Abstract
Objectives
Hospitalized neonates are often treated with nephrotoxic medications, a known risk factor for acute kidney injury (AKI). Nephrotoxic medications and AKI, especially in periviable neonates, could be detrimental to nephrogenesis. Our objectives were to evaluate the prevalence of neonatal treatment with nephrotoxic medications and its relationship with AKI in in the first 28 days of life, and to delineate the associated demographics and diagnoses.
Study design
Multicenter retrospective analysis using the national Pediatric Hospital Information System database, including 49 pediatric hospitals. Neonates admitted within the first two postnatal days were included. Treatment with 37 nephrotoxic medications across demographics and clinical variables, and relationship with AKI were evaluated. AKI was determined by using the International Classification of Diseases codes.
Results
Of 192,229 neonates, 74% were treated with at least one nephrotoxic medication. Incidence of AKI was significantly higher in the treated group (aRR 3.68 [95% CI: 2.85, 4.75]). The aRRs of treatment were increased in infants born < 32-week, and < 2000 g. Nephrotoxic medications were prescribed to 90–95% of neonates born ≤ 28-week gestational age. Most treatments (95–98%) occurred in the first 3 days. Intravascular aminoglycosides were the most frequent type; 28% of neonates were treated for ≥ 4 calendar days. Most common diagnoses were infections (25%) and patent ductus arteriosus (20%).
Conclusions
Neonatal treatment with nephrotoxic medications is common, especially among the smallest, most immature preterm neonates and demonstrates a need for initiatives to reduce neonatal exposure to these agents, when feasible. Across all gestational age categories, the prevalence of AKI is higher in the neonates treated with nephrotoxic drugs. The long-term effects of treatment with nephrotoxic medications and subsequent AKI on nephrogenesis and nephron endowment will need to be evaluated.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nephrotoxic medications (NMs) are frequently used during the management of hospitalized neonates and are associated with increased risk of acute kidney injury (AKI) [1,2,3,4,5]. AKI is an abrupt reduction in kidney function that acutely leads to abnormal fluid and electrolyte homeostasis [6, 7]. AKI in hospitalized, critically ill neonates, is independently associated with increased morbidity and mortality [6] and may lead to future development of chronic kidney disease (CKD) [7,8,9,10].
Although other researchers have documented associations between increased use of nephrotoxic medications and AKI in hospitalized infants [1,2,3,4,5], the prevalence and patterns of treatment with nephrotoxic medications for specific neonatal populations and diagnoses is underreported. Most prior investigations were limited to single centers. In one institution, exposure to nephrotoxic medications in very low birth weight (VLBW) neonates and infants was estimated at 78% and associated with neonatal AKI [1]. A recent study from a level IV Neonatal intensive care unit (NICU) showed that treatment with nephrotoxic medications occurred in 16.4 per 1000 patient-days and of those 31% developed AKI [2]. The collection and reporting of these data are essential to clinical neonatal AKI prevention efforts.
Therefore, we designed a multi-center retrospective study using the national Pediatric Hospital Information System (PHIS) database to characterize treatment with nephrotoxic medications in neonates admitted to the NICU. The objectives of our study were to identify the prevalence and patterns of nephrotoxic medication exposure in neonates in the first 28 postnatal days and to delineate the demographic characteristics and diagnoses of treated infants. We also aimed to study the relationship of nephrotoxic medications to neonatal AKI in hospitalized neonates.
Materials and methods
Data source
The Pediatric Hospital Information System (PHIS) database, maintained by the Children’s Hospital Association (Lenexa, KS), contains administrative, billing, and clinical data including patient demographics, diagnoses, medications, and procedures from member United States (U.S.) children’s hospitals. Recorded data for patients in 49 NICUs from 49 U.S. children’s hospitals were included in this analysis. Demographic variables, diagnoses, and daily drug-specific nephrotoxic medication administration were determined from each hospital’s daily charge records as listed in PHIS. The Nationwide Children’s Hospital Institutional Review Board determined that this preexisting de-identified data was not human subject research.
