Abstract
Purpose
This study analysed tree-ring stable carbon isotope composition (δ13C) of Pinus tabulaeformis Carr. and Pinus taiwanensis Hayata, from a subtropical forest located in central China for the last 130 years, to obtain the long-term trends of δ13C, carbon isotope discrimination by plants (Δ), leaf internal carbon dioxide (CO2) concentration (c i ) and intrinsic water-use efficiency (iWUE) in response to elevated atmospheric CO2 concentration (c a ) and climate change and explore how environmental changes affected long-term tree physiological responses and growth.
Materials and methods
Tree-ring cores were taken in Dabie Mountains, the border of Hubei, Henan and Anhui Provinces of China. δ13C was undertaken at every 3-year interval, and Δ, c i , iWUE and basal area increment (BAI) were determined. Regression analysis was used to quantify the trends in climate and the relationships of c i , iWUE and BAI with elevated c a and climate. Partial correlation analysis was used to distinguish the effects of c a and climate on c i and iWUE.
Results and discussion
δ13C of P. tabulaeformis and P. taiwanensis decreased in the past 130 years, but Δ had no obvious change over time for the two tree species. Both c i and iWUE increased significantly with the calendar year. BAI of P. tabulaeformis continuously increased during 1897–1993, but decreased slightly in the recent 20 years. However, BAI of P. taiwanensis did not present obvious change in the period 1882–2010. The c i and iWUE of P. tabulaeformis and P. taiwanensis also increased linearly with elevated c a in the past 130 years and with mean annual temperature during 1960–2007. Partial correlation analysis showed that elevated c a , not temperature, induced the changes in c a and iWUE. BAI of P. tabulaeformis since 1897 and that of P. taiwanensis during 1975–2010 responded quadratically to elevated c a . Warming-deduced drought in the study area in the recent 30 years resulted in increases in iWUE and decreases in BAI.
Conclusions
This study showed that while iWUE increased in the past 130 years, tree growth of two subtropical tree species in central China responded to rising c a non-linearly. Negative effects of some factors on tree growth, such as climate change (particularly warming-induced drought), nutrient limitation and physiological long-term acclimation to elevated c a , have overridden the CO2 fertilization effects in the past 30 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The elevated atmospheric carbon dioxide (CO2) concentration (c a ) not only makes a significant contribution to current global warming (Mann et al. 1998; Allen et al. 2009; Lacis et al. 2010; Montzka et al. 2011) but also plays an important role in tree physiological changes which might be associated with long-term trends in tree growth and forest productivity. Higher c a generally increases intrinsic water-use efficiency (iWUE), the ratio between the carbon assimilation (A) and stomatal conductance (g s ) (Francey and Farquhar 1982; LaMarche et al. 1984), and the increases in iWUE have been well-established in the literature in both controlled experiments (Morison 1993; Overdieck and Forstreuter 1994; Picon et al. 1996; Leavitt et al. 2003) and natural environments around the world (Bert et al. 1997; Feng 1999; Tang et al. 1999; Saurer et al. 2004; Liu et al. 2008; Gomez-Guerrero et al. 2013). Compared with short-term experimental results from studies of plants in growth chambers, the findings of long-lived trees growing in natural forests are more reliable and reveal more authentic behaviour of mature trees adjusting their physiological responses over time to gradually increasing c a .
Tree iWUE has received much attention due to the well-known theory of stable carbon isotope composition (δ13C), the relationships between leaf δ13C and leaf internal CO2 concentration (c i ), and the effects of g s and A on c i (Farquhar et al. 1982). As one of the most consistent responses of plants to elevated c a is the decrease in g s (Morsion 1987; Medlyn et al. 2001; Wullschleger et al. 2002; Long et al. 2004; Ainsworth and Long 2005; Zeppel et al. 2012), this water-saving mechanism can explain the improvement of iWUE. When the g s limitation of photosynthesis is strong, leaves or needles exhibit a low c i and a reduced carbon isotope discrimination by plants (Δ), along with improved iWUE (Saurer et al. 2004). Therefore, δ13C and Δ technologies in tree rings have been widely applied to examine long-term physiological changes, because changes of c i and Δ over time can be assessed for natural forests by measuring δ13C, and then potential changes of iWUE due to elevated c a and climate change can be assessed.
