk for much drier conditions, potentially leading to modify growth trends and response patterns of forests around the world, especially in the drought-prone regions (Gazol et al. 2015). Since around the middle twentieth century, the evidences for reduced sensitivity of tree growth to temperature have been reported on Picea glauca (Alaska), Picea abies (Alps), Picea rubens (southern Appalachian Mountains), Fagus sylvatica (European Mediterranean Basin) and so on (Lloyd and Fastie 2002; Büntgen et al. 2006; Tegel et al. 2014; White et al. 2014). And similar results had also been recorded by several dendroclimatic studies on Pinus tabulaeformis (Helan Mountains), Sabina przewalskii (Qilian Mountains), Picea crassifolia (northeast Tibet Plateau), Pinus koraiensis (Changbai Mountains) and so on in China (Zhang et al. 2009; Zhang and Wilmking 2010; Gao et al. 2013; Yu et al. 2013). Combining these instances of unstable growth–temperature relationships, warming-induced drought stress might be a driving factor in recent decades (D’Arrigo et al. 2008). Our results were also in good agreement with their studies.
The responses of tree growth to climate warming and the prediction of forest dynamics
As climate warming has progressed, the effects on tree growth have varied considerably over the period from 1958–2012. Based on the 10-year moving averages of the BAI, tree growth of mean (older than 50 ages) and four age groups (51–100 ages, 101–150 ages, 151–200 ages and older than 200 ages) showed the same trend, which increased slightly during the 1966–1988, then increased dramatically since the 1989, and decreased slowly after 2002 (Fig. 8). Since 1950, most sampling trees (between 50 and 200 years old) had already reached maturity and were far from their natural senescence. Both the relatively young and older trees showed the similar radial growth (BAI) trends during the same period. Therefore, the recent growth patterns observed could be attributed to climate factors, rather than age in our study region. Meanwhile, the mean temperature in the growing season was stable prior to 1989, with a rapid upward trend observed from 1989 to 2012. The trend in BAI was broadly synchronous with the variation of mean temperature before 2002, whereas this relationship diverged from 2003 to 2012. The conclusions were consistent with the investigation of tree growth patterns and the relationships between tree-ring width indices and climate factors in this study region.
Although plants can gradually adjust strategies to adapt to climate change, tree growth responses are likely to be limited by the range of the physiological threshold (Day et al. 2002). Within the range of threshold values, tree growth is positively related to temperature, but when the temperature exceeds a certain threshold value, a negative response is observed. The mean temperature range from 14.44° to 15.60° in the growing season from 1989 to 2002 generally promoted the growth rate of Siberia larch in this study region. Then, the growth rate declined with the rapid increase in temperature (16.10° ± 0.06°), exceeding the upper limits of the optimum range for Siberia larch during the period from 2003 to 2012. These results suggest that the optimal climate conditions for the growth of Siberia larch have passed and that its growth is limited at the warm, dry margins of its range by some combination of excessive temperature and limited moisture.
Differences in responses to climate change might explain the effects of climate warming on radial growth under different moisture restrictions. The total annual precipitation in the Tianshan Mountains in China slightly decreases from west to east, meaning that the eastern part suffers from drought stress more than the western part. In response to a similar increase in temperature, the spruce species, namely the Schrenk spruce, exhibited a higher growth speed after 1960 in the western and central Tianshan Mountains (Qi et al. 2015). In contrast, our results showed first an increase until 2002 and then a decrease in the growth rate of Siberia larch in the eastern Tianshan Mountains. This pattern of tree growth might imply high vulnerability to drought stress for forests in arid areas (Schuster and Oberhuber 2013).
Recent climate changes have already caused an increase in forest damage in many regions throughout Europe, North America and Inner Asia (D’Arrigo et al. 2009; Lebourgeois et al. 2012). If the temperature continues to rise, we speculate that the structure and function of forests will be changed and that tree mortality and forest die-off may tend to increase not only in arid areas but also in relatively humid areas (Liu et al. 2013). Based on the above discussion, we posit that an increase in temperature might trigger a shift in the response to climate factors via a type of threshold mechanism. Drought stress would slow tree growth, reduce the sensitivity of radial growth to temperature and lead to divergent growth–temperature relationships (Carrer and Urbinati 2006).