Patient selection and study variables
We conducted a multicenter, retrospective study using the PHIS database. Included neonates met the following criteria: admitted to the NICU at ≤ two days old with a recorded gestational age and birthweight and discharged between January 2005 and June 2016. Patient demographics included sex, race, ethnicity, gestational age, and birthweight. We chose to include race/ethnicity in our models because disparate outcomes, which may vary based on study start time (time zero) in the perinatal/neonatal period, have been reported [11,12,13]. Discharge years were classified into three groups: 2005–2008, 2009–2012 and 2013–2016. Patients were classified into six categories by gestational age: ≤ 24, 25–28, 29–32, 33–34, 35–36, and ≥ 37 weeks and also by birthweight: < 750, 750–999, 1000–1499, 1500–1999, 2000–2499, and ≥ 2500 g (g). Extremely low birthweight (ELBW) was defined as < 1000 g. Our list of nephrotoxic medications was selected in accordance with the Baby NINJA study [2]. From that list there were 37 nephrotoxic medications in PHIS during the study period. We grouped these 37 medications into 9 categories: non-steroidal anti-inflammatory drugs (NSAIDs), intravenous aminoglycosides, antivirals, anticonvulsants, angiotensin converting enzyme inhibitors (ACEi), vancomycin, other antibiotics, amphotericin B and others, eTable 1. Nephrotoxic medication exposure was defined as administration of at least one nephrotoxic medication within the first 28 days of life. High-risk exposure to nephrotoxic medications was defined as ≥ 4 consecutive calendar days of intravenous aminoglycosides or ≥ 3 nephrotoxic medications in 24 h. We chose ≥ 4 consecutive calendar days of intravenous aminoglycosides to minimize counting short courses of aminoglycosides that are usually empirically prescribed to neonates for 24–72 h while sepsis is ruled out. Fluid intake and output data as well as laboratory values were not available in the database, therefore AKI was defined using International Classification of Diseases (ICD)-9 and 10 codes. Associated diagnoses and comorbidities were selected using the ICD-9 and 10 codes and included AKI, infections, seizures, patent ductus arteriosus (PDA) and congenital heart disease (CHD), eTable2.
Statistical methods
Treatment frequency patterns were determined using basic univariate analysis. Comparisons between demographic characteristics and treatment exposure were measured with Pearson chi-squared tests. We estimated multivariable adjusted relative risks (aRRs) using modified Poisson regressions with a robust error variance and within-hospital clustering. This method was used in two separate models to both measure the associations between treatment status and AKI among neonates diagnosed with clinical risk factors, and to evaluate clinical and demographic factors associated with nephrotoxic medication exposure. Models were adjusted using gestational age birthweight, race, Hispanic ethnicity, sex, and 5-min Apgar scores as covariates. Analyses were conducted using Stata 16.1 (StataCorp, College Station, TX).
Results
Treatment with nephrotoxic medications is extremely common in hospitalized neonates
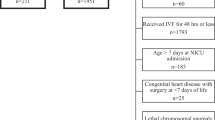
During the study period, 250, 962 neonates were admitted to the NICU, of which 192,229 met inclusion criteria. Of these neonates, 142,409 (74%) were treated with one or more nephrotoxic medications in the first 28 postnatal days, eFigure 1. In this cohort, 75% of males and 71% of females received one or more nephrotoxic medications. Use of nephrotoxic medications increased with decreasing birthweight and gestational age. The demographic characteristics per treatment groups are outlined in Table 1.
Treatment with nephrotoxic medications is most common in neonates with extreme prematurity and extremely low birthweight
Within the different gestational age and birthweight categories, the neonates with the lowest gestational ages (≤ 24 and 25–28 weeks; 9 and 95%, respectively) and the smallest birthweight (< 1000 g, Extremely low birthweight, 92%) were those most frequently treated with nephrotoxic medications. Frequency of treatment with nephrotoxic medications among different gestational age and birthweight categories is shown in eFigure 2.
Nephrotoxic medication categories and exposure patterns
Aminoglycosides were the most commonly administered nephrotoxic medication both in the entire cohort and across all gestational age categories. Aminoglycosides were followed by NSAIDs, vancomycin, and other antibiotics and antivirals depending on gestational age, Table 2.
Neonates were most commonly treated with nephrotoxic medications in the first 3 postnatal days both in the entire cohort and across gestational age categories, ranging from 95 to 98%, with a gradual decrease over the next 2–4 weeks. Treatment with ≥ 4 calendar days of aminoglycosides was the most common high-risk exposure pattern in the entire cohort (28%) and across all gestational age categories (18–33%), Table 2.
Infections and PDA are the most common diagnoses associated with nephrotoxic exposures
Nephrotoxic medications are prescribed to treat several conditions in neonates admitted to the NICU. In this cohort, infections and patent ductus arteriosus were the most prevalent diagnoses in the entire cohort and across gestational age categories, constituting 25% and 20% of treated neonates, respectively. Infections and patent ductus arteriosus were followed by either AKI, seizures or congenital heart disease depending on gestational age, Table 3.
