Abstract
Background
Increased interdialytic weight gain (IDWG) has been associated with poor outcomes in adults, but its impact on hemodialysis vasculopathy in children is unknown.
Methods
Nineteen patients (age 9 to 19 years old) with a median hemodialysis duration of 10.4 months were enrolled. Cardiovascular evaluation included left ventricular mass index (LVMI), pulse wave velocity (PWV), and carotid intima-media thickness (cIMT) measurements. PWV and cIMT were expressed as z-scores based on reference values in healthy children. Blood pressure (BP) evaluation consisted in a 24-h ambulatory BP monitoring. Mean IDGW and residual urine output during the 6 months prior to cardiovascular examination were calculated.
Results
Increased cIMT, LVMI, and PWV was observed in 11 (57.9%), 7 (36.8%), and 5 (26.3%) patients respectively, while BP was normal in all patients. Median IDWG was 3.5% (1.8–6.7). Residual urine output and BP status did not significantly differ between patients with IDWG ≥ or < 4%. After linear regression, IDWG was correlated to cIMT z-score (r2 = 0.485, p = 0.001), but not to PWV z-score (r2 = 0.04, p = 0.415) and LVMI (r2 = 0.092, p = 0.206). After univariate logistic regression, IDWG ≥ 4% was significantly associated to increased cIMT (above 1.65 SDS) (odds ratio 12.25, 95% confidence interval 1.08–138.988). The trend toward an increased cIMT with IDWG ≥ 4% was observed in both patients with short and long dialysis vintage.
Conclusions
High IDWG is associated with increased cIMT in hemodialyzed children independently of BP control and dialysis vintage. This observation reinforces the importance of interventions to avoid IDWG in hemodialyzed children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease is the most common cause of mortality in children with end stage renal disease (ESRD), leading to more than 30% of deaths among children on renal replacement therapy (RRT) [1]. While hypertrophic cardiomyopathy has been the most studied manifestation of cardiovascular disease, there is more recent evidence about the additional and significant role of vasculopathy, which evolves since the early stages of chronic kidney disease (CKD) [2,3,4,5,6,7,8]. Vascular injury may be partially reversible after kidney transplantation [3]. Nevertheless, the degree of vasculopathy at kidney transplantation is associated with a higher risk of permanent vascular damage in pediatric transplant recipients [4]. Avoidance of RRT by pre-emptive kidney transplantation seems to be the optimal strategy for preventing further vascular damage occurring during ESRD [9].
Multiple risk factors for vasculopathy have been reported, including glomerulopathy as primary renal disease, long dialysis duration, hypertension, and impaired bone and mineral metabolism [6]. Volume overload, expressed as interdialytic weight gain (IDWG), has been associated with increased incidence of cardiovascular events and arterial stiffness in hemodialyzed adult patients [10, 11]. Interdialytic weight gain was recently reported to be associated with hypertension and left ventricular hypertrophy in hemodialyzed children [12,13,14,15]. However, its vascular impact has not yet been elucidated in children. Thus, our study attempts to describe the impact of IDWG on the severity of the vasculopathy in children receiving hemodialysis.
Material and methods
This is a retrospective study of the 2016 annual cardiovascular assessment of all patients on hemodialysis at the University Children’s Hospital Robert Debré of Paris. The inclusion criteria involved patient’s age of 7 to 19 years old, hemodialysis duration longer than 3 months, and absence of congenital heart disease or primary myocardiopathy.
