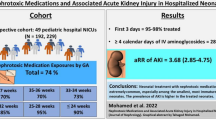
Abstract
Neonatal acute kidney injury (AKI) is common. Critically ill neonates are at risk for AKI for many reasons including the severity of their underlying illnesses, prematurity, and nephrotoxic medications. In this educational review, we highlight four clinical scenarios in which both the illness itself and the medications indicated for their treatment are risk factors for AKI: sepsis, perinatal asphyxia, patent ductus arteriosus, and necrotizing enterocolitis. We review the available evidence regarding medications commonly used in the neonatal period with known nephrotoxic potential, including gentamicin, acyclovir, indomethacin, vancomycin, piperacillin–tazobactam, and amphotericin. We aim to illustrate the complexity of decision-making involved for both neonatologists and pediatric nephrologists when managing infants with these conditions and advocate for ongoing multidisciplinary collaboration in the development of better AKI surveillance protocols and AKI mitigation strategies to improve care for these vulnerable patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Critically ill neonates represent a high-risk population with morbidity and mortality resulting from a number of underlying conditions such as prematurity, sepsis, perinatal asphyxia, patent ductus arteriosus (PDA), and necrotizing enterocolitis (NEC). Unfortunately, acute kidney injury (AKI) also occurs commonly in these patients, increasing their morbidity and mortality [1,2,3,4,5]. Many of the medications commonly used to treat these underlying conditions have nephrotoxic potential. Two recent studies have shown that > 80% of premature infants receive at least one nephrotoxic medication during their hospital stay [6, 7]. Whether or not risk of AKI is related to the underlying condition itself, nephrotoxic medications, or a combination of both, is unclear; how to balance the risks and benefits of nephrotoxic medications and non-nephrotoxic alternatives continues to be a significant challenge for the teams caring for these fragile patients. Given the widespread use of nephrotoxic medications and the fact that we have no treatment for AKI once it has occurred, understanding the mechanism of injury from both the underlying condition and the medications is critical for developing better AKI monitoring and mitigation strategies.
Here we present four clinical vignettes highlighting situations where infants are at particularly high risk for AKI and in which potentially nephrotoxic medications are indicated. We will aim to describe the risk of AKI from both the underlying condition and the associated nephrotoxic exposures, while also exploring the risk and benefits of using non-nephrotoxic alternatives. Finally, we will discuss AKI mitigation strategies and opportunities for multidisciplinary collaboration.
Neonatal early-onset sepsis: nephrotoxic antimicrobials
Baby A is a 5-day-old term infant presenting to the emergency department (ED) with fever at 102 °F. The infant was born via spontaneous vaginal delivery and discharged on postnatal day 2. Initially, the infant ate well but in the past day has had progressively poor oral intake with no wet diapers in the last 12 h. The patient’s mother’s prenatal labs were all within normal limits. Notably, a group B Streptococcus (GBS) screen was negative. There was a remote history of herpes simplex virus (HSV); however, the infant’s mother was compliant with prophylactic acyclovir, and there were no concerns for active infection or genital lesions at that time of delivery. In the ED, a sepsis evaluation is begun. Blood, urine, and cerebral spinal fluid (CSF) are collected for culture and HSV polymerase chain reaction testing. The infant is started on ampicillin, gentamicin, and acyclovir before admission to the neonatal intensive care unit (NICU).
The prevalence of bacteremia and meningitis among febrile infants 28 days of age or less is high, with observational studies showing a rate of meningitis of 0.3–3%, bacteremia or sepsis of 1–5%, and urinary tract infection (UTI) of 16–28% [8]. With such a high risk of infection, the standard of care includes aggressive treatment with empiric antibiotics, antiviral therapy when indicated, and admission to the hospital [9]. The recommended antimicrobial regimen for febrile neonates is ampicillin and gentamicin to provide coverage against the organisms most commonly responsible for early-onset neonatal sepsis (GBS, Escherichia coli, Listeria monocytogenes) with the addition of coverage for HSV when appropriate. Ampicillin and gentamicin are the two most commonly prescribed medications in the NICU [10].
Gentamicin, an aminoglycoside, works synergistically with ampicillin, binding the 30S ribosomal subunit and augmenting ampicillin binding of penicillin-binding proteins to inhibit bacterial cell wall synthesis (Table 1). Gentamicin is a well-recognized nephrotoxin. Because aminoglycosides (including gentamicin) are excreted nearly exclusively in the urine, high concentrations can occur in the renal cortex leading to proximal tubular damage [11]. This tubular damage is attributed to the binding affinity of aminoglycosides for the proximal tubular brush border membrane via the megalin complex. Through this binding, there is an accumulation of lipid within the proximal tubular cell lysosomes leading to renal damage. Gentamicin-associated AKI thus manifests as a tubulopathy with electrolyte wasting but often little change in urine output, making it a challenge to diagnose in neonates, especially if electrolytes and creatinine are not being regularly monitored.
Alternative, less nephrotoxic antibiotic regimens for empiric coverage of newborns, particularly those with kidney disease or at high risk for AKI, have been suggested. Cephalosporins are less nephrotoxic and have been utilized in some centers individually or in combination with other antibiotics, particularly when meningitis is a consideration. Cephalosporins often have improved CSF penetration compared to gentamicin. However, cephalosporins do not provide the most appropriate coverage. In a study comparing empiric antibiotic regimens in infants 60 days of age and younger with culture-positive infections, third generation cephalosporin use was unnecessarily broad (i.e., antimicrobial coverage for organisms not typically implicated in neonatal sepsis) for ~ 84% of patients and less effective than ampicillin and gentamicin or the combination of ampicillin and third generation cephalosporin [12, 13]. Furthermore, in a study of nearly 130,000 infants, empiric use of ampicillin and cefotaxime was associated with increased mortality (aOR 1.5, 95% CI 1.4–1.7) [14]. Across all gestational ages, infants who received empiric ampicillin with cefotaxime were more likely to die and less likely to be discharged than those who received empiric ampicillin and gentamicin. As a result, ampicillin and gentamicin remain standard of care for empiric antibiotic coverage for early sepsis evaluations in neonates in the USA and in many parts of the world [15, 16]. Since these patients are already at high risk for AKI due to their underlying sepsis, close kidney function and gentamicin level monitoring are critical for mitigating additional medication nephrotoxicity. Data regarding the long-term risks of the use of gentamicin, particularly in premature infants, is needed to guide both short-term clinical decision-making and long-term follow-up assessments.
The addition of acyclovir is indicated in this age group if there is clinical concern for HSV (history, ill-appearance, mucocutaneous vesicles, seizures or other focal neurologic signs, thrombocytopenia, elevated liver transaminases or liver failure, or CSF pleocytosis) [17]. Acyclovir is a specific inhibitor of herpesvirus DNA polymerase (Table 1). Nephrotoxicity occurs as the result of crystallization of the drug in the renal tubules causing obstruction and tubular damage and is more likely to occur in patients with impaired renal function, concurrent nephrotoxic exposures, or reduced intravascular volume [18]. To reduce or prevent nephrotoxicity, clinicians should ensure adequate hydration and even consider aggressive hydration regimens with close monitoring for signs of fluid overload (daily weights, intake/output). Additional measures to prevent nephrotoxicity include the following: adjust dosage for estimated glomerular filtration rate (GFR), decrease acyclovir administration rate to 1–2 h, and closely monitor renal function. Additionally, when possible, antimicrobial therapy should be narrowed as soon as an organism is isolated to decrease concurrent nephrotoxic medication exposure.