AKI is more common in neonates treated with nephrotoxic medications
The overall prevalence of neonatal AKI in the entire cohort was 2% and highest in neonates born ≤ 24 weeks (9%). Neonates who were treated with nephrotoxic medications were more likely to develop AKI during their hospital stay than those who were never treated (3 vs 1%). The prevalence of AKI in neonates with high-risk diagnoses was significantly higher in neonates treated with nephrotoxic medications than in those who were not treated: infections (4% vs 2%), patent ductus arteriosus (6% vs 2%), seizures (8% vs 5%), and congenital heart disease (4% vs 1%), Table 4.
When we adjusted for other risk-factors with our multivariable model, the aRRs for AKI were increased in neonates treated with nephrotoxic medications both within the entire cohort and for infants with high-risk diagnoses, Table 4.
Factors associated with elevated adjusted relative risks of treatment with nephrotoxic medication
The multivariable aRRs for treatment with any nephrotoxic medication were highest in ≤ 32-weeks gestational age preterm neonates (highest in 25–28 weeks) and those born < 2000 g (highest in < 1000 g). Similarly, neonates < 29 weeks GA and < 1500 g had significant probability of treatment with ≥ 3 NMs in 24 hours. The aRRs for treatment with ≥ 4 calendar days of aminoglycosides were significantly reduced in neonates with 29–36 weeks of gestational age and ≥ 1500 g birthweight, Table 5.
No significant difference in aRR of ever having been treated with nephrotoxic medications was noted across racial and ethnic categories. Administration of ≥ 3 nephrotoxic medications in 24 h was lower in Black relative to White neonates. Neonates of Hispanic ethnicity had a higher adjusted probability of treatment with ≥ 3 nephrotoxic medications in 24 h, relative to non-Hispanic neonates. Females were less likely to have ever been treated with nephrotoxic medications and to have received ≥ 4-days of aminoglycosides compared to males.
The adjusted risks of treatment with any nephrotoxic medication and ≥ 4 days of aminoglycosides were significantly higher in the years 2005–2012 (highest in 2005–2008) compared to 2013–2016.
Neonates with infections, patent ductus arteriosus, seizures, congenital heart disease and AKI all had an increased adjusted risk of ever being treated with nephrotoxic medications and of receiving ≥ 3 nephrotoxic medications in 24 h. Among these diagnoses, infections were associated with the greatest adjusted probability risk of receiving ≥ 3 nephrotoxic medications in 24 h or ≥ 4 calendar days of aminoglycosides. The multivariate aRRs of treatment with nephrotoxic medications by demographics and clinical diagnoses are shown in Table 5.
Discussion
When we evaluated treatment with nephrotoxic medications in the first 28 postnatal days and the relationship with neonatal AKI in neonates admitted to the NICU using a national cohort from the Pediatric Hospital Information System (PHIS), we found that 74% of all hospitalized neonates were treated with at least one nephrotoxic medication in the first 28 postnatal days. A key finding was that the smallest and most immature neonates, who were born at gestational age ≤ 28 weeks or < 1000 g, were the most likely to be exposed to one or more nephrotoxic medication with treatment ranging from 90 to 95%. Aminoglycosides were the most commonly prescribed nephrotoxic medications across all gestational age categories, followed by vancomycin, other nephrotoxic antibiotics, and NSAIDs. Most treatments with nephrotoxic medications occurred in the first 3 postnatal days (85–98%) and the proportion of treated infants remained above 30% throughout the 28 postnatal days in neonates born at ≤ 28 weeks. Infections and patent ductus arteriosus were the diagnoses most commonly associated with having ever received a nephrotoxic medication, both within the entire cohort and across all gestational ages. The aRR of having an AKI diagnosis was significantly higher in neonates treated with nephrotoxic medications compared to those who were not treated. To our knowledge, this is the first study to identify nephrotoxic medication exposure patterns by medication type across demographics and clinical diagnoses in neonates admitted to the Children’s Hospital NICUs across the United States.
