Abstract
Background
There are similarities in hemodialysis (HD) between adults and children and also unique pediatric aspects. In this systematic review, we evaluated the existing HD literature, including vascular access, indications, parameters, and outcomes as a reflection on real-life HD practices.
Methods
Medline, Embase, CINAHL, Web of Science, and Cochrane Library were systematically searched for literature on HD in children (1–20 years). Two reviewers independently assessed the literature and data on indications; vascular access, outcomes, and specific parameters for HD were extracted.
Results
Fifty-four studies (8751 patients) were included in this review. Studies were stratified into age groups 1–5, 6–12, and 13–20 years based on median/mean age reported in the study, as well as era of publication (1990–2000, 2001–2010, and 2011–2019). Across all age groups, both arteriovenous fistulas and central venous catheters were utilized for vascular access. Congenital abnormalities and glomerulopathy were the most common HD indications. HD parameters including HD session duration, dialysate and blood flow rates, urea reduction ratio, and ultrafiltration were characterized for each age group, as well as common complications including catheter dysfunction and intradialytic hypotension. Median mortality rates were 23.3% (3.3), 7.6% (14.5), and 2.0% (3.0) in ages 1–5, 6–12, and 13–20 years, respectively. Median transplantation rates were 41.6% (38.3), 52.0% (32.0), and 21% (25.6) in ages 1–5, 6–12, and 13–20, respectively.
Conclusion
This comprehensive systematic review summarizes available literature on HD in children and young adults, including best vascular access, indications, technical aspects, and outcomes, and reflects on HD practices over the last three decades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With significant advances in medical technology, hemodialysis (HD) has evolved to be a critical tool for managing children with kidney disease. HD is utilized in children with both acute kidney injury (AKI) and kidney failure, with nearly 50% of kidney failure patients receiving HD as a bridge to transplantation [1]. In the setting of pediatric AKI, situations such as severe electrolyte imbalance, fluid overload, hyperammonemia, and uremic encephalopathy require dialysis. Common etiologies of pediatric kidney failure requiring dialysis include congenital anomalies of the kidney and urinary tract (CAKUT) and glomerular diseases.
To choose an appropriate kidney replacement modality, important factors such as the indication for dialysis and clinical status of the patient must be considered. Although general principles of dialysis are similar between adult and pediatric populations, children have different growth, developmental, and psychosocial needs that should be considered [1]. Traditionally, older children and adolescents are more likely to receive HD while infants and younger children weighing < 10 kg often receive peritoneal dialysis (PD) [2]. The large size of HD extracorporeal circuits in relation to the blood volumes of small children results in a technically difficult procedure [1]. Additionally, clinicians favor HD in cases with contraindications to PD, such as diaphragmatic hernia, prior intra-abdominal surgery, or malignancy [3]. The unique technical challenges of HD in children require close monitoring to prevent potential complications with significant morbidity and mortality.
Overall, proper HD in children requires careful manipulation of the following technical parameters: vascular access, choice and size of dialysis machine, type of dialyzer, dialysate composition, and blood and dialysate flow rates. In addition, patient monitoring, assessment, and management of complications such as fluid overload or hypotension play an important role in efficient and safe hemodialysis. This systematic review summarizes the relevant published literature regarding the utilization of hemodialysis in children and young adults for acute and chronic kidney disease and provides a summary of widespread HD practices spanning over three decades. A review of existing guidelines utilized when treating children with HD is also provided.
Methods
Database source and search criteria
In this systematic review, we sought to summarize results of all published literature regarding type of access, indications, parameter, and outcomes of HD in children. This study was registered in the International Prospective Register for Systematic Reviews (#CRD42020141616). A systematic search was conducted in the following databases: Medline, Embase, CINAHL, Web of Science, and Cochrane Central Trials. Keywords and search terms were developed by an expert librarian. Online Resource 1 lists the full search criteria. All literature was downloaded to Rayyan QCRI and reviewed using the selection criteria. Included papers were assessed in Mendeley (version 1.19.4).
Study selection and eligibility
Two reviewers (CY, AF) independently reviewed the titles, abstracts, and full-text review of all citations. Any conflicts were resolved either by consensus or a third independent reviewer (RC). For our review, the eligible studies included children aged 1–20 years receiving hemodialysis for acute or chronic indications. We further narrowed our inclusion criteria to papers which had primary hemodialysis and vascular access outcomes, rather than single biomarker outcomes. The original search criteria involved children aged 1–17 years; however, given that the large percentage of studies included hemodialysis in subjects aged 1–20 years, our age limitations were extended. Studies prior to Jan 1990 or studies regarding other modalities of dialysis (hemodiafiltration, CRRT, PD, and SLED) were excluded. Non-English studies or those involving < 5 patients were excluded. Some studies included data on pediatric HD which involved subjects outside the defined age group (1–20 years) or data from mixed modalities (e.g., PD and HD combined). If the relevant data could not be extracted, the study was not included in the final analysis.
Data extraction and analysis
Data were extracted electronically from relevant literature by two reviewers (CY, AF). The appropriate data on vascular access, indications for HD, and specific parameters of HD, including dialysate composition, dialysate flow, blood flow, urea reduction rate, and ultrafiltration, were extracted from each study. Data were also collected regarding medications, anticoagulation, and blood transfusions along with outcomes of mortality and kidney transplantation. Data were stratified into age group (ages 1–5, 6–12, and 13–20 years) based on the mean/median age of the children included in individual studies, as well as the decade of publication (1990–2000, 2001–2010, and 2011–2019). Continuous data were presented as median (IQR), unless otherwise specified in the text.
This systematic review was completed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Online Resource 2) and the enhancing transparency in reporting the synthesis of qualitative research (ENTREQ) statement (Online Resource 3).