Perinatal asphyxia and hypoxic ischemic encephalopathy: inherent risk for AKI compounded by therapies
Baby A is a 24-h-old full-term female delivered via urgent Cesarean section for decreased fetal movement and persistent fetal bradycardia. At delivery, the baby was limp and pale with a heart rate of 72 beats per minute and no spontaneous respirations or movement. Despite positive pressure ventilation, she remained apneic at 4 min of life and was intubated. Apgar scores were 1, 4, and 5 at 1, 5, and 10 min, respectively. Physical examination revealed grossly abnormal neurologic findings. An umbilical cord arterial blood gas read 6.89/70/80/9/− 16. Perinatal asphyxia and hypoxic ischemic encephalopathy (HIE) was suspected and therapeutic hypothermia initiated.
Neonatal HIE results from in utero or perinatal asphyxia, a period of inadequate fetal and/or neonatal cerebral blood flow and oxygen delivery. HIE occurs in 1–8/1000 live births in developed countries but is substantially more common in underdeveloped parts of the world [19]. Long-term, significant neurodevelopmental impairment or even death can occur in the setting of neonatal HIE [20]. Therapeutic hypothermia, the standard of care in developed countries, has resulted in modest neuroprotection leading to improvements in survival and neurodevelopmental outcomes [21,22,23].
Neonates with HIE are predisposed to multi-organ dysfunction, including a predisposition to AKI, with an incidence ranging from 30 to 70% [1, 24,25,26,27,28,29,30]. With the initial insult, the kidneys may be deprived of adequate blood flow and/or oxygen delivery. The renal parenchyma has limited capacity for anaerobic metabolism, and injury can result from this initial offense [24, 31]. The kidneys, like the brain and other organ systems, are also at risk for reperfusion injury when a more normal circulatory pattern is established [31]. AKI in the setting of HIE is associated with adverse outcomes including increased mortality rates, length of stay, and length of mechanical ventilation, as well as poor neurologic outcomes [1, 25, 32,33,34]. In a retrospective review of 96 infants with HIE treated with therapeutic hypothermia, Selewski et al. noted AKI in 38% of affected infants [1]. While the rates of AKI in this group were high, they were lower than previously reported AKI rates in infants with HIE who did not undergo cooling, suggesting therapeutic hypothermia may be protective not only to the brain but possibly the kidneys as well. In the largest neonatal AKI cohort to date, 113 patients were noted to have HIE, and 42% of those developed AKI [33].
The etiology of the hypoxic/ischemic insult is not always apparent at delivery, but sepsis must always be considered in the differential diagnosis as sepsis can co-exist with HIE. Antibiotic administration is common, occurring in up to 100% of cases, with rates of gentamicin exposure greater than 90% in some series [1]. The decision to utilize antibiotics as a consistent practice in newborns with HIE varies from center to center with little supporting evidence for regular utilization. As with other neonates receiving antibiotics during early-onset sepsis evaluations, the standard antibiotic regimen in newborns with HIE is ampicillin and gentamicin. This nephrotoxic gentamicin exposure can compound the risk for AKI in infants with HIE. Careful consideration must be taken for these infants, weighing the risks of untreated sepsis against the risk for further renal injury in a high-risk newborn.
There are several important considerations for providers when prescribing gentamicin to infants with HIE. Gentamicin kinetics are altered in specific neonatal populations, including those with HIE [1, 35,36,37,38]. In a retrospective review of neonates with HIE treated with gentamicin, 56% of those treated and 72% of those with AKI had elevated gentamicin levels [1]. Extending the gentamicin dosing interval from multiple doses per day to doses every 24 or even every 36 h in infants with HIE is a consideration [36, 39]. These pharmacokinetic changes can be further complicated by therapeutic hypothermia, as those treated with therapeutic hypothermia have decreased gentamicin clearance [35, 37, 40]. Careful monitoring of gentamicin levels and kidney function with standardized protocols and clinical pharmacist involvement is paramount in such high-risk populations. Studies of alternative, less nephrotoxic antibiotic strategies for infants with HIE have not been conducted but are needed.
The use of newer technologies and biomarkers to evaluate for evidence of AKI earlier in the course would help with the risk–benefit assessment of nephrotoxin use in high-risk populations, such as neonates with HIE. Our current kidney function biomarker, serum creatinine (SCr), may be difficult to interpret in the first 48–72 h of life when decisions about HIE management need to be made. Detecting AKI in patients with HIE may be aided by the use of renal near-infrared spectroscopy or novel biomarkers. When renal oxygen saturation measures increase, this may reflect lower oxygen extraction by an injured kidney and help lead to the early diagnosis of AKI. In a small study of 38 infants undergoing therapeutic hypothermia for HIE, renal saturations were higher in neonates with AKI compared to those without AKI after 24 h of life [41]. Biomarkers such as neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 have shown promise in detecting AKI and may have a role in HIE as well [42].
Patent ductus arteriosus: nonsteroidal anti-inflammatories
Case A
Baby A is a 36-h-old ex-26 weeks of gestation male born with a birthweight of 925 g following preterm induction of labor for severe maternal pre-eclampsia. He remains on continuous positive airway pressure following a single dose of surfactant in the delivery room. Per institutional policy, he is receiving caffeine therapy for apnea of prematurity and is scheduled to receive indomethacin for PDA prophylaxis (three doses). However, following the second indomethacin dose, the infant produces < 0.5 ml/kg/h of urine for 6 h, and his SCr rises from 0.6 to 1.1 mg/dl. The third indomethacin dose is held.
Case B
Baby B is a 12-day-old ex-28 weeks of gestation male who, despite attempts at extubation, continues to require mechanical ventilation. Serial chest radiographs demonstrate worsening pulmonary edema, and a loud, machinery-style murmur is noted on cardiac auscultation. Echocardiography demonstrates a large PDA with left to right shunting, a dilated left atrium, and reversal of flow in the renal arteries. The medical team decides to attempt medical PDA closure.
The ductus arteriosus represents a critical component to fetal circulation in utero. It serves to connect the main pulmonary artery to the descending aorta, which allows blood from the right ventricle to bypass the fetal lungs. In utero, the patency of the ductus arteriosus is maintained through a variety of mechanisms including high circulating levels of prostaglandin E2 (PGE2) [43]. PGE2 promotes smooth muscle relaxation, which in turn promotes ductal patency in the newborn. Under normal physiologic conditions, the PDA closes soon after birth as a result of rapidly increasing arterial oxygen tension and decreasing PGE2 concentrations [43]. Medical management strategies for the PDA are predicated on strategies that lower levels of PGE2 (nonsteroidal anti-inflammatory drugs, NSAIDs).
The optimal management of the PDA remains a source of practice variation and a contentious issue in neonatology [44]. The PDA has been associated with adverse outcomes including bronchopulmonary dysplasia, pulmonary hemorrhage, NEC, intraventricular hemorrhage (IVH), poor neurodevelopmental outcomes, and AKI [43]. Despite this, studies evaluating the impact of PDA closure fail to demonstrate long-term improvements in many of these associated outcomes [45]. Nevertheless, medical and surgical PDA closure are common in many neonatal populations [46]. Currently, strategies to medically manage PDAs in premature infants include either PDA prevention (i.e., prophylaxis) or medical treatment of persistent PDA with NSAIDs. Surgical interventions include either PDA ligation or device closure.