Assessment of carotid intima-media thickness (cIMT), pulse wave velocity (PWV), and left ventricular mass index (LVMI) was performed on the same non-dialysis day in each patient by an experienced cardiologist with the same devices throughout the study. The collaborating cardiologist was blinded to patient with IDWG. Echocardiography was used to determine LVMI, calculated by dividing left ventricular mass by height raised to a power of 2.7 (g/m2.7). Left ventricular hypertrophy was defined as LVMI greater than the 95th percentile (perc.), based on perc. reference curves in healthy children of same sex and age [16]. Both cIMT and PWV were expressed as z-scores based on the specific median (M), skewness (L), and coefficient of variation (S) values for child’s height [17, 18]. Values above 1.65 SDS (95th perc.) according to sex and height in healthy children were defined as elevated [17, 18]. Pulse wave velocity recordings were obtained by applanation tonometry (SphygmoCor, AtCor Medical, Itasca, IL, USA). A vivid high-resolution ultrasound system (GE Healthcare, Horten, Norway) with 12-MHz transducer was used for cIMT of bilateral common carotid. The mean of five measurements of each vessel was used in the analysis. Blood pressure (BP) evaluation consisted in a 24-h ambulatory BP monitoring (ABPM), performed on a non-dialysis day within a delay of 2 weeks to cardiovascular tests, using the Spacelabs 90217 (Spacelabs Healthcare, Issaquah, WA, USA) device. Hypertension was defined as mean systolic BP and/or diastolic BP z-score greater than 1.65 SDS (95th perc.) for sex, age, and height [19].
All patients on either online hemodialysis or hemodiafiltration were dialyzed with high-flux filters for 4 h three times a week. Hemodiafiltration was preferentially applied in patients with higher IDWG. Routine clinical care included monthly measurement of residual urine output via 24-h urine collection on a day without hemodialysis and documentation of IDWG. Preserved residual urine output was defined as urine output ≥ 0.5 mL/kg/h per day and IDWG was calculated as the percentage of the weight increase between the end of the first (after a 3-day interdialytic interval) and the start of the second (mid-week) dialysis session. Dry weight was assessed by a weekly clinical examination by an experienced pediatric nephrologist and surveillance of body composition monitoring during hemodialysis. Finally, single pool Kt/V was calculated once a month in a mid-week dialysis session for each patient. We determined the mean of all IDWG and Kt/V data observed over the 6 months prior to cardiovascular testing or over the whole dialysis period in patients on dialysis for less than 6 months.
We collected data in the following laboratory results from the medical records: serum phosphorus (P) in mmol/L, serum calcium (Ca) in mmol/L, serum calcium × phosphorus product (Ca × P) (mmol2/L2) and parathyroid hormone (PTH) in pg/mL, serum 25-hydroxyvitamin D (25(OH)D) in ng/mL and 1.25-dihydroxy-vitamin D (1.25(OH)D) in ng/mL. All laboratory tests were drawn before dialysis sessions.
We calculated correlation between cIMT, PWV, and IDWG. Patients were then classified into two groups based on their mean IDWG using a cut-off of 4%, as previously reported in the literature [12]. We compared the distribution of all cardiovascular parameters as well as the clinical and laboratory parameters that are potentially associated with vasculopathy between the two groups of patients. Finally, we searched for risk factors of vasculopathy among all the included parameters.
Data were expressed as median values and ranges. All statistical evaluations were performed using SPSS Statistics software for Windows. Linear logistic regression was used to assess the correlations between the study parameters. Mann-Whitney and Fisher’s exact were used to compare the characteristics of the patients with and without high IDWG. Univariate logistic regression was performed in order to detect risk factors of vasculopathy. A p value of < 0.05 was considered statistically significant.
Results
Nineteen patients, 12 males and 7 females, were included in our study. The median age was 15.2 years (range 9.0–19.2) and the median body mass index (BMI) was 17.0 kg/m2 (15.3–22.0). None of the patients was overweight according to age-based pediatric reference charts. Primary renal disease was of glomerular origin in 12 (63.2%) and of non-glomerular origin in 7 (36.8%) patients. Four patients had received a prior kidney transplantation. Three children returned to hemodialysis 3, 5, and 7 years after kidney transplantation due to acute allograft rejection in one and chronic allograft failure in two cases. Hemodialysis was never discontinued in the last patient since renal allograft thrombosis occurred in early postoperative period. Median hemodialysis duration was 10.4 months (3.3–46.4). Hemodiafiltration was performed in eight patients, with an average convection fraction of 18.1% (15.3–21.4). Oligoanuria was observed in 13 (68.4%) patients. Median residual urine output in the non-oligoanuric patients was 0.63 mL/kg/h (0.5–3). The 24-h ABPM was normal in all patients (Fig. 1). Nine patients (47.4%) were under antihypertensive treatment: monotherapy in five and two medications in four patients. The number of patients with increased cIMT, LVMI, and PWV was 11 (57.9%), 7 (36.8%), and 5 (26.3%) respectively. The distribution of cIMT and PWV z-scores is illustrated in Fig. 1.