Results
The initial database search yielded 16,196 potential articles of interest (Fig. 1). After screening titles and abstracts, 101 full-text articles were assessed based on our inclusion criteria. Ultimately, 54 studies with a total of 8751 children were included, and appropriate data was collected (Tables 1 and 2, Online Resource 4–6). Two included studies had a mean or median age between 1 and 5 years of age (n = 100 children), 22 studies were included in the 6–12-year age group (n = 709), and 24 studies were included in the 13–20-year age group (n = 6058). Six studies did not list a mean or median age (n = 1884) [22,23,24,25,26,27]. HD parameters stratified by patient age and decade of publication are listed in Table 1. Figure 2 represents the gradual decrease in the age of dialysis initiation in various eras 1990–2000, 2001–2010, and 2011–2019, in the age groups 6–12 years and 13–20 years. There were only 2 studies in the group of 1–5 years, and thus, it is not graphically represented.
The median duration of HD session was 240.0 min in ages 1–5, 202.5 (30.0) minutes in ages 6–12, and 222.5 (34.0) minutes in ages 13–20. Across all age groups, median number of sessions was 3 sessions/week. Across the 3 eras from 1990 to 2019, frequency of dialysis sessions ranged from 3 to 4 times/week, and there was no clear trend in the change of hemodialysis duration over time. Cumulative duration of HD per patient in months was 27.7 (15.5) in ages 1–5, 25.0 (28.6) in ages 6–12, and 28.0 (24.7) in ages 13–20. A subset of studies described cumulative number of sessions/patient; median 50.0 (36.0) in ages 6–12 and a single study from ages 13–20 reported 86 sessions/patient [19].
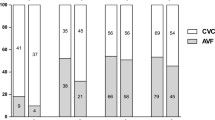
Recorded incidences of vascular access use varied greatly between papers. In age 1–5, there was 1 recorded event of arteriovenous fistula (AVF)/graft and 10 events of central venous catheter (CVC) use. In ages 6–12, 356 events of AVF/graft/basilic vein transposition occurred, and a total of 512 events of CVC were reported. From age 13–20, there were 1633 total events of AVF/graft/basilic vein transposition and 3349 recorded events of CVC use. Across the majority of studies, the internal jugular vein (IJV) was used. In eras 2001–2010 and 2011–2019, there was a predominance of CVC use observed. Total number of AVF/graft/basilic vein transposition use was 63 in studies from 1990 to 2000, with 18 events of CVC use. In the eras of 2001–2010 and 2011–2019, total events of AVF/graft/basilic vein transposition use across all ages were 1466 and 779 respectively, while events of CVC use were 3021 and 1172. In the 6 studies without a listed mean or median age, one study reported a session duration of 240 min [27], and two studies reported thrice weekly dialysis sessions [26, 27]. Cumulative duration was only listed in a single study as mean 8.6 months [25]. Recorded events of AVF/graft/basilic vein transposition ranged from 6 to 276 and 7 to 284 events for CVC use.
Indications for hemodialysis
Most of the studies (n = 47) reported data on HD in children with kidney failure. However, six studies assessed children undergoing acute HD, with a combined subset of 60 patients in total [6, 8, 9, 13, 16, 23]. Of these six studies, four were from the age group 6–12, one from age 13–20, and one did not have a recorded mean/median age. In studies of chronic hemodialysis patients, common etiologies of kidney failure across all ages included CAKUT, which included kidney dysplasia and obstructive uropathies, glomerulonephritis (GN), focal segmental glomerulosclerosis (FSGS), polycystic kidney disease, and HUS (Online Resource 4).
Hemodialysis parameters
Specific parameters of the HD prescription were recorded, including dialyzer and tubing type, volume of priming solution, surface area, dialysate composition and temperature, dialysate and blood flow, urea reduction ratio (URR), and ultrafiltration (UF) (Table 1). The two dialyzer machines reported in ages 1–5 were Gambro AK200 and Fresnius 2008K machines, with hollow-fiber, polysulfone dialyzer membranes. In ages 6–12, Fresnius (2000A, 2008H/K, 4008B/S) were used, with hollow-fiber polysulfone or acetate membranes. A single study mentioned the use of high-flux membranes [10]. Lastly, in ages 13–20, Gambro (AK100, AK10, AK200S), Fresnius (2008K, 4008H, 5008), Cobe, and Baxter machines were used. Dialyzer membranes included hollow-fiber low-flux polysulfone and high-flux dialyzers. In the era 1990–2000, a single study reported the use of low-flux dialyzers, while in the eras of 2001–2010 and 2011–2019, the use of both high- and low-flux dialyzers was reported. In the 6 studies without a reported mean/median age, dialyzer types listed were Gambro AK10/AK200/AK200S and Centry 3 [22, 25], and one study reported a range from low- to high-flux dialyzers (12–73% of patients) [27].
Only one study across all ages described tubing type, which was in accordance with the patient’s weight and body surface [17]. Two studies indicated priming of the system if extracorporeal volume exceeded 10%, one with normal saline and one with packed red blood cells diluted to a hematocrit of 40% [4, 25], with one study stating a minimum priming volume of 58 mL [25]. Dialyzer surface area was described in a single study (ages 6–12 years) and reported as 75–100% of body surface area [8].
In terms of dialysate solution, studies from ages 1–5 described using a standard dialysate solution, with one using a standard citrate dialysate [5]. In ages 6–12, a bicarbonate or acetate solution was used. In all solutions containing bicarbonate, concentration was 35 mmol/L, and calcium 1.25 mmol/L. Varying levels of glucose (ranging from glucose free—11 mmol/L) and sodium (138–148 mmol/L) were used. In ages 13–20, a varying concentration of bicarbonate (25–35 mmol/L) and sodium (134–140 mmol/L) was used, with a standard calcium concentration of 1.75 mmol/L. Temperature of the dialysate solution was only reported in two studies across all ages as 37.5 °C [10, 11]. In the 6 studies without a reported mean/median age, a single study reported using a range of Na (< 138, > 138 mmol/L), Ca (1.25–1.75 mmol/L), and bicarbonate (34–36 mmol/L) in their dialysate solutions [27].