Unfortunately, though moderately effective in achieving PDA closure, NSAIDs are known to cause nephrotoxicity (Table 1) [47]. NSAID administration causes inhibition of the enzyme cyclooxygenase (COX; also known as prostaglandin (PG) H synthase), suppressing production of homeostatic PGs (including PGI2, PGE2, and PGD2) which under normal circumstances lower vascular resistance and dilate renal vascular beds. With suppressed PG levels, renal vascular tone is altered leading to a redistribution of blood flow away from the nephrons and, in some circumstances, medullary ischemia and/or AKI. This process can be exacerbated by activation of renin–angiotensin–aldosterone axis and subsequent increased vasoconstriction through elevated angiotensin II levels and sympathetic stimulation, particularly in at-risk patients with baseline decreased renal function. In clinical situations where decreased renal blood flow already exists, such as in newborns, NSAID-related COX inhibition leads to a blunting of the PG-mediated associated renal vasodilation. In case A, the newborn premature infant has experienced typical postnatal renal adaptation, which includes the slow increase in renal blood flow and subsequent increase in GFR. This state of relative low blood flow to the kidneys increases the risk of AKI secondary to administration of indomethacin.
PDA prophylaxis, or early treatment to close the PDA soon after birth, provides the potential benefit of decreased symptomatic PDA, need for surgical ligation, and decreased incidence of comorbidities. Despite this, the long-term benefits have not been established. Chief among these concerns around the use of prophylaxis is that a protocolized PDA prophylaxis strategy will result in a large number of neonates being exposed to an unnecessary nephrotoxic medication; in the largest randomized controlled trial of indomethacin PDA prophylaxis, ~ 50% of neonates (n = 300/601) in the placebo arm never developed a PDA [48]. In a study of infants < 30 weeks of gestation who received indomethacin to close the PDA, 24% had acute renal failure (defined in this study by a rise in creatinine of > 25%) [49]. Additionally, the premature infants who typically receive PDA prophylaxis (often, very low birth weight (VLBW) infants) are some of the most vulnerable to neonatal AKI. In a study of neonates with PDA receiving gentamicin, NSAID therapy (indomethacin or ibuprofen) was shown to increase the risk of AKI by ~ 6% [50]. These competing risks have to be weighed against the fact that a hemodynamically significant PDA itself predisposes patients to AKI. In a study of infants < 28 weeks of gestation, the moderate-to-large PDA itself was strongly associated with all stages of AKI [51]. Currently, indomethacin (typical prophylactic dose 0.1 mg/kg every 24 h for three doses) is the drug of choice for PDA prophylaxis [45].
The utility of medical PDA treatment with NSAIDs, like that of prophylaxis, is a topic of much debate in the neonatal literature. Medical management is an attractive option to avoid surgical PDA closure which was, at one time, common; however, again some argue this approach may be unnecessary. Proponents of early medical therapy to close the PDA argue this strategy may prevent the PDA from becoming potentially hemodynamically significant later in the course. Others argue that targeted treatment of PDA or treatment of only the PDAs that providers anticipate may become hemodynamically significant in the future is best; scoring systems using clinical signs and symptoms and/or echocardiographic features of the PDA (i.e., PDA diameter, maximum flow velocity across PDA) to predict future hemodynamic significance, the occurrence of chronic lung disease, or death can be found in the literature [52, 53]. Yet conservative management strategies with a “watch and wait” approach are also regularly applied. In the recent PDA TOLERATE study, the authors found that early, targeted treatment of the moderate-to-large PDA in very preterm infants in the first 2 weeks of life did not reduce PDA ligations or presence of PDA at discharge, but did delay achievement of full feedings and increased the risk of late-onset sepsis and death [54].
In considering case B, this infant has developed signs of a hemodynamically significant PDA (hs-PDA). In cases such as this, providers must decide between attempting medical management (indomethacin, ibuprofen, or more recently acetaminophen) or careful monitoring. Indomethacin is the oldest and most studied PDA treatment. In a study of 421 premature infants with hs-PDAs, Gersony et al. found that administering indomethacin to infants with hs-PDAs results in a closure rate that is two times higher than patients without indomethacin treatment (p < 0.001) [55]. More recently, ibuprofen and acetaminophen have demonstrated similar efficacy to indomethacin with reduced risk of gastrointestinal and renal complications; acetaminophen appears to be the least nephrotoxic [56, 57]. Despite these benefits, ibuprofen and acetaminophen are not associated with the same reduction in severe IVH attributable to indomethacin [58, 59]. However, it must be acknowledged that at the time studies examining indomethacin and subsequent IVH were performed, interventions that are now common place and known to be associated with decreased rates of IVH, such as antenatal steroid exposure, were not yet widely established and further study is greatly needed [48]. An important consideration in treatment strategies is that NSAIDs appear to be less effective in achieving PDA closure as infants move beyond the newborn period [60].
Other therapeutics for PDA, including changes in fluid management and diuresis, dopaminergic agents, and surgical correction, have been utilized. Increased fluid volumes have been shown to be associated with increased incidence of PDA in premature infants [61]. Thus, restriction of fluid volumes or diuretic medication administration has been hypothesized to alter the incidence of PDA. Through activation of dopaminergic receptors in the renal vasculature, dopamine has been hypothesized to counteract the renal vasoconstriction associated with NSAIDs. In Cochrane reviews, none of these strategies in indomethacin-treated preterm infants have been shown to prevent AKI [62,63,64].
Surgical options, including device closure or PDA ligation, can be considered, particularly in older infants who have failed medical PDA management, but little is known about the associated renal impacts, and significant concern for death or neurodevelopmental impairment remains.
Regardless of the method used, few long-term benefits have been attributed to closure of the PDA. The American Academy of Pediatrics Committee on Fetus and Newborn Clinical Report does not support early, routine treatment to induce PDA closure in premature infants in the first 2 weeks of life [65]. Instead, this report suggests that there may be a role for more selective use of medical methods for induction of ductal closure for defined high-risk infants in the first 2 weeks of life or older infants with persistent PDAs, but this area requires further study. Questions remain about the best strategies to address the PDA in the premature infant. Studies to determine how AKI contributes to mortality rates among preterm infants exposed to differing PDA treatment regimens are needed.
Necrotizing enterocolitis: nephrotoxic antimicrobials used in combination
Baby A is a 20-day-old ex-24 weeks of gestational age infant who is receiving vancomycin, gentamicin, and piperacillin/tazobactam (PTZ, day 7 of a planned 14-day course) for NEC with intestinal perforation; the infant has required percutaneous abdominal drain placement. The infant acutely decompensates on day 7 of antibiotic treatment, requiring increased respiratory support with worsening hyperglycemia and new thrombocytopenia. Repeat blood and urine cultures are obtained, and due to concern for possible development of fungemia, the infant is started on amphotericin.
The above scenario illustrates an infant at significant risk to develop AKI as the result of multiple concomitant risk factors including extreme prematurity (incomplete nephrogenesis), sepsis, hypotension, and multiple nephrotoxic medications. AKI occurs in greater than 50% of neonates studied with NEC and is associated with significantly increased mortality [3].
In many clinical scenarios such as this one, infants receive concurrent nephrotoxic medications such as gentamicin, vancomycin, and PTZ for broad-spectrum antibiotic coverage for late-onset sepsis, especially when there is concern for NEC. In the neonatal population, the mortality rate attributable to NEC ranges from 20 to 30% and accounts for 10% of deaths in NICUs [66]. Additionally, 20–30% of neonates with NEC have concomitant bacteremia [67]. Empiric antimicrobial regimens must be broad to provide coverage for common pathogens that cause late-onset bacteremia as well as anaerobic coverage for intestinal perforation [68]. However, though broad-spectrum antimicrobial coverage is common in the setting of NEC, there is no consensus regarding the appropriate regimen. The choice of antimicrobials is typically guided by local pathogen identification and susceptibility patterns. Additionally, although 70–80% of neonates with NEC have negative blood cultures, the consensus guidelines support broad-spectrum antibiotic receipt for a total of 10–14 days [69].