We found that 92–95% of neonates with birthweight < 1000 g and gestational age ≤ 28 weeks were treated with nephrotoxic medications. Our findings are comparable to a previous cohort study involving 107 very low birth weight infants, that reported exposure to ≥ one nephrotoxic medication in 87% of patients and association with AKI [1]. We also found that high-risk nephrotoxic medication exposure, namely ≥ 4 calendar days of aminoglycosides, occurred in 28% of neonates overall and 22–33% of neonates when stratified by gestational age Neonatal empiric treatment with IV aminoglycosides to rule out sepsis in the first few postnatal days vary significantly among institutions. It has been shown that using clinical judgment in addition to implementing local sepsis risk scoring systems could result in reduced exposure to nephrotoxic medications without increasing the risk of sepsis [14, 15]. High-risk exposure to nephrotoxic medications (≥ 4 calendar days of aminoglycosides and/or ≥ 3 nephrotoxic medications in 24 h) was evaluated in a quality improvement fashion in a level IV NICU and was found to occur in 16.4 per 1000-patient days. In that cohort, 25% of patients with high nephrotoxic medication exposure developed AKI [2].
Our findings of high nephrotoxic medication use in neonates, and especially our finding that the most fragile and immature, extremely low birth weight preterm neonates are the most likely group to be prescribed nephrotoxic medications, are important because treatment with nephrotoxic medication is a preventable risk factor that is known to be associated with the development of neonatal AKI [1,2,3,4,5]. Neonatal AKI is a common comorbidity in hospitalized neonates, diagnosed by changes in serum creatinine concentrations and urine output as outlined by the Kidney Disease: Improving Global Outcomes [KDIGO] criteria [6]. Neonatal AKI occurs in up to 48% of critically ill neonates and is associated with short-term morbidities and increased neonatal mortality [6]. Exposure to nephrotoxic medications and resultant AKI, especially in extremely preterm infants who are in active nephrogenesis, might affect nephron endowment and lead to long-term risk of developing CKD [7,8,9,10]. Currently, there is no effective treatment for CKD, and management strategies focus on AKI prevention including reducing nephrotoxic medication exposure when feasible. In their recent study evaluating combined nephrotoxic medications and AKI, Salerno et al. found an association between exposure to the combination of gentamicin and indomethacin with neonatal AKI [5]. Downes et al. reported increased neonatal AKI incidence in neonates who received ≥ 2 nephrotoxic medications concomitantly during treatment with acyclovir [16]. Furthermore, Barhight et al. evaluated the association between treatment with nephrotoxic medications and AKI in preterm and low birth weight infants and reported that AKI occurred in 9% of infants treated with nephrotoxic medications [17]. The reported incidence of AKI in the latter study is higher than what we observed (3%) in our group treated with nephrotoxic medications. One potential explanation is that Barhight et al. used KDIGO serum creatinine criteria to identify AKI in their cohort while we used ICD codes. ICD codes rely on provider recognition and documentation of AKI which could have underestimated the true neonatal AKI prevalence in our cohort, as reported in previous studies [18, 19].
The adjusted probability of treatment with any nephrotoxic medication was similar between racial and ethnic groups. We found that the probability of treatment with ≥ 3 nephrotoxic medications in 24 h was lower in Black neonates compared to White, and higher in Hispanic compared to non-Hispanic. The significance and future implications of these findings are to be studied. Race, being a social construct does not affect biological outcomes, however racial disparities in neonatal health outcomes have been previously reported [11,12,13, 18,21,22] including in neonatal AKI [23].
The probability of treatment with ≥ 4 days of aminoglycosides was not high in neonates with AKI. This could be due to under-recognition of AKI [18, 19], close serum creatinine monitoring in neonates who received several days of aminoglycosides, which has been reported to decrease nephrotoxic medication-associated AKI [2, 24], close monitoring of aminoglycoside trough and peak levels during the treatment course [2, 24, 25] or because aminoglycosides may have likely been switched to a less nephrotoxic regimen within days when AKI was initially discovered. The decrease in neonatal treatment with nephrotoxic medications and with ≥ 4 days of aminoglycosides in the recent years could be due to recent efforts to reduce infant exposure to nephrotoxic medications [2].