Long-term trends in tree-ring δ13C and iWUE have been described across biomes (Peñuelas et al. 2011; Silva and Horwath 2013). However, the tropical and subtropical results are much under-represented in the field of the forest responses to rising c a , comprised only 11 % of all studies (Kӧrner 2009). One of the reasons is that trees growing in the non-seasonal tropical environment cannot display well-defined tree rings, which makes cross-dating between trees and between sites impossible. Hence, it has been generally assumed that regions, where climate does not exhibit a strong seasonal control on tree growth, are unsuitable for dendrochronology (Whitmore 1990). In spite of this, some tree species in tropical forests are able to generate the identified tree rings annually; therefore, some research has been done on the long-term iWUE variations in tropical and subtropical forests by δ13C analysis for trees in Brazil, Mexico and Thailand (Hietz et al. 2005; Silva et al. 2009; Brienen et al. 2011; Nock et al. 2011), and the iWUE changes of trees in these regions comply with the widespread increasing trends. In China, Sun et al. (2010) have evaluated long-term tree growth and iWUE of Masson pine (Pinus massoniana L.) in response to global climate change for subtropical forests, a very valuable supplement for tree-ring stable isotope studies in China, where most analogous research has been conducted in arid and semi-arid areas (e.g. Liu et al. 2007; Liu et al. 2008; Wang et al. 2012; Xu et al. 2013). Recently, two subtropical tree species, Chinese red pine (Pinus tabulaeformis Carr.) and Huangshan pine (Pinus taiwanensis Hayata) from the Dabie Mountains, central China, have been certified that they do produce annual rings (Zheng et al. 2012b) and can be used as proxy data to reconstruct the past climate (Zheng et al. 2012a). Here, we analysed δ13C of P. tabulaeformis and P. taiwanensis for the last 130 years, aiming to obtain the long-term trends of δ13C, Δ, c i and iWUE with elevated c a and climate change, and explore how environmental changes affected long-term tree physiological responses and growth.
2 Materials and methods
2.1 Study area and climate
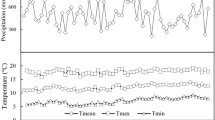
The study area is located in Dabie Mountains, the border of Hubei, Henan and Anhui Provinces of China (30° 10′ ∼ 32° 51′ N, 112° 40′ ∼ 117° 10′ E). The average elevation of Dabie Mountains is 500 ∼ 800 m.a.s.l., and the highest peak is about 1,792 m.a.s.l. The East Asian monsoon reaches this region from the North Pacific Ocean, resulting in abundant rainfall and high temperature during the summer (Ou and Qian 2006). At the meteorological station in Macheng (31° 11′ N, 115° 01′ E, 59 m.a.s.l.), the wettest and driest months were July (mean precipitation of 223.3 mm) and December (23.8 mm), while January (mean temperature of 3.29 °C) and July (28.2 °C) were the coldest and warmest months, respectively, based on the instrumental data from 1959 to 2009 (Fig. 1a). The average of annual mean temperature was 16.4 °C, and the average of annual rainfall was about 1,216.9 mm in this region from 1959 to 2009, with 43.6 % of the annual rainfall falling during the summer (June to August). An apparent warming trend was observed since 1980 in the study area (Fig. 1b); however, no significant increasing or decreasing trends could be detected for the annual mean temperature from 1959 to 1979 (Fig. 1b, P = 0.636) or annual rainfall since 1959 (Fig. 1c, P = 0.303).
2.2 Sampling and preparation
P. tabulaeformis and P. taiwanensis were sampled at two different sites in August 2010, the former from Shuidongwan (SDW) and the latter from Zherenfeng (ZRF), because these two tree species are not co-occurring at the same study site, but they are the dominant conifer species at each site. The soils of both SDW and ZRF are yellow brown earth although they are at a distance of about 18.5 km. Table 1 shows the locations and soil characteristics of the two samplings sites. Sixty increment cores from 25 P. tabulaeformis and 43 increment cores from 16 P. taiwanensis were sampled in total. Two thin cores were taken at breast height (1.3 m) for each tree using an increment borer (4.3 mm). In addition, 10 P. tabulaeformis and 10 P. taiwanensis were randomly selected from them, and a thicker core was taken from each tree by an increment borer (10 mm) and used for undertaking isotope analysis.
All the cores were air-dried, sanded and polished until wood cells were clearly visible under the microscope (Stokes and Smiley 1968). Then, they were visually cross-dated following the procedures described by Yamaguchi (1991). After a rigorous cross-dating, ring widths were measured at a resolution of 0.01 mm using a semi-automatic device Lintab 6 High Resolution digital positioning table and TSAP-Win Scientific Software. The quality of the cross-dating and ring-width measurements was verified using the COFECHA program (Holmes 1983).
2.3 δ13C analysis
Five intact thick cores of each species were selected for δ13C analysis. To minimize noise from short-term variations, rings from each 3-year period were pooled from the outer to pith (e.g. 2002–2004, 2005–2007 and 2008–2010). Wood samples representing 3-year increment for each core were carefully split out in sequence with a sharp blade. All the samples were oven-dried at 65° to constant weight and ground to fine powder with a ring grinder. The δ13C values were analysed by weighing an amount of 2 to 3 mg in tin capsules, using a Sercon Hydra 20-22 isotope ratio mass spectrometer coupled with a Europa EA GSL sample prep system in Stable Isotope Laboratory, Griffith University, Brisbane, Australia. The spectrometer for δ13C analysis was calibrated with sucrose (IAEA-CH-6) supplied by the Australian National University, and the mean standard deviation of δ13C analysis of the sucrose reference standards was 0.16 ‰. δ13C was calculated as follows:
where R sample is 13C/12C ratio of a sample and R standard 13C/12C ratio of the PeeDee Belemnite international standard (Xu et al. 2000).