Conclusion
Divergent responses of radial growth to climate change were clearly observed in the eastern Tianshan Mountains at the mid-latitude of the Northern Hemisphere. This study proved that the Siberia larch is a promising species for observing climate-driven influences on tree-ring growth. The radial growth of the Siberia larch was mainly controlled by temperature in this study region. In particular, the effects of minimum temperature gradually enhanced the radial growth. As the climate continues to warm, growth–climate relationships have changed, including a shift in the response of radial growth to the temperature of the growing season from positive to negative. The pattern of tree growth differed before and after the abrupt increase in temperature, first increasing and then decreasing. The drought stress and the adjustment of the physiological response could be important factors contributing to the recent decrease in the temperature sensitivity of Siberia larch in the growing season. Under conditions of further increases in temperature in the future, rapid warming may drastically accelerate tree growth decline not only in arid and semi-arid areas but also in humid areas. Furthermore, a deeper understanding of growth–climate relationships would help to increase the accuracy of simulating the growth pattern of dominant coniferous tree species and monitoring the distribution dynamics of the forests. Systematically analyzing the changes in the climate factors limiting radial growth and the reasons for divergent responses would assist in efforts to sustain the stability of the forest ecosystems and would support the regeneration of the more superior species. In addition, effectively using protective measures based on the principle of dendroclimatology would be advantageous for minimizing the impact of climate change on trees and enabling reasonable management of forest ecosystems.
Author contribution statement
Study conception and experimental design: J.L. and J.Y. Acquisition of samples: J.L., Z.W.T. and W.M.C. Analysis and interpretation of data: J.L., W.M.C., Z.L.N. and Z.S.D. Drafting of manuscript: J.L. and J.Y.
References
Andreu-Hayles L, D’Arrigo R, Anchukaitis KJ, Beck P, Frank D, Goetz S (2011) Varying boreal forest response to Arctic environmental change at the Firth River, Alaska. Environ Res Lett 6:045503. doi:10.1088/1748-9326/6/4/045503
Barber V, Juday G, Finney B (2000) Reduced growth of Alaska white spruce in the twentieth century from temperature-induced drought stress. Nature 405:668–672
Beniston M (2002) Climate modeling at various spatial and temporal scales: where can dendrochronology help? Drochronologia 20:117–132
Biondi F, Waikul K (2004) DENDROCLIM2002: a C++ program for statistical calibration of climate signals in tree-ring chronologies. Comput Geosci 30:303–311
Bonan GB (2008) Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320:1444–1449
Briffa KR, Melvin TM (2011) A closer look at regional curve standardization of tree-ring records: justification of the need, a warning of some pitfalls, and suggested improvements in its application. In: Hughes MK, Diaz HF, Swetnam TW (eds) Dendroclimatology: progress and prospects. Springer, New York, pp 113–145
Briffa KR, Schweingruber FH, Jones PD, Osborn TJ, Shiyatov SG, Vaganov EA (1998) Reduced sensitivity of recent tree-growth to temperature at high northern latitudes. Nature 391:678–682
Buermann W, Parida B, Jung M, MacDonald GM, Tucker CJ, Reichstein M (2014) Recent shift in Eurasian boreal forest greening response may be associated with warmer and drier summers. Geophys Res Lett 41:1995–2002
Büntgen ULF, Frank DC, Schmidhalter M, Neuwirth B, Seifert M, Esper JAN (2006) Growth/climate response shift in a long subalpine spruce chronology. Trees 20:99–110
Büntgen ULF, Frank DC, Wilson ROB, Carrer M, Urbinati C, Esper JAN (2008) Testing for tree-ring divergence in the European Alps. Global Change Biol 14:2443–2453
Carrer M, Urbinati C (2006) Long-term change in the sensitivity of tree-ring growth to climate forcing in Larix decidua. New Phytol 170:861–871
Chavardès RD, Daniels LD, Waeber PO, Innes JL, Nitschke CR (2013) Unstable climate-growth relations for white spruce in southwest Yukon, Canada. Clim Change 116:593–611
Cook ER (1985) A time series approach to tree-ring standardization. PhD thesis, University of Arizona, Tucson