Dialysate flow (mL/min) was reported once in ages 1–5 as a mean of 500 mL/min [4]. Median dialysate flow in age 6–12 years was 462.5 (75.0) mL/min and 500.0 mL/min in ages 13–20, and did not vary significantly across eras. From ages 1–5 years, mean blood flow was reported in two studies as 5 mL/kg/min [4] and 300 mL/min [5]. In the latter study, however, the age range of children (n = 90) was 1–15 years and mean age at start of HD was 5.6 ± 1.4 years and mean weight was 17.0 ± 2.7 kg (range 8–50). In ages 6–12, 3 studies reported blood flow in mL/kg/min with a median of 5.0 (0.6), and the remainder of studies reported a median of 142.5 (76.0) mL/min. In ages 13–20, a single study reported a mean 4.5 mL/kg/min [17], while the remainder had a median of 205.0 (100.0) mL/min. There was no specific trend noted in median blood flow across all eras, which ranged from a lowest value of 145.0 (38.5) mL/min in era 1990–2000 to a highest value of 215.0 (135.2) mL/min in era 2001–2010. In the 6 studies without a reported mean/median age, dialysate flow was listed in one study as 525 mL/min [26], and blood flow ranged from 125 to 200 mL/min.
Lastly, URR was reported in a single study between ages 1 and 5 as a mean of 62.1%. From ages 6 to 12, median URR was 58.5% (22.3), and from ages 13 to 20, 46.9% (26.3). In terms of era, there were no reported URR in studies from 1990 to 2000. Median URR in studies from 2001 to 2010 was 65.1% (11.4), and in 2011–2019, it was 64.5% (30.2). Ultrafiltration (UF) rate was reported sparsely. In studies where UF was reported as a percentage, it was calculated by dividing the ultrafiltration volume by the post-dialytic body weight. In ages 6–12, one study reported a mean UF volume of 5.4% [10], while another observed a mean UF rate of 321 mL/h [8]. In ages 13–20, one study reported a mean of 6.1 ± 2.6% UF rate [15]. In studies without a reported mean/median age, URR ranged from 65.1 to 76.4%, and UF rate was reported in one study as 9.3 mL/kg/h [27].
The most common medications administered included erythropoietin, iron supplementation, activated vitamin D (i.e., Calcitriol), anti-hypertensive medications, and growth hormone treatment (Online Resource 6). Mannitol was administered in 3 studies (one without a reported age, two studies aged 6–12) to avoid intravascular volume depletion and prevent disequilibrium syndrome and intradialytic hypotensive episodes [10, 11, 25]. In one study, 20% albumin was used in place of mannitol if hypoalbuminemia was present, and the line was primed with 5% albumin or normal saline if maximum extracorporeal volume exceeded 10% [25].
Anticoagulation in ages 1–5 was reported in a single study as heparin 10–30 U/kg/h [4]. If heparin was given in ages 6–12, dosing was variable and ranged from 45 to 70 U/kg as an initial bolus or an infusion with an upper limit of 0.2 units/kg/min. A single study used low molecular weight heparin (LMWH; dalteparin) dosed at 50 IU/kg [14]. Three studies reported low molecular weight heparin or unfractionated heparin (UFH) use but did not provide specific dosing. A single study used nafamostat for one patient [13]. In ages 13–20, LMWH and UFH were used in one study each for anticoagulation, as well as a citrate solution (3–30% range) in a single study [16]. Dosing of LMWH ranged from 33 to 110 U/kg, and heparin dose was titrated to achieve a clotting time of 150–170 s.
In terms of blood transfusion, a single study in ages 1–5 reported that at least 8 patients (out of 10 total patients) needed at least one transfusion to achieve Hb 11–13 g/dL, due to the presence of anemia despite erythropoietin treatment [4]. In ages 6–12, only one study commented on transfusion and specified that patients with Hb under 7 g/dL received transfusions during dialysis [8]. In ages 13–20, a single study reported a transfusion requirement in a mean of 13.4% of patients [20]. One study without a reported mean/median age reported mean transfusions of 7.4 ± 5.7 mL/kg/month in the first month of dialysis, which decreased to 1.6 ± 2.2 mL/kg/month after 6 months of treatment [25].
For long-term patient monitoring, 4 studies across all age ranges commented on their monitoring protocols. This included 30 min–hourly blood pressure and heart rate monitoring, as well as additional readings during any symptomatic event, and regular blood work monitoring weekly and monthly. One study monitored gross UF volume regularly [10].
Outcomes
Data regarding mortality, transplantation, and complication rates are described in Table 2. In the age group of 1–5 years, median mortality rate was 23.3% (3.3), while it was 7.6% (14.5) and 2.0% (3.0) in ages 6–12 and 13–20, respectively. Common causes were pulmonary edema, cardiovascular events, hyperkalemia, and infection. No deaths were directly attributed to HD therapy. Mortality in the era of 1990–2000 was reported in a single study as zero, in years 2001–2010 as median 3.2% (6.7) and in years 2011–2019 as median 6.5% (15). Median transplantation rates were 41.6% (38.3), 52.0% (32.0), and 21% (25.6) in ages 1–5, 6–12, and 13–20, respectively. In ages 1–5, transplantation rates ranged from 3.3 to 80%. In ages 6–12 and 13–20, transplantation rates ranged from 2 to 80% and 0 to 61.5%, respectively. In terms of era, transplantation rate was 55.7% (15.7) in 1990–2000, 20.9% (56.8) in 2001–2010, and 46.7% (23.1) in 2011–2019. In the 6 studies without a reported mean/median age, mortality was only reported in one study as 7.1%, with a transplantation rate of 92.9% [25].