2.4 Δ and iWUE
According to Farquhar et al. (1982), 12C was preferentially used over 13C during the plant’s photosynthesis, which resulted in the carbon isotope discrimination (Δ), and Δ was defined as follows:
where δ13Cair and δ13Cplant were the carbon isotope ratios in the atmospheric CO2 and plants, respectively. Farquhar et al. (1982) also described the dependence of Δ on plant physiological properties, in particular on c i /c a :
where the constant a (4.4 ‰) referred to the enrichment during CO2 diffusion, and b (27.0 ‰) was the fractionation by Rubisco against 13CO2. iWUE was calculated by the following (Ehleringer and Cerling 1995):
Annual c a was obtained from Law Dome Ice Core 2000-Year CO2, CH4 and N2O data set (Etheridge et al. 1996; Etheridge et al. 1998; MacFarling 2004; MacFarling et al. 2006), available at http://www.ncdc.noaa.gov/paleo/icecore/antarctica/law/law_data.html.
2.5 Tree growth
The variation in tree growth could be recorded by the width of the annual rings. However, ring width in mature trees might biologically decline with tree age or size; thus, a declining growth trend may be suspected if using ring width alone as growth decline detector (Phipps and Whiton 1988). To overcome this problem, ring width could be converted into the basal area increment (BAI), and unlike ring width, age-related trends in unstandardized BAI were generally positive, culminating in a linear level which could be maintained for many decades (Phipps and Whiton 1988; LeBlanc 1990; Pedersen 1998). Therefore, BAI had been suggested as a better means for assessing tree growth, and a negative trend in BAI was a strong indication of a real decline in tree growth (LeBlanc 1990; Pedersen 1998). The conversion from ring width into BAI is based on the assumption that increment was uniform along each ring and used the following formula:
where R was the tree radius at breast height, and n was the year of tree ring formation.
2.6 Statistical analysis
Regression analysis was used to quantify the significant temporal trends in climate from Macheng station during 1980–2009 and the significant relationships of c i , iWUE and BAI with elevated c a and climate. Partial correlation analysis was used to distinguish whether c a or climate factors, especially temperature, induced the changes of c i and iWUE.
3 Results
3.1 Variations of δ13C, Δ, c i , iWUE and BAI
Overall, the variations of δ13C, Δ, c i and iWUE over time were very similar for the two tree species in the past 130 years. δ13C of P. tabulaeformis and P. taiwanensis declined with the calendar year (Figs. 2a and 3a). The former decreased from −24.3 to −26.2 ‰ during 1897–2010 (Fig. 2a), while the latter from −23.8 to −26.4 ‰ during 1882–2010 (Fig. 3a). Both of them mainly reflected the trend of δ13Cair in the same period. When the atmospheric δ13C effect on plant carbon isotopic ratios was excluded, Δ was found to have no obvious change over time for P. tabulaeformis and P. taiwanensis (Figs. 2b and 3b). Conversely, c i increased significantly over time for the two tree species, and that of c i of P. tabulaeformis increased from 176 to 230 ppm during 1897–2010 (Fig. 2c) while P. taiwanensis from 167 to 233 ppm during 1882–2010 (Fig. 3c). Accordingly, iWUE also increased distinctly with the calendar year. In the past 130 years, iWUE of P. tabulaeformis increased from 71 to 97 μmol mol−1 (Fig. 2d) while that of P. taiwanensis from 75 to 94 μmol mol−1 (Fig. 3d), and the increases were 37 and 25 %, respectively.
Variations in carbon isotope ratios (δ13C, a), carbon isotope discrimination (Δ, b), leaf internal CO2 concentration (c i , c), intrinsic water-use efficiency (iWUE, d) and basal area increment (BAI, e) for Pinus tabulaeformis Carr. from 1897 to 2010. The upper curve in a is the variation of carbon isotope ratios in the atmospheric CO2
Variations in carbon isotope ratios (δ13C, a), carbon isotope discrimination (Δ, b), leaf internal CO2 concentration (c i , c), intrinsic water-use efficiency (iWUE, d) and basal area increment (BAI, e) for Pinus taiwanensis Hayata from 1882 to 2010. The upper curve in a is the variation of carbon isotope ratios in the atmospheric CO2
The variations of tree BAI were different for P. tabulaeformis and P. taiwanensis in the past 130 years. The former firstly increased with the calendar year during 1897–1993, but decreased slightly in the recent 20 years (Fig. 2e). However, BAI of P. taiwanensis did not present obvious change in the period 1882–2010 (Fig. 3e).