Cox PM, Pearson D, Booth BB, Friedlingstein P, Huntingford C, Jones CD, Luke CM (2013) Sensitivity of tropical carbon to climate change constrained by carbon dioxide variability. Nature 494:341–345
Dang HS, Zhang YJ, Zhang KR, Jiang MX, Zhang QF (2013) Climate-growth relationships of subalpine fir (Abies fargesii) across the altitudinal range in the Shennongjia Mountains, central China. Clim Change 117:903–917
D’Arrigo R, Wilson R, Liepert B, Cherubini P (2008) On the ‘divergence problem’ in northern forests: a review of the tree-ring evidence and possible causes. Global Planet Change 60:289–305
D’Arrigo R, Jacoby G, Buckley B, Sakulich J, Frank D, Wilson R, Curtis A, Anchukaitis K (2009) Tree growth and inferred temperature variability at the North American Arctic treeline. Global Planet Change 65:71–82
Day ME, Greenwood MS, Diaz-Sala C (2002) Age-and size-related trends in woody plant shoot development: regulatory pathways and evidence for genetic control. Tree Physiol 22:507–513
De Grandpre L, Tardif JC, Hessl A, Pederson N, Conciatori F, Green TR, Oyunsanaa B, Baatarbileg N (2011) Seasonal shift in the climate responses of Pinus sibirica, Pinus sylvestris, and Larix sibirica trees from semi-arid, north-central Mongolia. Can J Forest Res 41:1242–1255
Deslauriers A, Morin H (2005) Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees 19:402–408
Filipescue CN, Lowell EC, Koppenaal R, Mitchell AK (2014) Modeling regional and climatic variation of wood density and ring width in intensively managed Douglas-fir. Can J Forest Res 44:220–229
Franceschini T, Bontemps JD, Leban JM (2012) Transient historical decrease in earlywood and latewood density and unstable sensitivity to summer temperature for Norway spruce in northeastern France. Can J Forest Res 42:219–226
Franceschini T, Bontemps JD, Perez V, Leban JM (2013) Divergence in latewood density response of Norway spruce to temperature is not resolved by enlarged sets of climatic predictors and their non-linearities. Agric Forest Meteorol 180:132–141
Fritts HC (1976) Tree rings and climate. Academic Press, London
Fritts HC (1991) Reconstruction large-scale climate patterns from tree-ring data. University of Arizona Press, Tucson
Fritts HC, Shatz DJ (1975) Selecting and characterizing tree-ring chronologies for dendroclimatic analysis. Tree Ring Bull 35:31–40
Gao LL, Gou XH, Deng Y, Liu WH, Yang MX, Zhao ZQ (2013) Climate–growth analysis of Qilian juniper across an altitudinal gradient in the central Qilian Mountains, northwest China. Trees 27:379–388
Gazol A, Camarero JJ, Gutiérrez E, Popa I, Andreu-Hayles L, Motta R, Nola P, Ribas M, Sangüesa-Barreda G, Urbinati C, Carrer M (2015) Distinct effects of climate warming on populations of silver fir (Abies alba) across Europe. J Biogeogr 42:1150–1162
Gómez-Guerrero A, Silva LCR, Barrera-Reyes M, Kishchuk B, Velázquez-Martínez A, Martínez-Trinidad T, Plascencia-Escalante FO, Horwath WR (2013) Growth decline and divergent tree ring isotopic composition (δ13C and δ18O) contradict predictions of CO2 stimulation in high altitudinal forests. Global Change Biol 19:1748–1758
Goossens C, Berger A (1987) How to recognize an abrupt climatic change? In: Berger WH, Labeyrie LD (eds) Abrupt climatic change, evidence and implications. NATO ASI series C: mathematical and physical sciences, vol. 216. D. Reidel, Dordrecht, pp 31–45
Grissino-Mayer HD (2001) Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree Ring Res 57:205–221
He M, Yang B, Datsenko NM (2014) A six hundred-year annual minimum temperature history for the central Tibetan Plateau derived from tree-ring width series. Clim Dyn 43:641–655
IPCC (2013) Climate change 2013: the physical science basis: contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Jiang Y, Zhang WT, Wang MC, Kang MY, Dong MY (2014) Radial growth of two dominant montane conifer tree species in response to climate change in north-central China. PLoS ONE 9:e112537. doi:10.1371/journal.pone.0112537
Jiang Y, Wang BQ, Dong MY, Huang YM, Wang MC, Wang B (2015) Response of daily stem radial growth of Platycladus orientalis to environmental factors in a semi-arid area of North China. Trees 29:87–96
Lebourgeois F, Mérian P, Courdier F, Ladier J, Dreyfus P (2012) Instability of climate signal in tree-ring width in Mediterranean mountains: a multi-species analysis. Trees 26:715–729