Median IDWG was 3.5% (1.8–6.8). Patients with high IDWG (≥ 4%) were preferentially treated with hemodiafiltration and presented a longer dialysis vintage (Table 1). No association was observed between high IDWG and the following clinical and laboratory parameters: glomerular primary renal disease, preserved residual urine output, single pool Kt/V, systolic and diastolic BP z-scores, and antihypertensive medication (Table 1). Nevertheless, IDWG was higher in patients without residual diuresis and under antihypertensive treatment (Fig. 2). Finally, patients with high IDWG presented lower P levels and Ca × P product, whereas no significant association was observed between high IDWG and PTH, 25(OH)D and 1.25(OH)D levels (Table 1).
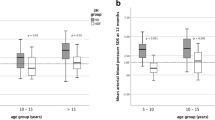
A significant linear correlation was found between IDWG and cIMT (r2 = 0.458, p = 0.001 and r2 = 0.485, p = 0.001 for cIMT values and z-scores respectively), but not between IDWG and PWV (r2 = 0.011, p = 0.671 and r2 = 0.04, p = 0.415 for PWV values and z-scores respectively) (Fig. 3). Although no significant linear correlation was observed between IDWG and LVMI (r2 = 0.092, p = 0.206), a trend toward a logarithmic correlation between those two variables was observed (r2 = 0.157, p = 0.094). Patients with IDWG ≥ 4% presented a significantly higher cIMT z-score (Fig. 4). After univariate logistic regression, IDWG ≥ 4% was significantly associated to increased cIMT (odds ratio 12.250, 95% confidence interval 1.078–138.988) (Table 2). No association was observed between the need for antihypertensive treatment and increased cIMT (Table 2). A trend toward an increased cIMT z-score with high IDWG was observed in both patients with short (< 12 months) and long (≥ 12 months) dialysis duration (Fig. 5).
Discussion
Vascular damage is detected in early stages of CKD, but its prevalence is higher in children with ESRD [2,3,4,5,6,7,8]. The underlying mechanism is uremia-toxin induced apoptosis of vascular smooth muscle cells, which are then transformed into osteo/chondrocyte-like cells due to perturbation in the levels of serum calcification inhibitors and promoters [20, 21]. As a result, vascular injury progresses to calcification primarily in the tunica media, but also in the tunica intima of the large vessels, inducing both structural and functional changes [21]. Carotid IMT and PWV are identified as surrogate markers of hypertrophic vasculopathy and arterial stiffness respectively in children with CKD [5]. Prevalence of increased cIMT and PWV has been recently reported in the order of 41.5 and 20.1% respectively in children with ESRD [7]. According to our findings, almost 90% of patients presented increased PWV and/or cIMT; the latter was the most prevalent early marker of cardiovascular disease. These results highlight the importance of large vessel arteriopathy assessment in hemodialyzed children and the need to identify potential risk factors.