3.2 Relationships of c i , iWUE and BAI with c a and climate
The regression analysis indicated that both c i and iWUE of P. tabulaeformis and P. taiwanensis increased linearly with elevated c a in the past 130 years (Figs. 4a, c and 5a, c) and with mean annual temperature during 1960–2007 (Figs. 4b, d and 5b, d), but there was no significant relationship between c i or iWUE with annual precipitation. Partial correlation analysis showed that both c i and iWUE significantly correlated with c a when mean annual temperature was controlled during 1960–2007 (Table 2); nevertheless, the correlation of mean annual temperature with c i or iWUE would be negligible when c a was controlled (Table 2). This demonstrated that elevated c a , not temperature, induced the changes in c a and iWUE.
Relationships between leaf internal CO2 concentration (c i ) and atmospheric CO2 concentration (c a , a for Pinus tabulaeformis Carr. from 1897 to 2010, c for Pinus taiwanensis Hayata from 1882 to 2010) and between c i and mean annual temperature (MAT, b for Pinus tabulaeformis Carr., d for Pinus taiwanensis Hayata) from 1960 to 2007
Relationships between intrinsic water-use efficiency (iWUE) and atmospheric CO2 concentration (c a , a for Pinus tabulaeformis Carr. from 1897 to 2010, c for Pinus taiwanensis Hayata from 1882 to 2010) and between iWUE and mean annual temperature (MAT, b for Pinus tabulaeformis Carr., d for Pinus taiwanensis Hayata) from 1960 to 2007
BAI of P. tabulaeformis responded quadratically to elevated c a in the past 130 years (Fig. 6a). BAI increased with rising c a until it peaked at about 350 ppm (around 1990) and decreased thereafter. Although very unusual BAI variations were present for P. taiwanensis in the earlier period (hollow points in Fig. 6b), BAI also responded quadratically to c a since 1975, when c a reached about 330 ppm (solid points in Fig. 6b). The coming decrease in BAI of P. taiwanensis indicated that tree growth was transforming from increases to decreases in the recent several years. However, BAI of P. tabulaeformis and P. taiwanensis correlated with neither annual mean temperature nor annual precipitation during 1960–2007.
4 Discussion
4.1 Long-term trends in iWUE and BAI
In this study, iWUE of two subtropical tree species from central China were found to increase in the past 130 years. The increases in iWUE have demonstrated a global scale (Peñuelas et al. 2011; Silva and Horwath 2013), especially during the last century, which has been well-established by studies in the boreal (Barber et al. 2000; Saurer et al. 2004; Ivlev and Voronin 2007; Gagen et al. 2008; Kirdyanov et al. 2008; Sidorova et al. 2008), temperate (Bert et al. 1997; Duquesnay et al. 1998; Feng 1999; Waterhouse et al. 2004; Peñuelas et al. 2008; Battipaglia et al. 2013), Mediterranean (Saurer et al. 2003; Peñuelas et al. 2008; Linares et al. 2009; Andreu-Hayles et al. 2011; Maseyk et al. 2011), arid regions (Liu et al. 2007; Liu et al. 2008) and high-altitudinal forests (Gomez-Guerrero et al. 2013). Long-term improved iWUE trends were not unexpected for subtropical and tropical trees (Hietz et al. 2005; Silva et al. 2009; Sun et al. 2010; Brienen et al. 2011; Nock et al. 2011), although only a few studies on this existed to date. Here, we showed more evidence to confirm that iWUE of subtropical trees did increase with elevated c a in the past century.
Trees of Melia azedarch, Chukrasia tabularis and Toona ciliate in a tropical monsoon forest in western Thailand showed to have iWUE improved 0.33, 0.38 and 0.38 μmol mol−1 per ppm increase in c a , respectively (Nock et al. 2011). Hietz et al. (2005) reported that iWUE of Cedrela odorata and Swietenia macrophylla of a tropical moist forest in northern Brazil increased 0.24 and 0.30 μmol mol−1 per ppm, respectively, from 1850 to 1990. Another tree species, Araucaria angustifolia from southern Brazil was found that the increases of iWUE in forest and grassland ecosystem were 0.47 and 0.33 μmol mol−1 per ppm, respectively (Silva et al. 2009). In subtropical forest of southern China, the increase in iWUE of Pinus massoniana was 0.44 μmol mol−1 per ppm (Sun et al. 2010). In this study, iWUE of P. tabulaeformis and P. taiwanensis increased about 0.46 and 0.34 μmol mol−1 per ppm, within the range (0.33–0.47 μmol mol−1 per ppm) of exiting studies on long-term iWUE trends in tropical and subtropical tree species. Higher increase was detected in the recent study (Brienen et al. 2011) that iWUE of Mimosa acantholoba from a tropical dry forest in south Mexico increased 0.52 μmol mol−1 per ppm. These different increases in iWUE showed that besides tree species difference, divergences in temperature, air humidity and soil water availability were also important factors that caused the differences in the physiological responses of plants to elevated c a (Arneth et al. 2002; Saurer et al. 2004; Waterhouse et al. 2004).