Li XX, Liang EY, Gricar J, Prislan P, Rossi S, Cufar K (2013) Age dependence of xylogenesis and its climatic sensitivity in Smith fir on the south-eastern Tibetan Plateau. Tree Physiol 33:48–56
Liang EY, Shao XM, Eckstein D, Huang L, Liu XH (2006) Topography- and species-dependent growth responses of Sabina przewalskii and Picea crassifolia to climate on the northeast Tibetan Plateau. Forest Ecol Manag 236:268–277
Liang EY, Ren P, Zhang SB, Shao XM, Eckstein D (2013) How can Populus euphratica cope with extremely dry growth conditions at 2,800 m a.s.l. on the northern Tibetan Plateau? Trees 27:447–453
Lindeman RH, Merenda PF, Gold RZ (1980) Introduction to Bivariate and Multivariate Analysis. Scott, Foresman, Glenview
Lioubimtseva E (2004) Climate change in arid environments: revisiting the past to understand the future. Prog Phys Geogr 28:502–530
Liu HY, Park Williams A, Allen CD, Guo DL, Wu XC, Anenkhonov OA, Liang EY, Sandanov DV, Yin Y, Qi ZH, Badmaeva NK (2013) Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Global Change Biol 19:2500–2510
Lloyd AH, Fastie CL (2002) Spatial and temporal variability in the growth and climate response of treeline trees in Alaska. Clim Change 52:481–509
Ma L, Wu JL, Abuduwaili J (2013) Climate and environmental changes over the past 150 years inferred from the sediments of Chaiwopu Lake, central Tianshan Mountains, northwest China. Int J Earth Sci 102:959–967
Oberhuber W, Kofler W, Pfeifer K, Seeber A, Gruber A, Wieser G (2008) Long-term changes in tree-ring–climate relationships at Mt. Patscherkofel (Tyrol, Austria) since the mid-1980s. Trees 22:31–40
Palombo C, Battipaglia G, Cherubini P, Chirici G, Garfi V, Lasserre B, Lombardi F, Marchetti M, Tognetti R (2014) Warming-related growth responses at the southern limit distribution of mountain pine (Pinus mugo Turra subsp. mugo). J Veg Sci 25:571–583
Peng JF, Gou XH, Chen FH, Li JB (2005) Climatic records of tree-ring width in Picea schrenkiana Fisch and Larix sibirica Ledb. Ecol Environ 14:460–465 (in Chinese, with English abstract)
Peng SS, Piao SL, Ciais P, Myneni RB, Chen AP, Chevallier F, Dolman AJ, Janssens IA, Peñuelas J, Zhang GX, Vicca S, Wan SQ, Wang SP, Zeng H (2013) Asymmetric effects of daytime and night-time warming on Northern Hemisphere vegetation. Nature 501:88–92
Phipps RL, Whiton JC (1988) Decline in long-term growth trends of white oak. Can J Forest Res 18:24–32
Qi ZH, Liu HY, Wu XC, Hao Q (2015) Climate-driven speedup of alpine treeline forest growth in the Tianshan Mountains, Northwestern China. Global Change Biol 21:816–826
Rubino DL, McCarthy BC (2000) Dendroclimatological analysis of white oak (Quercus alba L., Fagaceae) from an old-growth forest of southeastern Ohio, USA. J Torrey Bot Soc 127:240–250
Sánchez-Salguero R, Navarro RM, Camarero JJ, Fernández-Cancio Á (2010) Drought-induced growth decline of Aleppo and maritime pine forests in south-eastern Spain. Forest Syst 19:458–470
Schaberg PG (2000) Winter photosynthesis in red spruce (Picea rubens Sarg.): limitations, potential benefits, and risks. Arct Antarct Alp Res 32:375–380
Schneider L, Esper J, Timonen M, Büntgen U (2014) Detection and evaluation of an early divergence problem in northern Fennoscandian tree-ring data. Oikos 123:559–566
Schuster R, Oberhuber W (2013) Drought sensitivity of three co-occurring conifers within a dry inner Alpine environment. Trees 27:61–69
Stoffel M, Corona C (2014) Dendroecological dating of geomorphic disturbance in trees. Tree Ring Res 70:3–20
Stokes MA, Smiley TL (1968) An introduction to tree-ring dating. The University of Chicago Press, Chicago
Tegel W, Seim A, Hakelberg D, Hoffmann S, Panev M, Westphal T, Büntgen U (2014) A recent growth increase of European beech (Fagus sylvatica L.) at its Mediterranean distribution limit contradicts drought stress. Eur J Forest Res 133:61–71
Trindade M, Bell T, Laroque C (2010) Changing climatic sensitivities of two spruce species across a moisture gradient in Northeastern Canada. Dendrochronologia 29:25–30
Wang T, Liang Y, Ren HB, Yu D, Ni J, Ma KP (2004) Age structure of Picea schrenkiana forest along an altitudinal gradient in the central Tianshan Mountains, northwestern China. Forest Ecol Manag 196:267–274