Strengths of our study include both using a national database with a large sample size of neonates across gestational ages and birthweights, which is likely generalizable to most US Children’s Hospital NICUs and likely to those in other developed countries, and screening a wide range of classes and types of nephrotoxic medications. Pediatric Hospital Information System (PHIS) provides daily medication exposures for each infant. One limitation is that, unfortunately laboratory and daily fluid intake and output data to identify AKI by either changes in serum creatinine and/or urine output as outlined by KDIGO were not available. Prior research has shown that neonatal AKI is underreported in the hospital encounter diagnoses, problem lists and hospital charges [18, 19] and therefore we speculate that using PHIS and relying on ICD codes for neonatal AKI identification likely resulted in selection bias in the form of underestimation of neonatal AKI in our cohort. In the presence of more sensitive diagnoses of AKI via closer monitoring of kidney function by clinicians and/or with more sensitive AKI biomarkers, the associated prevalence of AKI in neonates treated with nephrotoxic medications is likely higher than we were able to detect. A second limitation is that we did not exclude infants diagnosed with significant congenital anomalies of the kidney and urinary tract from our analysis. The inclusion of these patients is unlikely to change our central findings, i.e., of an association between nephrotoxic medication use and AKI, or of increased nephrotoxic medication use in neonates who are born at a gestational age≤ 28 weeks and/or < 1000 g since survival with clinically significant anomalies is thought to be rare in extremely preterm infants. Another limitation is due to the observational nature of our investigation—we were able to identify associations between use of nephrotoxic medications and AKI, which may not be a causative relationship. Medication doses were not available in PHIS and there is likely a significant variation in dosing among NICUs [25,26,27]. Even tertiary references provide different recommendations [28,29,30]. NSAID exposures including treatment with prophylactic indomethacin and NSAID shortly after birth and for patent ductus arteriosus typically follow standard dosing patterns per FDA-labeling and as used in large, randomized trials [31,32,33,34,35,36,37,38]. Additional studies are needed to evaluate the pharmacokinetics of medications to minimize adverse drug effects while maintaining efficacy especially in extremely premature neonates.
This study, inclusive of 192,229 neonates as recorded in a large national U.S. database, underscores the frequent use of nephrotoxic medications in neonates admitted to the NICU and the association of nephrotoxic medications with neonatal AKI. It shows the wide prevalence of treatment with nephrotoxic medications in a high-risk population and subsequent development of AKI. The most vulnerable neonates who are born at gestational age ≤ 28 weeks and/or < 1000 g who have the most immature kidneys and are in active nephrogenesis were the most likely to be treated with nephrotoxic medications. This demonstrates the need for quality improvement initiatives to reduce exposure to nephrotoxic medications in these fragile neonates, when feasible. Additionally, the long-term sequelae of treatment with nephrotoxic medications mediated by subsequent AKI, on nephrogenesis and nephron endowment will need to be evaluated in future studies.
References
Rhone ET, Carmody JB, Swanson JR, Charlton JR (2014) Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med 27(14):1485–1490
Stoops C, Stone S, Evans E et al (2019) Baby NINJA (nephrotoxic injury negated by just-in-time action): reduction of nephrotoxic medication-associated acute kidney injury in the neonatal intensive care unit. J Pediatr 215:223-228.e226
Charlton JR, Boohaker L, Askenazi D et al (2019) Incidence and risk factors of early onset neonatal AKI. Clin J Am Soc Nephrol 14(2):184–195
Brenner BM, Garcia DL, Anderson S (1988) Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1(4 Pt 1):335–347
Salerno SN, Liao Y, Jackson W et al (2021) Association between nephrotoxic drug combinations and acute kidney injury in the neonatal intensive care unit. J Pediatr 228:213–219
Jetton JG, Boohaker LJ, Sethi SK et al (2017) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1(3):184–194
Harer MW, Pope CF, Conaway MR, Charlton JR (2017) Follow-up of Acute kidney injury in Neonates during Childhood Years (FANCY): a prospective cohort study. Pediatr Nephrol 32(6):1067–1076
Mammen C, Al Abbas A, Skippen P et al (2012) Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis 59(4):523–530
Maqsood S, Fung N, Chowdhary V, Raina R, Mhanna MJ (2017) Outcome of extremely low birth weight infants with a history of neonatal acute kidney injury. Pediatr Nephrol 32(6):1035–1043
Carmody JB, Charlton JR (2013) Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics 131(6):1168–1179
Sigurdson K, Mitchell B, Liu J et al (2019) Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. https://doi.org/10.1542/peds.2018-3114