Long-term trends in BAI for P. tabulaeformis since 1897 and P. taiwanensis during 1975–2010 continuously increased for many decades but decreased in the recent decades, which were common either in tropical and subtropical forests (Silva et al. 2009; Sun et al. 2010; Nock et al. 2011) or other terrestrial ecosystems (Peñuelas et al. 2008; Silva et al. 2010; Gomez-Guerrero et al. 2013). Widespread declines in tree BAI in recent years can be attributed to the fact that the negative effects of some factors on tree growth, such as climate change (particularly warming-induced drought) (Silva et al. 2010; Sun et al. 2010; Nock et al. 2011), nutrient limitation and physiological long-term acclimation to elevated c a , have overridden the potential growth benefits from a CO2-rich environment (Xu et al. 2009). However, abnormal BAI trends in P. taiwanensis in the earlier period could not be explained reasonably at present and a paradoxical cause may be that very anomalous, not concentrically radial growths led to large errors in conversion from ring width to BAI, especially when trees were younger.
4.2 Effects of elevated c a and climate on iWUE and BAI
The three scenarios of gas exchange responses proposed by Saurer et al. (2004) are useful for better understanding of physiological reactions of trees to elevated c a : (1) a constant c i leads to decreasing c i /c a and increasing iWUE, (2) a constant c i /c a , due to c i increasing in a proportional way with c a , indicates that the linkage between g s and A is largely maintained under elevated c a (Medlyn et al. 2001) and also causes increasing iWUE, and (3) a constant c a − c i , due to c i increasing at the same rate with c a , with no improvement in iWUE and no active stomatal responses. The variations in c i /c a and c a − c i of P. tabulaeformis and P. taiwanensis (Fig. 7) showed that they experienced two phases in the past 130 years: c a − c i remained constant before 1940 (hollow points in Fig. 7b, d), and c i /c a were relatively stable after 1940 (solid points in Fig. 7a, c). This indicates that the stomatal responses were converted around 1940, when c a reached approximately 310 ppm. In the first stage, c i increased at the same rate with c a ; therefore, no active stomatal responses occurred and iWUE did not present obvious change (Figs. 2d and 3d). After c a exceeded 310 ppm, the relationship between c i and c a was transformed from constant c a − c i to constant c i /c a , which meant that stomata began to actively respond to elevated c a , but c i could not keep peace with c a and led to evident increase in iWUE (Figs. 2d and 3d).
Higher iWUE may be caused from reduced g s , increased A or a combination of both. Although the reason that caused the increasing iWUE could not be determined by δ13C analysis alone, variations in c i over time may provide some insights into changes of A over time. The c i of both P. tabulaeformis and P. taiwanensis increased in the past 130 years (Figs. 2b and 3b), which could prove indirectly that A of two tree species increased over time. For P. tabulaeformis, increased A could be considered as the primary factor that resulted in the increase in iWUE during 1940–1990. Nevertheless, A may not increase any more in the recent 20 years because a reduced BAI was detected with elevated c a , and reduced g s may contribute to higher iWUE more than increased A. It is not contradictory between reduced g s and increased c i , since individual open stomata can absorb more CO2 under very high c a and keep c i increasing, even though stomata partially close (Xu et al. 2009). Water deficit is usually regarded as the cause of stomata closing. Although the precipitation did not change in the study area from 1959 to 2009 (Fig. 1c), an obvious warming trend was found during 1980–2009 (Fig. 1b), which could cause the evaporation and transpiration increase. Therefore, the plants need to conserve water by reducing g s . Similar stomatal responses were applied to P. taiwanensis, but A continued increasing until around 2005 and g s began to decrease about a few years ago. In this study, the sampling site of P. taiwanensis is located at a higher elevation than P. tabulaeformis by approximately 620 m. As the lower temperature and more rainfall at the higher locations are beneficial to reduce plant evaporation and transpiration, P. taiwanensis responded to close parts of stomata later than P. tabulaeformis, and correspondingly, the decreases in BAI with c a lagged behind P. tabulaeformis. Overall, our research findings have been consistently with those of Linares and Camarero (2012) and Silva and Anand (2013), which have highlighted that about 66–81 % of the iWUE and 28–56 % of the BAI could be attributed to the rising c a , with 11–21 % of the BAI explained by the c a (Linares and Camarero 2012) and that the iWUE increased by 10–60 % with the rising c a , but tree growth varied widely, from the increases in high latitudes (>40°N), to progressively lower increases towards lower latitudes, as compared with 15–55 and 7–10 % decreases in tree growth for tropical and subtropical forests, respectively, with the mixed tree growth responses for the other regions (Silva and Anand 2013).