Wang T, Ren HB, Ma KP (2005) Climatic signals in tree ring of Picea schrenkiana along an altitudinal gradient in the central Tianshan Mountains, northwestern China. Trees 19:735–741
Wang H, Shao XM, Jiang Y, Fang XQ, Wu SH (2013) The impacts of climate change on the radial growth of Pinus koraiensis along elevations of Changbai Mountain in northeastern China. Forest Ecol Manag 289:333–340
White PB, Soulé P, van de Gevel S (2014) Impacts of human disturbance on the temporal stability of climate–growth relationships in a red spruce forest, southern Appalachian Mountains, USA. Dendrochronologia 32:71–77
Wigley TM, Briffa KR, Jones PD (1984) On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J Clim Appl Meteorol 23:201–213
Wilson R, Elling W (2004) Temporal instability in tree-growth/climate response in the Lower Bavarian Forest region: implications for dendroclimatic reconstruction. Trees 18:19–28
Wu GJ, Xu GB, Chen T, Liu XH, Zhang YF, Wang LA, Wang WZ, Fang ZA, Yu SL (2013a) Age-dependent tree-ring growth responses of Schrenk spruce (Picea schrenkiana) to climate-A case study in the Tianshan Mountain, China. Dendrochronologia 31:318–326
Wu XC, Liu HY, Wang YF, Deng MH (2013b) Prolonged limitation of tree growth due to warmer spring in semi-arid mountain forests of Tianshan, northwest China. Environ Res Lett 8:024016
Xu JM, Lu JX, Bao FC, Evans R, Downes G, Huang RF, Zhao YK (2012) Cellulose microfibril angle variation in Picea crassifolia tree rings improves climate signals on the Tibetan plateau. Trees 26:1007–1016
Xu GB, Liu XH, Qin DH, Chen T, Wang WZ, Wu GJ, Sun WZ, An WL, Zeng XM (2014a) Tree-ring δ18O evidence for the drought history of eastern Tianshan Mountains, northwest China since 1700 AD. Int J Climatol 34:3336–3347
Xu Y, Li WJ, Shao XM, Xu ZH, Nugroho P (2014b) Long-term trends in intrinsic water-use efficiency and growth of subtropical Pinus tabulaeformis Carr. and Pinus taiwanensis Hayata in central China. J Soils Sediments 14:917–927
Youngblut DK, Luckman BH (2013) Evaluating the temperature sensitivity of radial growth patterns from whitebark pine in the western Canadian Cordillera. Dendrochronologia 31:16–28
Yu DP, Liu JQ, Benard JL, Zhou L, Zhou WM, Fang XM, Wei YW, Jiang SW, Dai LM (2013) Spatial variation and temporal instability in the climate–growth relationship of Korean pine in the Changbai Mountain region of Northeast China. Forest Ecol Manag 300:96–105
Yuan YJ, Jin LY, Shao XM, He Q, Li ZZ, Li JF (2003) Variations of the spring precipitation day numbers reconstructed from tree rings in the Urumqi River drainage, Tianshan Mts. over the last 370 years. Chin Sci Bull 48:1507–1510
Zha TS, Kellomaki S, Wang KY, Ryyppoe A, Niinistoe S (2004) Seasonal and annual stem respiration of scots pine trees under boreal conditions. Ann Bot 94:889–896
Zhang YX, Wilmking M (2010) Divergent growth responses and increasing temperature limitation of Qinghai spruce growth along an elevation gradient at the northeast Tibet Plateau. Forest Ecol Manag 260:1076–1082
Zhang YX, Wilmking M, Gou XH (2009) Changing relationships between tree growth and climate in Northwest China. Plant Ecol 201:39–50
Zhang WT, Jiang Y, Dong MY, Kang MY, Yang HC (2012) Relationship between the radial growth of Picea meyeri and climate along elevations of the Luyashan Mountain in North-Central China. Forest Ecol Manag 265:142–149
Zhang TW, Zhang RB, Yuan YJ, Gao YQ, Wei WS, Diushen M, He Q, Shang HM, Wang J (2015) Reconstructed precipitation on a centennial timescale from tree rings in the western Tien Shan Mountains, Central Asia. Quatern Int 358:58–67
Acknowledgments
This work was funded by the National Natural Science Foundation of China (Projects No. 41171067 and 41361010). The authors would like to thank Xiaolong Shang for his assistance in the field work. We are grateful to Wen Xiao and Ao Shen for the construction of the tree-ring width chronology. We also thank the anonymous referees for their careful and helpful comments and suggestions regarding this paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Liang.
Rights and permissions
About this article
Cite this article
Jiao, L., Jiang, Y., Zhang, WT. et al. Divergent responses to climate factors in the radial growth of Larix sibirica in the eastern Tianshan Mountains, northwest China. Trees 29, 1673–1686 (2015). https://doi.org/10.1007/s00468-015-1248-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1248-6