Janevic T, Zeitlin J, Auger N et al (2018) Association of race/ethnicity with very preterm neonatal morbidities. JAMA Pediatr 172(11):1061–1069
Ryan RM, Feng R, Bazacliu C et al (2019) Black race is associated with a lower risk of bronchopulmonary dysplasia. J Pediatr 207:130-135.e132
Flidel-Rimon O, Galstyan S, Juster-Reicher A, Rozin I, Shinwell ES (2012) Limitations of the risk factor-based approach in early neonatal sepsis evaluations. Acta Paediatr 101(12):e540-544
Kuzniewicz MW, Puopolo KM, Fischer A et al (2017) A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr 171(4):365–371
Downes KJ, Boge CLK, Baro E et al (2020) Acute kidney injury during treatment with intravenous acyclovir for suspected or confirmed neonatal herpes simplex virus infection. J Pediatr 219:126-132.e122
Barhight M, Altaye M, Gist KM, Isemann B, Goldstein SL, Akinbi H (2017) Nephrotoxic medications and associated acute kidney injury in very low birth weight infants. J Clin Nephrol Res 4(4):1070
Travers CP, Carlo WA, McDonald SA et al (2020) Racial/ethnic disparities among extremely preterm infants in the United States from 2002 to 2016. JAMA Netw Open 3(6):e206757
David RJ, Collins JW (1991) Bad outcomes in black babies: race or racism? Ethn Dis 1(3):236–244
Beck AF, Edwards EM, Horbar JD, Howell EA, McCormick MC, Pursley DM (2020) The color of health: how racism, segregation, and inequality affect the health and well-being of preterm infants and their families. Pediatr Res 87(2):227–234
Elgendy MM, Othman HF, Younis M, Puthuraya S, Matar RB, Aly H (2021) Trends and racial disparities for acute kidney injury in premature infants: the US national database. Pediatr Nephrol. https://doi.org/10.1007/s00467-021-04998-w
Roy JP, Goldstein SL, Schuh MP (2020) Under-recognition of neonatal acute kidney injury and lack of follow-up. Am J Perinatol. https://doi.org/10.1055/s-0040-1716841
Carmody JB, Swanson JR, Rhone ET, Charlton JR (2014) Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol 9(12):2036–2043
Norris AH, Shrestha NK, Allison GM et al (2019) 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis 68(1):1–4
Stark A, Childers J, England M et al (2020) Dosing of antimicrobials in the neonatal intensive care unit: does clinical practice reflect pharmacokinetics-based recommendations? Pediatr Infect Dis J 39(8):713–717
Metsvaht T, Nellis G, Varendi H et al (2015) High variability in the dosing of commonly used antibiotics revealed by a Europe-wide point prevalence study: implications for research and dissemination. BMC Pediatr 15:41
Liem TB, Krediet TG, Fleer A, Egberts TC, Rademaker CM (2010) Variation in antibiotic use in neonatal intensive care units in the Netherlands. J Antimicrob Chemother 65(6):1270–1275
Bradley JS, Nelson JD (eds) (2019) Nelson’s pediatric antimicrobial therapy, 25th edn. American Academy of Pediatrics, Itasca
American Academy of Pediatrics (AAP) (2018) In: Kimberlin DW, Brady MT, Jackson MA, Long SA (eds) Red Book: 2018 Report of the Committee on Infectious Diseases, 31st edn. American Academy of Pediatrics, Itasca
Micromedex® (electronic version). IBM Watson Health, Greenwood Village, Colorado
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021903
Schmidt B, Davis P, Moddemann D et al (2001) Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med 344(26):1966–1972
Ohlsson A, Walia R, Shah SS (2020) Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev 2:CD003481
Ohlsson A, Shah SS (2020) Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev 1:CD004213
Ment LR, Oh W, Ehrenkranz RA et al (1994) Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics 93(4):543–550
Evans P, O’Reilly D, Flyer JN, Soll R, Mitra S (2021) Indomethacin for symptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev 1:CD013133
Acknowledgements
This research was supported by the Ohio Perinatal Research Network of Nationwide Children's Hospital
Author information
Authors and Affiliations
Contributions
TM conceptualized and designed the study, analyzed the data, drafted the initial manuscript, and reviewed and revised the manuscript. JS conceptualized and designed the study, collected data, analyzed the data and reviewed and revised the manuscript. HA assisted with the study design, carried out the analysis and reviewed and revised the manuscript for important intellectual content. JM and PP assisted with the study design and reviewed and revised the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Pavel Prusakov has received research grant support from Merck & Co. and Pfizer. All other authors report no conflict of interest.
Ethical statement
The Nationwide Children’s Hospital Institutional Review Board determined that this preexisting de-identified data was not human subject research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohamed, T.H., Abdi, H.H., Magers, J. et al. Nephrotoxic medications and associated acute kidney injury in hospitalized neonates. J Nephrol 35, 1679–1687 (2022). https://doi.org/10.1007/s40620-022-01264-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-022-01264-6