Individual dialysis complications for each study are listed in Online Resource 5. Intradialytic hypotension was reported in only one study between ages 1 and 5 as 60.0% [4]. Median proportion of intradialytic hypotension in ages 6–12 was 14.6% (18.9), and in ages 13–20, the rate was 26.8% (26.8). Catheter dysfunction was relatively common across all age ranges. It was reported in a single study in ages 1–5 as a dysfunction rate of 56.0% [4]. In ages 6–12, catheter dysfunction rate was median 41.0% (18.9), and in ages 13–20, the median rate was 53.0% (14.0). Common reasons for removal and/or replacement were catheter dislodgement or malposition, as well as obstruction, secondary to kinking or thrombosis. Catheter infection rate was not reported in any studies aged 1–5. Infection rate was observed to be a median of 1.4 (1.4) per 1000 catheter days in ages 6–12 and was only reported in one study aged 13–20 as 0.9 per 1000 catheter days [18]. In terms of era, catheter infection rate was exclusively reported in studies from 2011 to 2019, as a median 1.23 (0.6) events/1000 catheter days. Overall CVC survival time in patients aged 1–5 was reported in one study as 42.7 days [4]. For ages 6–12, catheter survival was a median of 53.8 (30.2) days, and in ages 13–20, it was a median 303.8 (79.2) days. In studies without a mean/median age reported, one study reported a catheter dysfunction rate of 52% with mean 42 day CVC survival [23].
Discussion
While the concept of HD is similar in adults, specific technical factors must be taken into account in children, including the choice of dialyzer, size of extracorporeal circuit, and vascular access. In adults, HD prescription is relatively standardized and has standard dialysis flow rates (> 500 mL/min) and blood flow rates (> 300 mL/min). In contrast, children have blood flow rates based on body weight (5–7 mL/min/kg) [26]. Another important difference between pediatric and adult hemodialysis is the increased risk of loss of plasma proteins, especially in infants, when the priming volume is not returned back due to various reasons. Early onset kidney disease also predisposes infants and children to recurrent blood transfusion which sensitizes them and adversely affects their transplant outcomes. These issues, in part, are due to lack of age-appropriate HD equipment and tubing for younger infants and children. More recently, HD has been increasingly used successfully in infants and young children, due to significant advances in extracorporeal therapy, such as smaller dialyzers and tubing systems that allow for smaller circuit volumes [28]. Further, given the chronic nature, early onset of pediatric kidney disease and high risk for malnutrition, special care in hemodialysis prescription is required to achieve ideal nutritional, growth, and psychosocial outcomes as the child matures. There is increasing evidence that more intensified HD regimens in children can have a positive impact on cardiovascular, nutritional, and growth status [29, 30]. Of note, specialized, multi-disciplinary care can be difficult to achieve given a relative paucity of pediatric dialysis centers in the USA, compared to adult center availability [31]. There are many gaps in high-quality evidence to support pediatric hemodialysis guidelines upon which current practices are based.
As of 2017, HD was the most common initiating therapy (54.4%) for children requiring dialysis in the USA, in comparison to PD (29.5%) or transplantation (16.1%) [32]. A recent study of Canadian children showed a decline in the use of PD for AKI in favor of increasing HD or continuous kidney replacement therapy use [40]. Similarly, various other national registries have shown HD as the most common initial modality followed by PD and transplantation (Table 3) [33, 35,36,37]. As demonstrated in our review, median age of dialysis initiation decreased from earlier years (1990–2000) to later eras (2011–2019), in both age groups 6–12 and 13–20 years. Advancements in technological expertise in pediatric HD may have contributed to this trend.
Regarding vascular access, existing literature supports AVF/AVG as the preferred pediatric vascular access [41]. There are several reported benefits of AVF in comparison to CVCs including lower risk of infection, thrombosis and better solute clearance [12, 24]. Due to the young age of patients at onset of dialysis, the importance of maintaining vasculature for future access was emphasized. Despite these guidelines, the 2019 USRDS data reported that 87.6% of pediatric patients with kidney failure used a CVC for initial vascular access over AVF (11.0%) and AVG (1.4%) [33], with similar trends in NAPRTCS and ANZDATA reports (Table 3) [34, 35]. Our data observed greater CVC use across all three age groups, and in eras 2001–2010 and 2011–2019 there was also a predominance of CVC use observed. Unfortunately, data on catheter type was too sparse to draw conclusions about trends. There are several factors that may contribute to predominant CVC use, which include technical difficulty in fistula/graft creation in children with small vasculature, rapid onset of kidney failure, and an expected short period of time until transplantation [21]. The location of the access is also important. The right IJV was reported to be most preferable, as it allows for higher blood flow and the blood flow remains insignificantly affected when the patient becomes mobile, in comparison to femoral veins. It is crucial to utilize the subclavian vein as a last option due to high risk of stenosis and other complications.
The studies in this review largely focused on chronic HD; however, a few studies did report their use of HD in acute situations such as AKI, rapidly progressive kidney failure, electrolyte disturbances, drug ingestions/intoxications, tumor lysis syndrome, and HUS [42]. In terms of long-term chronic HD, the most common indications reported in this review included CAKUT and glomerulonephritis. This is in accordance with etiologies reported by the various registries listed in Table 3 [33,34,35,36,37,38,39,40].
Current guidelines recommend that delivered dialysis dose be measured monthly in children and adolescents, with a minimum single-pool Kt/V target of 1.2 or URR > 65%. To provide optimal dialysis, various factors need to be considered and modified based on the child’s size and availability of resources in dialysis centers, including appropriate dialyzer surface area, dialysate and blood flow rate, and frequency and time of dialysis sessions. In our review, HD session frequency and duration did not vary significantly over different eras, and the majority of studies reported 3–4 sessions/week. The surface area of dialyzer commonly correlates to approximately 75–100% of the total body surface of the child [28]. In addition, the type of dialyzer utilized is generally tailored to the patient’s size, solute clearance, and ultrafiltration requirements. In our review, not all studies reported on type and flux of dialyzer used but there was an emerging trend of increasing high-flux dialyzer use in the more recent eras for children aged 6–12 and 13–20 years old. Use of high-flux dialyzers has increased as they enable improved clearance of middle molecules such as β-2-microglobulin and have been shown to reduce cardiovascular mortality in patients [43].