The nephrotoxic burden in the above scenario is not only limited to drug choice. The risk also lies in the combination of drugs prescribed. The combination of vancomycin and PTZ is indicated when coverage for both methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa is needed. Nephrotoxicity from vancomycin alone has been documented in the literature (Table 1). In a study by McKamy et al., 14% of 167 pediatric patients receiving vancomycin developed nephrotoxicity, with higher risk noted when vancomycin was given in conjunction with other nephrotoxins and diuretics [70]. Moreover, the combination of vancomycin and PTZ has been associated with higher rates of AKI in adult patients [71]. The mechanism of nephrotoxicity-related to PTZ combined with vancomycin is not understood. Piperacillin inhibits tubular secretion and clearance (Table 1). It is hypothesized that an interaction between piperacillin and vancomycin may contribute to toxicity in the proximal tubule cells. In children aged 6 months to 18 years old, Downes et al. found that the incidence of AKI was > 10% in the group of patients who received the combination of vancomycin and PTZ [72]. This is an area where greater study is needed, particularly in neonates. In the meantime, a plan for daily monitoring of kidney function, careful dosage adjustment for GFR in these patients when AKI occurs, and measurement of vancomycin troughs should be agreed upon by the neonatologist, nephrologist, and pharmacist, in order to mitigate renal injury.
The risk of fungal infections and their association with significant morbidity and mortality further complicates this scenario. The risk is particularly high for VLBW infants who may be more vulnerable due to their immature immune systems and poorly developed epithelial and mucosal barriers. Invasive procedures such as abdominal drain placement, central venous catheter placement, and intubation, all of which compromise skin integrity and therefore host defenses, add additional risk factors in this population [73]. Moreover, the prolonged use of broad-spectrum antibiotics is an important risk factor for invasive Candida infection.
Amphotericin B is used as empiric therapy for neonatal fungal infections (Table 1) [18, 74, 75]. The nephrotoxicity from amphotericin B is due to a combination of direct distal tubular toxicity and vasoconstriction [18, 76]. Nephrotoxicity results in hypokalemia, hyponatremia, acidosis, and hypomagnesemia and may be associated with significant morbidity. Because of this adverse side-effect profile, fluconazole is often considered a reasonable alternative to amphotericin B as long as it was not previously used for fungal prophylaxis [77]. Benefits of fluconazole include its less nephrotoxic profile as well as good oral bioavailability. Similarly, liposomal formulations of amphotericin may be preferred because of the less nephrotoxic side-effect profile. However, some studies suggest that survival rates are better when amphotericin B compared to liposomal formulations is used [78]. When considering liposomal formulations, consider current renal function and concern for systemic infections versus UTI. Liposomal formulations may be less effective for UTI because of decreased penetration of the drug in the kidneys [77]. Decreased kidney penetrance allows for the decreased rate of nephrotoxicity of liposomal formulations of amphotericin, though nephrotoxicity may still occur at varying rates 0–20% [79,80,81,82]. The risk factors for nephrotoxicity are similar to those in other drugs, including longer treatment duration, concurrent nephrotoxic medication exposure, and severity of illness [83].
Potential nephroprotective agents: caffeine and theophylline
Two medications, theophylline and caffeine, show promise in their ability to prevent or ameliorate AKI in certain neonatal populations (Table 2). Caffeine is frequently utilized prophylactically in premature neonates to prevent apnea of prematurity. Caffeine is an adenosine receptor antagonist that inhibits the preglomerular vasoconstrictive effects of adenosine and is theorized to be nephroprotective. Recent work has shown that caffeine exposure in the first week of life is associated with less AKI in VLBW infants. In a study of 140 VLBW infants, AKI occurred less frequently in neonates exposed to caffeine than those who did not receive caffeine (all patients 17.8 vs 43.6%, p = 0.002; patients requiring prolonged invasive respiratory support 29.2 vs 75%, p = 0.002) [84]. These findings were replicated by Harer et al. in a secondary analysis of 675 neonates, < 33 weeks of gestation in the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study [85]. In this study, neonates exposed to caffeine within the first week of life were less likely to develop AKI (11.2 vs 31.6%, p < 0.01; aOR 0.20, 95% CI 0.11–0.34). Furthermore, among those neonates who developed early AKI, those who received caffeine were less likely to develop more severe stage 2 or 3 AKI (aOR 0.20, 95% CI 0.12–0.34).
Four randomized controlled trials of neonates with perinatal asphyxia have shown benefit from a single dose of theophylline [26,27,28,29, 86]. Theophylline, like caffeine, is a nonselective adenosine receptor antagonist. Theophylline inhibits adenosine-induced vasoconstriction preventing the development of AKI and oliguria following perinatal asphyxia. In a systematic review and meta-analysis (4 trials, n = 197 patients), prophylactic theophylline receipt was associated with less severe renal dysfunction compared to placebo (pooled relative risk 0.38, 95% CI 0.25–0.57; p < 0.001) [87]. Of note, the current studies are limited in that they did not include any populations that received therapeutic hypothermia, which has become the standard of care where available and, as mentioned above, may be renal-protective. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines currently recommend a single dose of theophylline in “neonates with severe perinatal asphyxia who are at high risk of AKI” [88]. However, there remain concerns about the side-effect profile of theophylline (convulsions, gastrointestinal complications, arrhythmia), which limits broad adoption. Larger randomized trials to assess the long-term neurodevelopmental and renal outcomes as well as the effect of theophylline in conjunction with therapeutic hypothermia are needed before widespread adoption of this therapy to prevent AKI in this population, particularly given that studies of aminophylline, a drug with a similar mechanism of action, have failed to prevent postsurgical renal dysfunction in pediatric patients with cardiac disease [89]. To our knowledge, there are no studies of caffeine use to prevent AKI in neonates with HIE.
Surveillance and prevention: strategies for improving the care of neonates at risk for AKI
Despite major advances in our understanding of the epidemiology and short-term outcomes associated with neonatal AKI, we still have no medical intervention for established AKI nor do we have information on the long-term impact of neonatal AKI on kidney function. Such information is needed and would aid clinical decision-making in challenging cases, such as the ones described above. Until we have better interventions or more data to guide risk–benefit analyses in management, the cornerstone of our current AKI mitigation strategies includes early recognition, surveillance, prevention of AKI where feasible, and accurate identification of at-risk infants for long-term follow-up. Nephrotoxic medication stewardship and targeted AKI surveillance in the highest risk populations thus represent two important opportunities to improve the care and outcomes of neonates at risk for AKI.
The monitoring of SCr is key to the reliable diagnosis and recognition of AKI. Despite this, there remains large practice variation in SCr monitoring in critically ill neonates across centers. The AWAKEN study found that a quarter of centers checked a median of only two SCrs during the entirety of a NICU admission [2]. The incidence of AKI was also significantly higher in centers that measured SCr often (median SCr > 5/infant) compared to centers that measured SCr less often (median SCr < 5/infant; 347/840 (41.3%) vs 258/182 (21.8%), p < 0.0001) suggesting AKI is being missed in some centers because SCr is not being measured. Regular monitoring of SCr in hospitalized infants is paramount, particularly those known to be at increased for AKI because of underlying disease and/or nephrotoxic exposure. Accurate identification of AKI episodes in the medical record will also facilitate appropriate long-term follow-up and our ability to determine what impact neonatal AKI may have on chronic kidney disease in older children and adults.