5 Conclusions
In this study, similar trends in δ13C, Δ, c i and iWUE with elevated c a and climate change were found for P. tabulaeformis and P. taiwanensis from a subtropical forest located in central China. Two tree species demonstrated declining trends in BAI in recent years, although the variations of BAI were not uniform in the past 130 years. Continuous increases in iWUE could not prevent the decreases in tree growth in recent years, because negative effects of some factors on tree growth, such as climate change (particularly warming-induced drought), nutrient limitation and physiological long-term acclimation to elevated c a , have overridden the CO2 fertilization effects.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytol 165:351–371
Allen MR, Frame DJ, Huntingford C, Jones CD, Lowe JA, Meinshausen M, Meinshausen N (2009) Warming caused by cumulative carbon emissions towards the trillionth tonne. Nature 458:1163–1166
Andreu-Hayles L, Planells O, Gutierrez E, Muntan E, Helle G, Anchukaitis KJ, Schleser GH (2011) Long tree-ring chronologies reveal 20th century increases in water-use efficiency but no enhancement of tree growth at five Iberian pine forests. Glob Change Biol 17:2095–2112
Arneth A, Lloyd J, Santruckova H, Bird M, Grigoryev S, Kalaschnikov YN, Gleixner G, Schulze ED (2002) Response of central siberian scots pine to soil water deficit and long-term trends in atmospheric CO2 concentration. Global Biogeochem Cy 16:5-1–5-13
Barber VA, Juday GP, Finney BP (2000) Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature 405:668–673
Battipaglia G, Saurer M, Cherubini P, Calfapietra C, McCarthy HR, Norby RJ, Cotrufo MF (2013) Elevated CO2 increases tree-level intrinsic water use efficiency: insights from carbon and oxygen isotope analyses in tree rings across three forest FACE sites. New Phytol 197:544–554
Bert D, Leavitt SW, Dupouey JL (1997) Variations of wood δ13C and water-use efficiency of Abies alba during the last century. Ecology 78:1588–1596
Brienen RJW, Wanek W, Hietz P (2011) Stable carbon isotopes in tree rings indicate improved water use efficiency and drought responses of a tropical dry forest tree species. Trees-struct Funct 25:103–113
Duquesnay A, Bréda N, Stievenard M, Dupouey JL (1998) Changes of tree-ring δ13C and water-use efficiency of beech (Fagus sylvatica L.) in north-eastern France during the past century. Plant Cell Environ 21:565–572
Ehleringer JR, Cerling TE (1995) Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Physiol 15:105–111
Etheridge DM, Steele LP, Langenfelds RL, Francey RJ, Barnola JM, Morgan VI (1996) Natural and anthropogenic changes in atmospheric CO2 over the last 1000 years from air in Antarctic ice and firn. J Geophys Res 101:4115–4128
Etheridge DM, Steele LP, Francey RJ, Langenfelds RL (1998) Atmospheric methane between 1000 A.D. and present: evidence of anthropogenic emissions and climatic variability. J Geophys Res 103:15979–15993
Farquhar GD, Oleary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the inter-cellular carbon-dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Feng XH (1999) Trends in intrinsic water-use efficiency of natural trees for the past 100–200 years: a response to atmospheric CO2 concentration. Geochim Cosmochim Ac 63:1891–1903
Francey RJ, Farquhar GD (1982) An explanation of 13C/12C variations in tree rings. Nature 297:28–31
Gagen M, McCarroll D, Robertson I, Loader NJ, Jalkanen R (2008) Do tree ring δ13C series from Pinus sylvestris in northern Fennoscandia contain long-term non-climatic trends? Chem Geol 252:42–51
Gomez-Guerrero A, Silva LCR, Barrera-Reyes M, Kishchuk B, Velazquez-Martinez A, Martinez-Trinidad T, Ofelia Plascencia-Escalante F, Horwath WR (2013) Growth decline and divergent tree ring isotopic composition (δ13C and δ18O) contradict predictions of CO2 stimulation in high altitudinal forests. Glob Change Biol 19:1748–1758
Hietz P, Wanek W, Dunisch O (2005) Long-term trends in cellulose δ13C and water-use efficiency of tropical Cedrela and Swietenia from Brazil. Tree Physiol 25:745–752
Holmes RL (1983) Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull 43:69–78
Ivlev A, Voronin VV (2007) The mechanism of carbon isotope fractionation in photosynthesis and carbon dioxide component of the greenhouse effect. Biol Bull 34:603–609
Kirdyanov AV, Treydte KS, Nikolaev A, Helle G, Schleser GH (2008) Climate signals in tree-ring width, density and δ13C from larches in Eastern Siberia (Russia). Chem Geol 252:31–41
Kӧrner C (2009) Responses of humid tropical trees to rising CO2. Annu Rev Ecol Evol Syst 40:61–79