Furthermore, the tubing size is commonly selected according to the patient’s size and blood flow to maintain the extracorporeal circuit within normal limits [2]. For patients < 10 kg, neonatal tubing (25 mL) is often used while pediatric tubing (75 mL) is often utilized for patients weighing 10–20 kg [44, 45]. It is generally accepted that a maximum of 10% of a patient’s blood volume may be in the extracorporeal circuit at one time [45]. Accordingly, several studies in this review noted that if > 10% is noted in the circuit, the system is commonly primed with pRBCs and 5% albumin or a normal saline solution to avoid hypotensive complications [4, 25]. The specific volume and content of the blood prime are commonly dependent on the extracorporeal volume, the patient’s perfusion status, and risk of hemodynamic instability [44]. For example, the combination of neonatal lines with a F3 dialyzer is a total of 25 mL + 29 mL = 54 mL. This is safe for a 7.5-kg child, (blood volume of 7.5 × 80 mL = 600 mL), whose maximum allowable extracorporeal blood volume is 60 mL. However, a child under 7.5 kg in weight cannot use this circuit or needs to have consideration for a blood prime.
The composition of dialysate is another important component and is commonly tailored according to the patient’s characteristics. Typically, a standard bicarbonate solution is used with a concentration of 35 mmol/L, with physiological glucose and calcium concentration (1.25 mmol/L) [2, 7], and was utilized by a majority of studies in our review. Of note, lowering the bicarbonate concentration to the 22–25 mmol/L range is often considered with citrate anticoagulation to reduce the risk of metabolic alkalosis [45]. Higher levels of dialysate bicarbonate are suggested to increase cardiovascular risk in adults; however, there is a lack of evidence to suggest the same in children [46].
Additionally, dialysate flow is an important component of dialysis adequacy. Standard flow rates independent of body size typically range between 500 and 800 mL/min [26, 45]. A flow rate of 500 mL/min is suitable for pediatric patients; however, a flow rate of 800 mL/min may be required to achieve optimal clearance in larger patients. In our study, the mean dialysate flow was found to be fairly consistent across all eras and age groups; ages 1–5 were reported in only one study as a mean of 500 mL/min, while median dialysate flow in ages 6–12 years was 462.5 (75.0) mL/min and 500.0 (0) mL/min in ages 13–20. Dialysate temperature is another component to providing optimal dialysis. In our review, two studies (both ages 6–12) reported using a temperature of 37.5 °C [10, 11], consistent with the standard dialysate temperature, which typically ranges from 36 to 37.5 °C. A temperature of 37.5 °C is preferred and recommended for dialysate usage in infants to avoid the risk of dialysis-associated hypothermia [44]. However, in situations of intradialytic hypotension, it is thought that patients may benefit from a cooler dialysate temperature of 35 °C [47, 48].
Blood flow rate is one of the key factors in dialysis adequacy. Generally, a blood flow rate of 150–200 mL/min/m2 is sufficient and based on the body weight of the child (5–7 mL/kg/min) [26]. In our review, studies which reported blood flow in mL/kg/min ranged from 4.0 to 5.1 mL/kg/min across all age groups. No specific change in blood flow rate in children over time was identified in our review. Flow rate can be slowly increased over the first few treatments; initiating at 2–3 mL/kg/min and then increasing by approximately 1 mL/kg/min during the first 3 sessions [28]. There may be a limitation to blood flow due to the catheter size/position; however, no such instances were reported in our study.
Dialysis urea clearance is measured by URR, which is calculated using the pre- and post-dialysis BUN values, and generally, the desired URR is > 65% per session [49]. In our review, URR was reported in one study between ages 1 and 5 as a mean of 62.1%. From ages 6 to 12, median URR was 58.5% (22.3), and from ages 13 to 20, 46.9% (26.3), indicating that not all studies were reaching the desired target, although the majority of studies did achieve URR > 65%. In a few studies, it was noted a lower urea clearance was targeted (30–40%) in the first few sessions of dialysis and titrated up to the desired goal [44]. Overall, our data suggests that efficacy may decrease as age increases, as indicated by URR% attained. Kt/V measurements were not obtained in this review. Gotta et al. similarly observes that HD efficacy varies with age and weight, and adolescents and larger children may be at higher risk of receiving insufficient HD, based on spKt/V measurements [26]. When assessing URR based on era, median URR% was not reported in 1990–2000, but was 61.4% (11.4) from 2001 to 2010, and 62% (32.4) in 2011–2019 suggesting that overall efficacy was nearing > 65% in later eras.
Furthermore, the dialysis sessions (duration and frequency) are often designed to achieve a target dry weight while staying within the acceptable limit of fluid ultrafiltration. In our review, the ultrafiltration (UF) was sparsely reported, suggesting a lack of consistent data exists for this parameter. While traditionally, an ultrafiltration cut-off of 5% was considered to be a target to avoid hypotension; one study in our review achieved a much higher rate (up to 9.7%) which was tolerated with interventions such as intravenous mannitol and sodium ramping [10]. Data on fluid overload and hemodiafiltration were not included in this study, and thus, potential trends cannot be commented on.