The contribution of urine output to the diagnosis of AKI in critically ill neonates warrants special discussion. Until recently, the contribution of urine output to the staged definitions of AKI in children and neonates has not been systematically studied. This has stemmed at least in part from the difficulty involved in measuring urine output with precision in neonates and the difficulty of obtaining this data from the electronic medical record. The AWAKEN study was the first multicenter study to study systematically the independent contribution of AKI defined by urine output [2]. This study clearly showed that urine output-based AKI definitions identify clinically relevant kidney injury that is associated with poor outcomes. While this study represents a critical first step, important questions remain about how best to use this biomarker, including the optimal urine output thresholds, duration of oliguria, and optimal urine surveillance protocols.
A recent survey of neonatologists and nephrologists highlighted differences between specialties regarding awareness of systematic AKI definitions and recognition of AKI when it occurs [90]. Neonatologists were less likely to use a categorical definition of neonatal AKI (p < 0.00001) than pediatric nephrologists and less likely to recognize and diagnose stage 1 AKI (p < 00001). This survey also identified differences across centers in the implementation of guidelines for SCr monitoring for neonates exposed to two of the most well-recognized nephrotoxins, gentamicin and indomethacin. Guidelines for SCr monitoring in patients receiving these medications were reported by only 34% (aminoglycosides) and 62% (indomethacin) of respondents [90]. These practice variations highlight an opportunity to improve our care of these infants: interdisciplinary teams of neonatologists, nephrologists, pharmacists, and nursing staff could develop kidney function monitoring protocols for patients receiving specific nephrotoxic medications, especially for those infants with clinical conditions that put them at highest risk for AKI (such as those described in the vignettes). These protocols could include identification of at-risk infants through documentation in the daily progress note, daily SCr monitoring, assessment of urine output, and daily monitoring of drug levels when possible. Such protocols would allow for better surveillance of the most at-risk populations without increasing the lab burden on the general NICU population, with the ultimate goal being mitigation of nephrotoxic medication-induced AKI. These infants could also be identified as those who would benefit from long-term follow-up.
Once protocols are developed, they should be regularly assessed for efficacy as well as consistency in implementation. Several systematic, quality improvement strategies for AKI surveillance have been developed for use in older children and adults. In 2013, Goldstein et al. published a report on an electronic medical record (EMR) surveillance system that identified patients at risk for nephrotoxic medication-induced AKI in the Nephrotoxic Injury Negated by Just-in-time Action (NINJA) study [91]. This system works by sending an EMR alert to the pharmacists to order daily SCr for any noncritically ill, hospitalized child receiving IV aminoglycoside for > 3 days or > 3 nephrotoxins simultaneously. This EMR-centric intervention to increase SCr surveillance reduced AKI intensity by 42%. This study showed that the rates of AKI could be improved simply by identifying patients at risk for nephrotoxic medication-induced AKI and led to the development of a sustainable quality improvement project that has decreased the rates of nephrotoxic medication-induced AKI. This system has been used in pediatric patients but has not yet been implemented in neonatal cohorts. More broadly, new consensus recommendations for improving the care of adult patients with or at risk for AKI through quality improvement programs have been published by the 22nd Acute Disease Quality conference group [92]. These guidelines describe AKI-related care processes with measurable quality indicators that can be implemented then assessed for efficacy. These indicators include the identification of at-risk populations at the time of hospital admission, early identification of AKI, “nephrotoxin stewardship,” and appropriate long-term follow-up. While these frameworks are written for adult populations, the concepts could easily be applied to neonatal cohorts. Neonates represent a high-risk population with serious conditions for which withholding potentially beneficial nephrotoxins may not be justified; thus, improving our surveillance represents a more attainable short-term strategy.
Conclusion
This review highlights the complexity of neonatal care with regard to nephrotoxic medication exposure: the high potential for AKI in commonly occurring clinical scenarios, the complication of need for nephrotoxic medication as part of the therapeutic plan, and the fact that non-nephrotoxic alternatives do not always offer more benefit than risk. However, in cases where a less nephrotoxic alternative exists and is equally effective, such as the case of ibuprofen and indomethacin for PDA closure, the less nephrotoxic medication should be strongly considered. There is ample opportunity for the neonatology, the pediatric nephrology, and the pharmacist communities to share current knowledge and develop research pathways and clinical protocols to improve the identification of risk factors for and, ultimately, prevention of AKI in critically ill neonates. Establishing AKI surveillance and mitigation protocols could help both the subspecialty teams as they weigh the risks and benefits of necessary nephrotoxic medication use and work in collaboration to improve outcomes for these patients.
Key summary points
-
1.
Critically ill neonates are at risk for neonatal acute kidney injury due to underlying illnesses such as sepsis, hypoxic ischemic encephalopathy, patent ductus arteriosus, and necrotizing enterocolitis, and often nephrotoxic medications are indicated for the treatment of these illnesses.
-
2.
Nephrotoxic medications are prevalent in the neonatal intensive care unit and often necessary as non-nephrotoxic alternatives do not always offer more benefit than risk.
-
3.
Multidisciplinary teams are necessary to monitor and care for infants at high risk for neonatal acute kidney injury and to develop protocols to improve the identification of and ultimately reduce and prevent acute kidney injury.
Multiple-choice study questions
-
1.
Manifestations of amphotericin nephrotoxicity include the following:
-
a)
Hyperkalemia, hyperphosphatemia, and acidosis
-
b)
Hypokalemia, hypomagnesemia, and acidosis
-
c)
Hypernatremia, hyperkalemia, and alkalosis
-
d)
Hyponatremia, hyperphosphatemia, and alkalosis
-
2.
All of the following organisms are common causes of early-onset neonatal sepsis except:
-
a)
Pseudomanas aeruginosa
-
b)
Listeria monocytogenes
-
c)
Group B Streptococcus
-
d)
Escherichia coli
-
3.
Strategies to minimize the potential nephrotoxic effects of acyclovir include (select all that apply):
-
a)
Ensure adequate hydration
-
b)
Monitor serum drug levels
-
c)
Discontinuation of the medication once the culture and sensitivity results identify an organism that requires treatment with a different agent
-
d)
Regular monitoring of serum creatinine levels
-
4.
In patients with hypoxic ischemic encephalopathy (HIE), theophylline may be nephron-protective through what mechanism?
-
a)
Nonselective adenosine receptor antagonism
-
b)
Nonselective adenosine receptor activation
-
c)
Afferent arteriole vasoconstriction
-
d)
Increased cardiac output
-
5.
Treatment of patent ductus arteriosus (PDA) with indomethacin or ibuprofen may achieve closure of the PDA through what mechanism?