Lacis AA, Schmidt GA, Rind D, Ruedy RA (2010) Atmospheric CO2: principal control knob governing earth’s temperature. Science 330:356–359
LaMarche VC, Graybill DA, Fritts HC, Rose MR (1984) Increasing atmospheric carbon dioxide: tree ring evidence for growth enhancement in natural vegetation. Science 225:1019–1021
Leavitt SW, Idso SB, Kimball BA, Burns JM, Sinha A, Stott L (2003) The effect of long-term atmospheric CO2 enrichment on the intrinsic water-use efficiency of sour orange trees. Chemosphere 50:217–222
LeBlanc DC (1990) Relationships between breast-height and whole-stem growth indexes for red spruce on Whiteface Mountain, New York. Can J Forest Res 20:1399–1407
Linares JC, Camarero JJ (2012) From pattern to process: linking intrinsic water-use efficiency to drought-induced forest decline. Glob Change Biol 18:1000–1015
Linares JC, Delgado-Huertas A, Camarero JJ, Merino J, Carreira JA (2009) Competition and drought limit the response of water-use efficiency to rising atmospheric carbon dioxide in the Mediterranean fir Abies pinsapo. Oecologia 161:611–624
Liu XH, Shao XM, Liang EY, Zhao LJ, Chen T, Qin D, Ren JW (2007) Species-dependent responses of juniper and spruce to increasing CO2 concentration and to climate in semi-arid and arid areas of northwestern China. Plant Ecol 193:195–209
Liu XH, Shao XM, Wang LL, Liang E, Qin DH, Ren JW (2008) Response and dendroclimatic implications of δ13C in tree rings to increasing drought on the northeastern Tibetan Plateau. J Geophys Res 113:doi:10.1029/2007JG000610
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants face the future. Annu Rev Plant Biol 55:591–628
MacFarling MC (2004) The natural and anthropogenic variations of carbon dioxide, methane and nitrous oxide during the Holocene from ice core analysis. PhD thesis, University of Melbourne, Melbourne, Australia
MacFarling MC, Etheridge D, Trudinger C, Steele P, Langenfelds R, van Ommen T, Smith A, Elkins J (2006) Law dome CO2, CH4 and N2O ice core records extended to 2000 years BP. Geophys Res Lett 33: doi: 10.1029/2006GL026152
Mann ME, Bradley RS, Hughes MK (1998) Global-scale temperature patterns and climate forcing over the past six centuries. Nature 392:779–787
Maseyk K, Hemming D, Angert A, Leavitt SW, Yakir D (2011) Increase in water-use efficiency and underlying processes in pine forests across a precipitation gradient in the dry Mediterranean region over the past 30 years. Oecologia 167:573–585
Medlyn BE, Barton CVM, Broadmeadow MSJ, Ceulemans R, De Angelis P, Forstreuter M, Freeman M, Jackson SB, Kellomaki S, Laitat E, Rey A, Roberntz P, Sigurdsson BD, Strassemeyer J, Wang K, Curtis PS, Jarvis PG (2001) Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol 149:247–264
Montzka SA, Dlugokencky EJ, Butler JH (2011) Non-CO2 greenhouse gases and climate change. Nature 476:43–50
Morison JIL (1993) Response of plants to CO2 under water limited conditions. Vegetatio 104:193–209
Morsion JIL (1987) Intercellular CO2 concentration and stomatal response to CO2. In: Zeiger E, Farquhar GD, GCowan IR (eds) Stomatal function. Stanford University Press, Stanford, CA, USA, pp 229–251
Nock CA, Baker PJ, Wanek W, Leis A, Grabner M, Bunyavejchewin S, Hietz P (2011) Long-term increases in intrinsic water-use efficiency do not lead to increased stem growth in a tropical monsoon forest in western Thailand. Glob Change Biol 17:1049–1063
Ou TH, Qian WH (2006) Vegetation variations along the monsoon boundary zone in East Asia. Chinese J Geophys-ch 49:698–705
Overdieck D, Forstreuter M (1994) Evapotranspiration of beech stands and transpiration of beech leaves subject to atmospheric CO2 enrichment. Tree Physiol 14:997–1003
Pedersen BS (1998) The role of stress in the mortality of midwestern oaks as indicated by growth prior to death. Ecology 79:79–93
Peñuelas J, Hunt JM, Ogaya R, Jump AS (2008) Twentieth century changes of tree-ring δ13C at the southern range-edge of Fagus sylvatica: increasing water-use efficiency does not avoid the growth decline induced by warming at low altitudes. Glob Change Biol 14:1076–1088
Peñuelas J, Canadell JG, Ogaya R (2011) Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Global Ecol Biogeogr 20:597–608
Phipps RL, Whiton JC (1988) Decline in long-term growth trends of white oak. Can J Forest Res 18:24–32
Picon C, Guehl JM, Aussenac G (1996) Growth dynamics, transpiration and water-use efficiency in Quercus robur plants submitted to elevated CO2 and drought. Ann Des Sci For 53:431–446
Saurer M, Cherubini P, Bonani G, Siegwolf R (2003) Tracing carbon uptake from a natural CO2 spring into tree rings: an isotope approach. Tree Physiol 23:997–1004