Additionally, anticoagulation may be required for HD to prevent extracorporeal circuit clotting and bleeding. Unfractionated heparin and LMWH were both used in the studies of this review, across all age groups and in studies published in 2001–2010 and 2011–2019. Of studies which reported heparin dose, reported dosing ranged from 10 to 30 U/kg/h in ages 1–5 years, to 12 U/kg/h in ages 6–12 years. LMWH types and dosing were variable across studies. The general HD practical guidelines in children by Fischbach et al. recommended the use of conventional heparin (continuous infusion rate of 20 to 30 IU/kg/h) or LMWH (1 mg/kg) for anticoagulation [2]. Non-heparin-based anticoagulation can be considered for patients with increased risk of bleeding [45]. Two studies in our review used alternate anticoagulation; one used nafamostat [13] and one used a range from 3 to 30% citrate [16].
There were also various complications of HD reported by the studies included in this review. Catheter infection rate was only reported in studies published from 2011 to 2019, making it difficult to comment on trends over time. Intradialytic hypotension was reported in only one study of ages 1–5 as 60.0%, and a median of 14.6% (18.9) in ages 6–12 and 26.8% (26.8) in ages 13–20. Techniques for prevention may include a lower dialysate temperature and limiting the amount of ultrafiltration per session. Dialysate composition, specifically bicarbonate and potassium, has also been shown to influence cardiovascular stability during dialysis sessions in adults, although this has not been well studied in pediatric patients [46, 50]. If hypotension did occur, interventions included a normal saline bolus, 5% albumin, and mannitol [45]. It is also important to monitor vital signs closely, at least every 30 min throughout the session.
Our systematic review encompassed the entire pediatric population (ages 1–20) and followed the PRISMA checklist for the conduct and reporting of systematic reviews. We electronically searched multiple databases with our specific search criteria to minimize selection bias and included a wide range of studies in this review. One limitation is that not all studies in this review included details regarding each relevant HD parameter. The information presented here is the most accurate estimation of values given the data that was provided. Given that mortality and transplantation data were variably reported in the literature and most of the studies included in this review did not report mortality data in relation to dialysis vintage, this review is also limited in providing robust data on outcomes compared to more comprehensive registries such as the North American Pediatric Renal Trials and Collaborative Studies. Data regarding Kt/V and fluid overload were not included in this study. In the future, it would be helpful to assess similar parameters in related modalities such as hemodiafiltration.
In conclusion, HD is a commonly utilized method for kidney replacement therapy in children, for both acute and chronic conditions. This systematic review summarizes the various parameters of HD included in a pediatric dialysis prescription, and how existing practices utilize these parameters. It also reviews current choice of vascular access and patient outcomes in these studies. The common complications during HD were reviewed along with existing techniques for prevention. Ultimately, HD is a safe and effective treatment for pediatric patients, and further studies and advancements in technology will continue to refine this process. Optimal hemodialysis prescription and equipment are imperative for improving long-term clinical outcomes among infants and children.
Data availability
All data involved in this systematic review can be found within the article and/or in online supplementary material.
References
Müller D, Goldstein SL (2011) Hemodialysis in children with end-stage renal disease. Nat Rev Nephrol 7:650–658. https://doi.org/10.1038/nrneph.2011.124
Fischbach M, Edefonti A, Schröder C, Watson A (2005) Hemodialysis in children: general practical guidelines. Pediatr Nephrol 20:1054–1066. https://doi.org/10.1007/s00467-005-1876-y
Walters S, Porter C, Brophy PD (2009) Dialysis and pediatric acute kidney injury: choice of renal support modality. Pediatr Nephrol 24:37–48. https://doi.org/10.1007/s00467-008-0826-x
Kovalski Y, Cleper R, Krause I, Davidovits M (2007) Hemodialysis in children weighing less than 15 kg: a single-center experience. Pediatr Nephrol 22:2105–2110. https://doi.org/10.1007/s00467-007-0614-z
Youssef DM, Neemat-Allah MAA (2013) Hemodialysis in children: eleven years in a single center in Egypt. Iran J Kidney Dis 7:468–474
Kurien R, George J, Jacob CK, Shastry J (1996) Vascular access in pediatric hemodialysis. Indian Pediatr 33:767–770
Fischbach M, Terzic J, Cohen CB, Cousandier E, Hamel G, Battouche D, Geisert J (1998) Glucose-charged dialysate for children on hemodialysis: acute dialytic changes. Pediatr Nephrol 12:60–62. https://doi.org/10.1007/s004670050404
Hari P, Kanitkar M, Mantan M, Bagga A (2002) Hemodialysis in children. Indian Pediatr 39:375–380
Peynircioglu B, Ozkan F, Canyigit M, Pamuk GA, Geyik S, Cil BE, Balkanci F (2007) Radiologically placed tunneled internal jugular catheters in the management of chronic hemodialysis and long-term infusion therapies in the pediatric population. J Vasc Interv Radiol 18:875–881. https://doi.org/10.1016/j.jvir.2007.04.016