-
a)
Efferent arteriole vasoconstriction
-
b)
Reduction in prostaglandin (PGE2) concentrations
-
c)
Increases in prostaglandin (PGE2) concentrations
-
d)
Nonselective adenosine receptor antagonism
References
Selewski DT, Jordan BK, Askenazi DJ, Dechert RE, Sarkar S (2013) Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J Pediatr 162:725–729.e721. https://doi.org/10.1016/j.jpeds.2012.10.002
Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, Chishti AS, Woroniecki R, Mammen C, Swanson JR, Sridhar S, Wong CS, Kupferman JC, Griffin RL, Askenazi DJ (2017) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1:184–194. https://doi.org/10.1016/s2352-4642(17)30069-x
Criss CN, Selewski DT, Sunkara B, Gish JS, Hsieh L, McLeod JS, Robertson JO, Matusko N, Gadepalli SK (2018) Acute kidney injury in necrotizing enterocolitis predicts mortality. Pediatr Nephrol 33:503–510. https://doi.org/10.1007/s00467-017-3809-y
Gadepalli SK, Selewski DT, Drongowski RA, Mychaliska GB (2011) Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg 46:630–635. https://doi.org/10.1016/j.jpedsurg.2010.11.031
Carmody JB, Swanson JR, Rhone ET, Charlton JR (2014) Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol 9:2036–2043. https://doi.org/10.2215/cjn.05190514
Rhone ET, Carmody JB, Swanson JR, Charlton JR (2014) Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med 27:1485–1490. https://doi.org/10.3109/14767058.2013.860522
Barhight M, Altaye M, Gist KM, Isemann B, Goldstein SL, Akinbi H (2017) Nephrotoxic medications and associated acute kidney injury in very low birth weight infants. J Clin Nephrol Res 4:1070
Greenhow TL, Hung YY, Pantell RH (2016) Management and outcomes of previously healthy, full-term, febrile infants ages 7 to 90 days. Pediatrics 138(6). https://doi.org/10.1542/peds.2016-0270
Powell EC, Mahajan PV, Roosevelt G, Hoyle JD Jr, Gattu R, Cruz AT, Rogers AJ, Atabaki SM, Jaffe DM, Casper TC, Ramilo O, Kuppermann N (2018) Epidemiology of bacteremia in febrile infants aged 60 days and younger. Ann Emerg Med 71:211–216. https://doi.org/10.1016/j.annemergmed.2017.07.488
Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK Jr, Smith PB (2014) Medication use in the neonatal intensive care unit. Am J Perinatol 31:811–821. https://doi.org/10.1055/s-0033-1361933
Kent A, Turner MA, Sharland M, Heath PT (2014) Aminoglycoside toxicity in neonates: something to worry about? Exper Rev Anti Infect Ther 12:319–331
Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C (2015) Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care 31:568–571. https://doi.org/10.1097/pec.0000000000000400
Sullins AK, Abdel-Rahman SM (2013) Pharmacokinetics of antibacterial agents in the CSF of children and adolescents. Paediatr Drugs 15:93–117. https://doi.org/10.1007/s40272-013-0017-5
Clark RH, Bloom BT, Spitzer AR, Gerstmann DR (2006) Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics 117:67–74. https://doi.org/10.1542/peds.2005-0179
Baker CJ, Byington CL, Polin RA (2011) Policy statement—recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics 128:611–616. https://doi.org/10.1542/peds.2011-1466
Pocket book of hospital care for children: guidelines for the management of common childhood illnesses (2013). Geneva, Switzerland
Caviness AC, Demmler GJ, Almendarez Y, Selwyn BJ (2008) The prevalence of neonatal herpes simplex virus infection compared with serious bacterial illness in hospitalized neonates. J Pediatr 153:164–169. https://doi.org/10.1016/j.jpeds.2008.02.031
Hanna MH, Askenazi DJ, Selewski DT (2016) Drug-induced acute kidney injury in neonates. Curr Opin Pediatr 28:180–187. https://doi.org/10.1097/mop.0000000000000311
Kurinczuk JJ, White-Koning M, Badawi N (2010) Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 86:329–338. https://doi.org/10.1016/j.earlhumdev.2010.05.010
Douglas-Escobar M, Weiss MD (2015) Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr 169:397–403. https://doi.org/10.1001/jamapediatrics.2014.3269
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ (2005) Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365:663–670. https://doi.org/10.1016/s0140-6736(05)17946-x
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH (2005) Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 353:1574–1584. https://doi.org/10.1056/NEJMcps050929
Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, Higgins RD (2012) Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 366:2085–2092. https://doi.org/10.1056/NEJMoa1112066
LaRosa DA, Ellery SJ, Walker DW, Dickinson H (2017) Understanding the full spectrum of organ injury following intrapartum asphyxia. Front Pediatr 5:16. https://doi.org/10.3389/fped.2017.00016
Sarkar S, Askenazi DJ, Jordan BK, Bhagat I, Bapuraj JR, Dechert RE, Selewski DT (2014) Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr Res 75:431–435. https://doi.org/10.1038/pr.2013.230
Jenik AG, Ceriani Cernadas JM, Gorenstein A, Ramirez JA, Vain N, Armadans M, Ferraris JR (2000) A randomized, double-blind, placebo-controlled trial of the effects of prophylactic theophylline on renal function in term neonates with perinatal asphyxia. Pediatrics 105:E45
Bakr AF (2005) Prophylactic theophylline to prevent renal dysfunction in newborns exposed to perinatal asphyxia—a study in a developing country. Pediatr Nephrol 20:1249–1252. https://doi.org/10.1007/s00467-005-1980-z
Eslami Z, Shajari A, Kheirandish M, Heidary A (2009) Theophylline for prevention of kidney dysfunction in neonates with severe asphyxia. Iran J Kidney Dis 3:222–226
Raina A, Pandita A, Harish R, Yachha M, Jamwal A (2016) Treating perinatal asphyxia with theophylline at birth helps to reduce the severity of renal dysfunction in term neonates. Acta Paediatr 105:e448–e451. https://doi.org/10.1111/apa.13469
Sarkar S, Barks JD, Bhagat I, Donn SM (2009) Effects of therapeutic hypothermia on multiorgan dysfunction in asphyxiated newborns: whole-body cooling versus selective head cooling. J Perinatol 29:558–563. https://doi.org/10.1038/jp.2009.37
Saikumar P, Venkatachalam MA (2003) Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol 23:511–521
Alaro D, Bashir A, Musoke R, Wanaiana L (2014) Prevalence and outcomes of acute kidney injury in term neonates with perinatal asphyxia. Afr Health Sci 14:682–688. https://doi.org/10.4314/ahs.v14i3.26
Kirkley MJ, Boohaker L, Griffin R, Soranno DE, Gien J, Askenazi D, Gist KM (2019) Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr Nephrol 34:169–176. https://doi.org/10.1007/s00467-018-4068-2
Hadzimuratovic E, Skrablin S, Hadzimuratovic A, Dinarevic SM (2014) Postasphyxial renal injury in newborns as a prognostic factor of neurological outcome. J Matern Fetal Neonatal Med 27:407–410. https://doi.org/10.3109/14767058.2013.818646
Choi DW, Park JH, Lee SY, An SH (2018) Effect of hypothermia treatment on gentamicin pharmacokinetics in neonates with hypoxic-ischaemic encephalopathy: a systematic review and meta-analysis. J Clin Pharm Ther 43:484–492. https://doi.org/10.1111/jcpt.12711