Saurer M, Siegwolf RTW, Schweingruber FH (2004) Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob Change Biol 10:2109–2120
Sidorova OV, Siegwolf RTW, Saurer M, Naurzbaev MM, Vaganov EA (2008) Isotopic composition (δ13C, δ18O) in wood and cellulose of Siberian larch trees for early medieval and recent periods. J Geophys Res 113:doi:10.1029/2007JG000473
Silva LCR, Anand M (2013) Probing for the influence of atomospheric CO2 and climate change on forest ecosystems across biomes. Glob Change Biol 22:83–92
Silva LCR, Anand M, Oliveira JM, Pillar VD (2009) Past century changes in Araucaria angustifolia (Bertol.) Kuntze water use efficiency and growth in forest and grassland ecosystems of southern Brazil: implications for forest expansion. Glob Change Biol 15:2387–2396
Silva LCR, Anand M, Leithead MD (2010) Recent widespread tree growth decline despite increasing atmospheric CO2. PLoS One 5: doi:10.1371/journal.pone.0011543
Silva LCR, Horwath WR (2013) Explaining global increases in water use efficiency: why have we overestimated responses to rising atmospheric CO2 in natural forest ecosystems? PLoS One 8: doi:10.1371/journal.pone.0053089
Stokes MA, Smiley TL (1968) An introduction to tree-ring dating. An introduction to tree-ring dating. University of Chicago Press, Chicago
Sun FF, Kuang YW, Wen DZ, Xu ZH, Li JL, Zuo WD, Hou EQ (2010) Long-term tree growth rate, water use efficiency, and tree ring nitrogen isotope composition of Pinus massoniana L. in response to global climate change and local nitrogen deposition in Southern China. J Soils Sediments 10:1453–1465
Tang KL, Feng XH, Funkhouser G (1999) The δ13C of tree rings in full-bark and strip-bark bristlecone pine trees in the white mountains of California. Glob Change Biol 5:33–40
Wang W, Liu X, An W, Xu G, Zeng X (2012) Increased intrinsic water-use efficiency during a period with persistent decreased tree radial growth in northwestern China: causes and implications. Forest Ecol Manag 275:14–22
Waterhouse JS, Switsur VR, Barker AC, Carter AHC, Hemming DL, Loader NJ, Robertson I (2004) Northern European trees show a progressively diminishing response to increasing atmospheric carbon dioxide concentrations. Quaternary Sci Rev 23:803–810
Whitmore TC (1990) An introduction to tropical rainforests. Oxford University, Oxford, UK
Wullschleger SD, Tschaplinski TJ, Norby RJ (2002) Plant water relations at elevated CO2—implications for water-limited environments. Plant Cell Environ 25:319–331
Xu G, Liu X, Qin D, Chen T, An W, Wang W, Wu G, Zeng X, Ren J (2013) Climate warming and increasing atmospheric CO2 have contributed to increased intrinsic water-use efficiency on the northeastern Tibetan Plateau since 1850. Trees-struct Funct 27:465–475
Xu ZH, Chen CR, He JZ, Liu JX (2009) Trends and challenges in soil research 2009: linking global climate change to local long-term forest productivity. J Soils Sediments 9:83–88
Xu ZH, Saffigna PG, Farquhar GD, Simpson JA, Haines RJ, Walker S, Osborne DO, Guinto D (2000) Carbon isotope discrimination and oxygen isotope composition in clones of the F1 hybrid between slash pine and Caribbean pine in relation to tree growth, water-use efficiency and foliar nutrient concentration. Tree Physiol 20:1209–1217
Yamaguchi DK (1991) A simple method for cross-dating increment cores from living trees. Can J Forest Res 21:414–416
Zeppel MJB, Lewis JD, Chaszar B, Smith RA, Medlyn BE, Huxman TE, Tissue DT (2012) Nocturnal stomatal conductance responses to rising [CO2], temperature and drought. New Phytol 193:929–938
Zheng Y, Zhang Y, Shao X, Yin Z-Y, Zhang J (2012a) Temperature variability inferred from tree-ring widths in the Dabie Mountains of subtropical central China. Trees-struct Funct 26:1887–1894
Zheng Y, Zhang Y, Shao X, Yin Z-Y, Zhang J (2012b) Climate significance of tree ring width of Huangshan pine and Chinese pine in the Dabie Mountains. Prog Geography 31:72–77 (in Chinese with English abstract)
Acknowledgments
This research was supported by the Australian Research Council (DP1092470), Griffith University (Australia). We thank Prof. Zhi-Yong Yin, Dr. Yonghong Zheng and Dr. Yong Zhang for their assistance in the field work and Mr Rene Diocares for his assistance in stable isotope analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Yan Xu and Wenjing Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, Y., Li, W., Shao, X. et al. Long-term trends in intrinsic water-use efficiency and growth of subtropical Pinus tabulaeformis Carr. and Pinus taiwanensis Hayata in central China. J Soils Sediments 14, 917–927 (2014). https://doi.org/10.1007/s11368-014-0878-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-0878-4