Hothi DK, Harvey E, Goia CM, Geary DF (2008) Evaluating methods for improving ultrafiltration in pediatric hemodialysis. Pediatr Nephrol 23:631–638. https://doi.org/10.1007/s00467-007-0716-7
Hothi DK, Harvey E, Goia CM, Geary D (2009) The value of sequential dialysis, mannitol and midodrine in managing children prone to dialysis failure. Pediatr Nephrol 24:1587–1591. https://doi.org/10.1007/s00467-009-1151-8
Ma A, Shroff R, Hothi D, Lopez MM, Veligratli F, Calder F, Rees L (2013) A comparison of arteriovenous fistulas and central venous lines for long-term chronic haemodialysis. Pediatr Nephrol 28:321–326. https://doi.org/10.1007/s00467-012-2318-2
Shin HS, Oh JY, Park SJ, Kim JH, Lee JS, Shin J II (2015) Outcomes of hemodialysis in children: a 35-year experience at Severance Hospital. Yonsei Med J 56:1007–1014. https://doi.org/10.3349/ymj.2015.56.4.1007
Lutkin M, Stronach L, Yadav P, Hothi DK (2018) Dalteparin anticoagulation in paediatric home haemodialysis. Pediatr Nephrol 33:2337–2341. https://doi.org/10.1007/s00467-018-4032-1
Marsenić OD, Pavličić D, Peco-Antić A, Bigović G, Jovanović O (2000) Prediction of equilibrated urea in children on chronic hemodialysis. ASAIO J 46:283–287. https://doi.org/10.1097/00002480-200005000-00008
Kreuzer M, Bonzel K-E, Büscher R, Offner G, Ehrich JHH, Pape L (2010) Regional citrate anticoagulation is safe in intermittent high-flux haemodialysis treatment of children and adolescents with an increased risk of bleeding. Nephrol Dial Transplant 25:3337–3342. https://doi.org/10.1093/ndt/gfq225
Hoppe A, Von Puttkamer C, Linke U, Kahler C, Booß M, Braunauer-Kolberg R, Hofmann K, Joachimsky P, Hirte I, Schley S, Utsch B, Thumfart J, Briese S, Gellermann J, Zimmering M, Querfeld U, Müller D (2011) A hospital-based intermittent nocturnal hemodialysis program for children and adolescents. J Pediatr 158:64–68. e1. https://doi.org/10.1016/j.jpeds.2010.06.036
Rus RR, Novljan G, Buturović-Ponikvar J, Kovač J, Premru V, Ponikvar R (2011) Vascular access in children on chronic hemodialysis: a Slovenian experience. Ther Apher Dial 15:292–297. https://doi.org/10.1111/j.1744-9987.2011.00954.x
Marsenic O, Anderson M, Couloures KG, Hong WS, Kevin Hall E, Dahl N (2016) Effect of the decrease in dialysate sodium in pediatric patients on chronic hemodialysis. Hemodial Int 20:277–285. https://doi.org/10.1111/hdi.12384
Laskin BL, Huang G, King E, Geary DF, Licht C, Metlay JP, Furth SL, Kimball T, Mitsnefes M (2017) Short, frequent, 5-days-per-week, in-center hemodialysis versus 3-days-per week treatment: a randomized crossover pilot trial through the Midwest Pediatric Nephrology Consortium. Pediatr Nephrol 32:1423–1432. https://doi.org/10.1007/s00467-017-3656-x
Boehm M, Bonthuis M, Noordzij M, Harambat J, Groothoff JW, Melgar ÁA, Buturovic J, Dusunsel R, Fila M, Jander A, Koster-Kamphuis L, Novljan G, Ortega PJ, Paglialonga F, Saravo MT, Stefanidis CJ, Aufricht C, Jager KJ, Schaefer F (2019) Hemodialysis vascular access and subsequent transplantation: a report from the ESPN/ERA-EDTA Registry. Pediatr Nephrol 34:713–721. https://doi.org/10.1007/s00467-018-4129-6
Smyet SW, Evans JHC, Will E, Brocklebank JT (1992) Paediatric haemodialysis: estimation of treatment efficiency in the presence of urea rebound. Clin Phys Physiol Meas 13:51–62. https://doi.org/10.1088/0143-0815/13/1/005
Muñoz ET, Moreno CX (2000) Management of temporary haemodialysis catheters in paediatrics. EDTNA-ERCA J 26:16–18. https://doi.org/10.1111/j.1755-6686.2000.tb00085.x
Chand DH, Brier M, Strife CF (2005) Comparison of vascular access type in pediatric hemodialysis patients with respect to urea clearance, anemia management, and serum albumin concentration. Am J Kidney Dis 45:303–308. https://doi.org/10.1053/j.ajkd.2004.10.017
Feinstein S, Rinat C, Becker-Cohen R, Ben-Shalom E, Schwartz SB, Frishberg Y (2007) The outcome of chronic dialysis in infants and toddlers - advantages and drawbacks of haemodialysis. Nephrol Dial Transplant 23:1336–1345. https://doi.org/10.1093/ndt/gfm734
Gotta V, Marsenic O, Pfister M (2018) Age- and weight-based differences in haemodialysis prescription and delivery in children, adolescents and young adults. Nephrol Dial Transplant 33:1649–1660. https://doi.org/10.1111/hdi.12589
Shroff R, Smith C, Ranchin B, Bayazit AK, Stefanidis CJ, Askiti V, Azukaitis K, Canpolat N, Ağbaş A, Aitkenhead H, Anarat A, Aoun B, Aofolaju D, Bakkaloglu SA, Bhowruth D, Borzych-Dużałka D, Bulut IK, Büscher R, Deanfield J, Dempster C, Duzova A, Habbig S, Hayes W, Hegde S, Krid S, Licht C, Litwin M, Mayes M, Mir S, Nemec R, Obrycki L, Paglialonga F, Picca S, Samaille C, Shenoy M, Sinha MD, Spasojevic B, Stronach L, Vidal E, Vondrák K, Yilmaz A, Zaloszyc A, Fischbach M, Schmitt CP, Schaefer F (2019) Effects of hemodiafiltration versus conventional hemodialysis in children with ESKD: the HDF, heart and height study. J Am Soc Nephrol 30:678–691. https://doi.org/10.1681/asn.2018100990
Raina R, Vijayaraghavan P, Kapur G, Sethi SK, Krishnappa V, Kumar D, Bunchman TE, Bolen SD, Chand D (2018) Hemodialysis in neonates and infants: a systematic review. Semin Dial 31:289–299. https://doi.org/10.1111/sdi.12657
Müller D, Zimmering M, Chan CT, McFarlane PA, Pierratos A, Querfeld U (2008) Intensified hemodialysis regimens: neglected treatment options for children and adolescents. Pediatr Nephrol 23:1729–1736. https://doi.org/10.1007/s00467-008-0783-4