Bijleveld YA, de Haan TR, van der Lee HJ, Groenendaal F, Dijk PH, van Heijst A, de Jonge RC, Dijkman KP, van Straaten HL, Rijken M, Zonnenberg IA, Cools F, Zecic A, Nuytemans DH, van Kaam AH, Mathot RA (2016) Altered gentamicin pharmacokinetics in term neonates undergoing controlled hypothermia. Br J Clin Pharmacol 81:1067–1077. https://doi.org/10.1111/bcp.12883
Ting JY, Kwan E, McDougal A, Osiovich H (2015) Pharmacokinetics of gentamicin in newborns with moderate-to-severe hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia. Indian J Pediatr 82:119–125. https://doi.org/10.1007/s12098-014-1527-z
Cies JJ, Habib T, Bains V, Young M, Menkiti OR (2018) Population pharmacokinetics of gentamicin in neonates with hypoxemic-ischemic encephalopathy receiving controlled hypothermia. Pharmacotherapy 38:1120–1129. https://doi.org/10.1002/phar.2186
Rao SC, Srinivasjois R, Moon K (2016) One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev 12:Cd005091. https://doi.org/10.1002/14651858.CD005091.pub4
Frymoyer A, Meng L, Bonifacio SL, Verotta D, Guglielmo BJ (2013) Gentamicin pharmacokinetics and dosing in neonates with hypoxic ischemic encephalopathy receiving hypothermia. Pharmacotherapy 33:718–726. https://doi.org/10.1002/phar.1263
Chock VY, Frymoyer A, Yeh CG, Van Meurs KP (2018) Renal saturation and acute kidney injury in neonates with hypoxic ischemic encephalopathy undergoing therapeutic hypothermia. J Pediatr 200:232–239.e231. https://doi.org/10.1016/j.jpeds.2018.04.076
Sweetman DU, Molloy EJ (2013) Biomarkers of acute kidney injury in neonatal encephalopathy. Eur J Pediatr 172:305–316. https://doi.org/10.1007/s00431-012-1890-6
Benitz W (2015) Patent ductus arteriosus. Fanaroff and Martin’s neonatal-perinatal medicine, vol 10th. Elsevier Saunders, Philadelphia, PA
Gien J (2008) Controversies in the management of patent ductus arteriosus. NeoReviews 9:e477–e482. https://doi.org/10.1542/neo.9-10-e477
Fowlie PW, Davis PG, McGuire W (2010) Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev (7):Cd000174. https://doi.org/10.1002/14651858.CD000174.pub2
Slaughter JL, Reagan PB, Bapat RV, Newman TB, Klebanoff MA (2016) Nonsteroidal anti-inflammatory administration and patent ductus arteriosus ligation, a survey of practice preferences at US children’s hospitals. Eur J Pediatr 175:775–783. https://doi.org/10.1007/s00431-016-2705-y
Whelton A (1999) Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med 106:13s–24s
Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, Solimano A, Vincer M, Wright LL (2001) Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med 344:1966–1972. https://doi.org/10.1056/nejm200106283442602
Akima S, Kent A, Reynolds GJ, Gallagher M, Falk MC (2004) Indomethacin and renal impairment in neonates. Pediatr Nephrol 19:490–493. https://doi.org/10.1007/s00467-003-1402-z
Constance JE, Reith D, Ward RM, Balch A, Stockmann C, Korgenski EK, Thorell EA, Sherwin CMT (2017) Risk of nonsteroidal anti-inflammatory drug-associated renal dysfunction among neonates diagnosed with patent ductus arteriosus and treated with gentamicin. J Perinatol 37:1093–1102. https://doi.org/10.1038/jp.2017.80
Majed B, Bateman DA, Uy N, Lin F (2019) Patent ductus arteriosus is associated with acute kidney injury in the preterm infant. Pediatr Nephrol 34:1129–1139. https://doi.org/10.1007/s00467-019-4194-5
El-Khuffash A, James AT, Corcoran JD, Dicker P, Franklin O, Elsayed YN, Ting JY, Sehgal A, Malikiwi A, Harabor A, Soraisham AS, McNamara PJ (2015) A patent ductus arteriosus severity score predicts chronic lung disease or death before discharge. J Pediatr 167:1354–1361.e1352. https://doi.org/10.1016/j.jpeds.2015.09.028
Polat TB, Celik IH, Erdeve O (2016) Early predictive echocardiographic features of hemodynamically significant patent ductus arteriosus in preterm VLBW infants. Pediatr Int 58:589–594. https://doi.org/10.1111/ped.12915
Clyman RI, Liebowitz M, Kaempf J, Erdeve O, Bulbul A, Hakansson S, Lindqvist J, Farooqi A, Katheria A, Sauberan J, Singh J, Nelson K, Wickremasinghe A, Dong L, Hassinger DC, Aucott SW, Hayashi M, Heuchan AM, Carey WA, Derrick M, Fernandez E, Sankar M, Leone T, Perez J, Serize A (2019) PDA-TOLERATE trial: an exploratory randomized controlled trial of treatment of moderate-to-large patent ductus arteriosus at 1 week of age. J Pediatr 205:41–48.e46. https://doi.org/10.1016/j.jpeds.2018.09.012
Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS (1983) Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr 102:895–906
El-Mashad AE, El-Mahdy H, El Amrousy D, Elgendy M (2017) Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. Eur J Pediatr 176:233–240. https://doi.org/10.1007/s00431-016-2830-7
Ohlsson A, Shah PS (2018) Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst Rev 4:Cd010061. https://doi.org/10.1002/14651858.CD010061.pub3
Van Overmeire B (2007) Common clinical and practical questions on the use of intravenous ibuprofen lysine for the treatment of patent ductus arteriosus. J Pediatr Pharmacol Ther 12:194–206. https://doi.org/10.5863/1551-6776-12.3.194
Ohlsson A, Walia R, Shah SS (2018) Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev 9:Cd003481. https://doi.org/10.1002/14651858.CD003481.pub7
Van Overmeire B, Van de Broek H, Van Laer P, Weyler J, Vanhaesebrouck P (2001) Early versus late indomethacin treatment for patent ductus arteriosus in premature infants with respiratory distress syndrome. J Pediatr 138:205–211. https://doi.org/10.1067/mpd.2001.110528
Stephens BE, Gargus RA, Walden RV, Mance M, Nye J, McKinley L, Tucker R, Vohr BR (2008) Fluid regimens in the first week of life may increase risk of patent ductus arteriosus in extremely low birth weight infants. J Perinatol 28:123–128. https://doi.org/10.1038/sj.jp.7211895
Barrington K, Brion LP (2002) Dopamine versus no treatment to prevent renal dysfunction in indomethacin-treated preterm newborn infants. Cochrane Database Syst Rev (3):Cd003213. https://doi.org/10.1002/14651858.Cd003213
Brion LP, Campbell DE (2001) Furosemide for symptomatic patent ductus arteriosus in indomethacin-treated infants. Cochrane Database Syst Rev (3):Cd001148. https://doi.org/10.1002/14651858.Cd001148
Anabrees JA, Aifaleh KM (2012) Fluid restriction and prophylactic indomethacin in extremely low birth weight infants. J Clin Neonatol 1:1–5. https://doi.org/10.4103/2249-4847.92228
Benitz WE (2016) Patent ductus arteriosus in preterm infants. Pediatrics 137:e20153730. https://doi.org/10.1542/peds.2015-3730
Hull MA, Fisher JG, Gutierrez IM, Jones BA, Kang KH, Kenny M, Zurakowski D, Modi BP, Horbar JD, Jaksic T (2014) Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. J Am Coll Surg 218:1148–1155. https://doi.org/10.1016/j.jamcollsurg.2013.11.015
Heida FH, Hulscher JB, Schurink M, van Vliet MJ, Kooi EM, Kasper DC, Pones M, Bos AF, Benkoe TM (2015) Bloodstream infections during the onset of necrotizing enterocolitis and their relation with the pro-inflammatory response, gut wall integrity and severity of disease in NEC. J Pediatr Surg 50:1837–1841. https://doi.org/10.1016/j.jpedsurg.2015.07.009
Lin PW, Stoll BJ (2006) Necrotising enterocolitis. Lancet 368:1271–1283. https://doi.org/10.1016/s0140-6736(06)69525-1
Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG (2010) Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 50:133–164. https://doi.org/10.1086/649554
McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J (2011) Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr 158:422–426. https://doi.org/10.1016/j.jpeds.2010.08.019
Carreno J, Smiraglia T, Hunter C, Tobin E, Lomaestro B (2018) Comparative incidence and excess risk of acute kidney injury in hospitalised patients receiving vancomycin and piperacillin/tazobactam in combination or as monotherapy. Int J Antimicrob Agents 52:643–650. https://doi.org/10.1016/j.ijantimicag.2018.08.001
Downes KJ, Cowden C, Laskin BL, Huang YS, Gong W, Bryan M, Fisher BT, Goldstein SL, Zaoutis TE (2017) Association of acute kidney injury with concomitant vancomycin and piperacillin/tazobactam treatment among hospitalized children. JAMA Pediatr 171:e173219. https://doi.org/10.1001/jamapediatrics.2017.3219
Maldonado NA, Cano LE, De Bedout C, Arbelaez CA, Roncancio G, Tabares AM, Robledo CG, Robledo J (2014) Association of clinical and demographic factors in invasive candidiasis caused by fluconazole-resistant Candida species: a study in 15 hospitals, Medellin, Colombia 2010-2011. Diagn Microbiol Infect Dis 79:280–286. https://doi.org/10.1016/j.diagmicrobio.2014.02.003
Butler KM, Rench MA, Baker CJ (1990) Amphotericin B as a single agent in the treatment of systemic candidiasis in neonates. Pediatr Infect Dis J 9:51–56. https://doi.org/10.1097/00006454-199001000-00012
Autmizguine J, Tan S, Cohen-Wolkowiez M, Cotten CM, Wiederhold N, Goldberg RN, Adams-Chapman I, Stoll BJ, Smith PB, Benjamin DK Jr (2018) Antifungal susceptibility and clinical outcome in neonatal candidiasis. Pediatr Infect Dis J 37:923–929. https://doi.org/10.1097/inf.0000000000001913
Horwitz E, Shavit O, Shouval R, Hoffman A, Shapiro M, Moses AE (2012) Evaluating real-life clinical and economical burden of amphotericin-B deoxycholate adverse reactions. Int J Clin Pharm 34:611–617. https://doi.org/10.1007/s11096-012-9654-y
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD (2016) Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:409–417. https://doi.org/10.1093/cid/civ1194
Ascher SB, Smith PB, Watt K, Benjamin DK, Cohen-Wolkowiez M, Clark RH, Benjamin DK Jr, Moran C (2012) Antifungal therapy and outcomes in infants with invasive Candida infections. Pediatr Infect Dis J 31:439–443. https://doi.org/10.1097/INF.0b013e3182467a72
Manzoni P, Galletto P, Rizzollo S, Franco C, Gallo E, Antonucci R, Fanos V, Farina D (2012) Liposomal amphotericin B does not induce nephrotoxicity or renal function impairment in premature neonates. Early Hum Dev 88(Suppl 2):S86–S91. https://doi.org/10.1016/s0378-3782(12)70024-5
Karadag-Oncel E, Ozsurekci Y, Yurdakok M, Kara A (2013) Is liposomal amphotericin B really safety in neonates? Early Hum Dev 89:35–36. https://doi.org/10.1016/j.earlhumdev.2012.07.015
Scarcella A, Pasquariello MB, Giugliano B, Vendemmia M, de Lucia A (1998) Liposomal amphotericin B treatment for neonatal fungal infections. Pediatr Infect Dis J 17:146–148
Silver C, Rostas S (2018) Comprehensive drug utilization review in neonates: liposomal amphotericin B. J Pharm Pharmacol 70:328–334. https://doi.org/10.1111/jphp.12878
Goldman RD, Koren G (2004) Amphotericin B nephrotoxicity in children. J Pediatr Hematol Oncol 26:421–426
Carmody JB, Harer MW, Denotti AR, Swanson JR, Charlton JR (2016) Caffeine exposure and risk of acute kidney injury in a retrospective cohort of very low birth weight neonates. J Pediatr 172:63–68.e61. https://doi.org/10.1016/j.jpeds.2016.01.051
Harer MW, Askenazi DJ, Boohaker LJ, Carmody JB, Griffin RL, Guillet R, Selewski DT, Swanson JR, Charlton JR (2018) Association between early caffeine citrate administration and risk of acute kidney injury in preterm neonates: results from the AWAKEN study. JAMA Pediatr 172:e180322. https://doi.org/10.1001/jamapediatrics.2018.0322
Bhat MA, Shah ZA, Makhdoomi MS, Mufti MH (2006) Theophylline for renal function in term neonates with perinatal asphyxia: a randomized, placebo-controlled trial. J Pediatr 149:180–184. https://doi.org/10.1016/j.jpeds.2006.03.053
Al-Wassia H, Alshaikh B, Sauve R (2013) Prophylactic theophylline for the prevention of severe renal dysfunction in term and post-term neonates with perinatal asphyxia: a systematic review and meta-analysis of randomized controlled trials. J Perinatol 33:271–277. https://doi.org/10.1038/jp.2012.97
KDIGO (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138
Bellos I, Iliopoulos DC, Perrea DN (2019) Pharmacological interventions for the prevention of acute kidney injury after pediatric cardiac surgery: a network meta-analysis. Clin Exp Nephrol 23:782–791. https://doi.org/10.1007/s10157-019-01706-9
Kent AL, Charlton JR, Guillet R, Gist KM, Hanna M, El Samra A, Fletcher J, Selewski DT, Mammen C (2018) Neonatal acute kidney injury: a survey of neonatologists’ and nephrologists’ perceptions and practice management. Am J Perinatol 35:1–9. https://doi.org/10.1055/s-0037-1604260
Goldstein SL, Kirkendall E, Nguyen H, Schaffzin JK, Bucuvalas J, Bracke T, Seid M, Ashby M, Foertmeyer N, Brunner L, Lesko A, Barclay C, Lannon C, Muething S (2013) Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics 132:e756–e767. https://doi.org/10.1542/peds.2013-0794
Kashani K, Rosner MH, Haase M, Lewington AJP, O’Donoghue DJ, Wilson FP, Nadim MK, Silver SA, Zarbock A, Ostermann M, Mehta RL, Kane-Gill SL, Ding X, Pickkers P, Bihorac A, Siew ED, Barreto EF, Macedo E, Kellum JA, Palevsky PM, Tolwani AJ, Ronco C, Juncos LA, Rewa OG, Bagshaw SM, Mottes TA, Koyner JL, Liu KD, Forni LG, Heung M, Wu VC (2019) Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol 14:941–953. https://doi.org/10.2215/cjn.01250119
Acknowledgments
The authors thank Liza Kramer, PharmD Candidate, Class of 2019, University of Iowa College of Pharmacy; and Whitni Patterson, Pharm D Candidate, Class of 2019, University of Iowa College of Pharmacy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Study questions’ answers
1. b; 2. a; 3. a, c, d; 4. a; 5. b
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murphy, H.J., Thomas, B., Van Wyk, B. et al. Nephrotoxic medications and acute kidney injury risk factors in the neonatal intensive care unit: clinical challenges for neonatologists and nephrologists. Pediatr Nephrol 35, 2077–2088 (2020). https://doi.org/10.1007/s00467-019-04350-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04350-3