Fischbach M, Fothergill H, Zaloszyc A, Menouer S, Terzic J (2011) Intensified daily dialysis: the best chronic dialysis option for children? Semin Dial 24:640–644. https://doi.org/10.1111/j.1525-139X.2011.01020.x
Chand DH, Swartz S, Tuchman S, Valentini RP, Somers MJG (2017) Dialysis in children and adolescents: the pediatric nephrology perspective. Am J Kidney Dis 69:278–286. https://doi.org/10.1053/j.ajkd.2016.09.023
US Renal Data System (2018) 2018 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD
US Renal Data System (2019) US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD
North American Pediatric Renal Trials and Collaborative Studies (2011) NAPRTCS 2011 annual dialysis report. In: North Am. Pediatr. Ren. Trials Collab. Stud. https://naprtcs.org/system/files/2011_Annual_Dialysis_Report.pdf
McTaggart S, Kennedy S, McDonald S, Henning P, Dent H (2009) Paediatric report - ANZDATA Registry 2009 report. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, South Australia
ERA-EDTA Registry (2010) ERA-EDTA registry annual report 2008. Academic Medical Center, Department of Medical Informatics, Amsterdam, The Netherlands
Plumb L, Casula A, Pyart R, Evans KM, Inward C, Medcalf J, Marks SD (2020) The 21st UK Renal Registry annual report: a summary of analyses of paediatric data in 2017. Nephron 144:67–71. https://doi.org/10.1159/000504852
Ardissino G, Daccò V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, Marra G, Edefonti A, Sereni F (2003) Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics 111:e382–e387. https://doi.org/10.1542/peds.111.4.e382
Mong Hiep TT, Ismaili K, Collart F, Van Damme-Lombaerts R, Godefroid N, Ghuysen MS, Van Hoeck K, Raes A, Janssen F, Robert A (2010) Clinical characteristics and outcomes of children with stage 3-5 chronic kidney disease. Pediatr Nephrol 25:935–940. https://doi.org/10.1007/s00467-009-1424-2
Chanchlani R, Nash DM, McArthur E, Zappitelli M, Archer V, Kuwornu JP, Garg AX, Greenberg JH, Goldstein SL, Thabane L, Wald R (2019) Secular trends in incidence, modality, and mortality with dialysis receiving AKI in children in Ontario a population-based cohort study. Clin J Am Soc Nephrol 14:1288–1296. https://doi.org/10.2215/CJN.08250718
Chand DH, Valentini RP (2008) International pediatric fistula first initiative: a call to action. Am J Kidney Dis 51:1016–1024. https://doi.org/10.1053/j.ajkd.2008.02.309
Guzzo I, de Galasso L, Mir S, Bulut IK, Jankauskiene A, Burokiene V, Cvetkovic M, Kostic M, Bayazit AK, Yildizdas D, Schmitt CP, Paglialonga F, Montini G, Yilmaz E, Oh J, Weber L, Taylan C, Hayes W, Shroff R, Vidal E, Murer L, Mencarelli F, Pasini A, Teixeira A, Afonso AC, Drozdz D, Schaefer F, Picca S (2019) Acute dialysis in children: results of a European survey. J Nephrol 32:445–451. https://doi.org/10.1007/s40620-019-00606-1
Palmer S, Rabindranath K, Craig J, Roderick P, Locatelli F, Strippoli G (2012) High-flux versus low-flux haemodialysis membranes for end-stage kidney disease. Cochrane Database Syst Rev:1–102. https://doi.org/10.1002/14651858.CD005016.pub2
Raina R, Krishnappa V (2019) Hemodialysis treatment prescription. In: Critical care pediatric nephrology and dialysis: a practical handbook. Springer, Singapore, pp 95–106
Sethi SK, Bunchman T, Raina R, Kher V (2014) Unique considerations in renal replacement therapy in children: core curriculum 2014. Am J Kidney Dis 63:329–345. https://doi.org/10.1053/j.ajkd.2013.08.018
Basile C, Rossi L, Lomonte C (2016) The choice of dialysate bicarbonate: do different concentrations make a difference? Kidney Int 89:1008–1015. https://doi.org/10.1016/j.kint.2016.01.010
Hayes W, Hothi DK (2011) Intradialytic hypotension. Pediatr Nephrol 26:867–879. https://doi.org/10.1007/s00467-010-1661-4
Mustafa RA, Bdair F, Akl EA, Garg AX, Thiessen-Philbrook H, Salameh H, Kisra S, Nesrallah G, Al-Jaishi A, Patel P, Patel P, Mustafa AA, Schünemann HJ (2016) Effect of lowering the dialysate temperature in chronic hemodialysis: a systematic review and meta-analysis. Clin J Am Soc Nephrol 11:442–457. https://doi.org/10.2215/CJN.04580415
National Kidney Foundation (2006) KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis 48(suppl 1):S1–S322. https://doi.org/10.1053/j.ajkd.2006.04.040
Gabutti L, Salvadé I, Lucchini B, Soldini D, Burnier M (2011) Haemodynamic consequences of changing potassium concentrations in haemodialysis fluids. BMC Nephrol 12:14. https://doi.org/10.1186/1471-2369-12-14
Acknowledgments
Work of CY was supported by the Michael G. DeGroote School of Medicine–McMaster Medical Student Research Excellence Awards.
Author information
Authors and Affiliations
Contributions
All authors contributed to this manuscript and approved the final version. S. Sanger performed database search; C. Y. and A. F. independently reviewed literature and completed data extraction with assistance from R. Chanchlani. C.Y. and A.F. drafted the initial manuscript; A. T. completed statistical analysis and figure preparation. R. R., R. Chanchlani, R. Chakraborty, S. Sethi, and A. T. provided data interpretation and analysis, figure preparations, manuscript revisions, and critical feedback.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 381 kb)
Rights and permissions
About this article
Cite this article
Chanchlani, R., Young, C., Farooq, A. et al. Evolution and change in paradigm of hemodialysis in children: a systematic review. Pediatr Nephrol 36, 1255–1271 (2021). https://doi.org/10.1007/s00467-020-04821-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04821-y