Excessive IDWG, which represents water and sodium overload between two hemodialysis sessions, has been implicated in the development of arterial hypertension, left ventricular hypertrophy, and mortality [12,13,14,15]. Paglialonga et al. reported a logarithmic correlation between IDWG and LVMI in a small cohort of dialysis patients and we found a similar trend in our study. Water overload may also induce vascular damage by increasing systemic BP, while sodium overload has been proven to promote hypertrophy of vascular smooth muscle cell both in vitro and in vivo studies by enhancing tissue angiotensin-II activity and provoking changes in shear stress and endothelial function [22, 23]. Toward this direction, our results highlighted a significant correlation between IDWG and cIMT and an association between high IDWG (≥ 4%) and increased cIMT. Although the small sample size did not permit multivariate statistical analysis, several observations suggest an independent impact of IDWG on cIMT. Elevated serum P and Ca × P have been incriminated for vascular calcification in pediatric CKD [6, 24, 25]. In our study, patients with high IDWG presented relatively lower serum P levels and Ca × P product. Thus, abnormalities in bone and mineral metabolism cannot explicate the correlation between IDWG and cIMT. In our center, patients with high IDWG are preferentially treated with hemodiafiltration, which precludes us to assess the effect of the type of treatment on cIMT. However, given the suggested cardiovascular protective effect of hemodiafiltration compared to hemodialysis in children by 3H trial, this indication bias will tend to decrease the association between IDWG and cIMT in our study [26]. Thus, difference in the type of treatment received cannot justify the association we found between high IDWG and increased cIMT. Finally, patients on long-term hemodialysis are at higher risk for increased cIMT [8]. Nevertheless, the same trend toward an increased cIMT in patients with high IDWG was observed both in patients with short (< 12 months) or long (≥ 12 months) dialysis vintage. These remarks are indicative of an independent association between high IDWG and increased cIMT.
Blood pressure status was within normal range in all patients. Thus, our study suggests that high IDWG induces vascular damage despite a well-controlled BP. Our results are in concordance with those published in the literature, where high IDWG or chronic volume overload was significantly related to mortality and poor cardiovascular outcome in hemodialyzed adults presenting either hypertension or normal BP [27,28,29]. We also observed that patients with higher IDWG and without residual diuresis needed more antihypertensive treatment. There is evidence that reducing IDWG by applying salt restriction is superior to antihypertensive medications for optimal preservation of left ventricular function in hemodialyzed adults [30]. Therefore, the ideal management of patients with hypertension due to volume overload should firstly aim at minimizing IDWG, whereas antihypertensive drugs may be added carefully after reaching optimal dry weight [31].
In our study, high IDWG was not correlated to increased PWV. This contrasts with adult studies that found an association between chronic volume overload and arterial stiffness, independently of BP status, in hemodialyzed patients [11, 32, 33]. This discrepancy of results may be due to the changes in the vascular compliance with age. Large artery elasticity index increases until the age of 30, then it slowly declines during young and middle adulthood and abruptly decreases during late adulthood [34]. We hypothesize that the higher arterial compliance in children may explain the absence of association between IDWG and PWV in normotensive children. Further studies are needed to confirm our results.
The cut-off for a high IDWG was set at 4% in this study. Clinical guidelines recommend avoiding an IDWG > 10% because it demands large ultrafiltration rates, rising the risk for intradialytic hypotension [31]. However, due to the emerging evidence of IDWG effect on cardiovascular function, the target of IDWG needs to be lower. In several adult studies, an IDWG ≥ 5.7%, which represents a 4-kg IDWG in a patient with a post-HD weight of 70 kg, was associated with increased mortality risk [22, 32]. In a recent pediatric study, an IDWG ≥ 4%, which represents a 1-kg IDWG in a patient with a post-HD weight of 25 kg, was associated with increased risk of left ventricular hypertrophy [11, 14]. Our results underline the importance of maintaining an IDWG < 4% in hemodialyzed children.
Accurate assessment of dry weight, which is necessary for IDWG monitoring, remains a challenge in clinical practice. Surveillance of BP may be helpful, but volume overload, expressed as > 15% of extracellular weight measured at bio-impedance spectroscopy is not always related to hypertension [35,36,37]. Along with physical examination, there are a variety of technologies that have been used to improve assessment of volume status, including blood volume monitoring during hemodialysis, measurement of bioelectrical impedance analysis, and lung ultrasound [13]. Reduction of IDWG can be achieved by decreasing dietary sodium intake and enhancing sodium removal during hemodialysis, by increasing frequency and/or duration of hemodialysis and optimizing dialysate sodium concentration [12, 25]. Residual urine output is generally felt to be useful for minimizing IDWG, but the presence of residual urine output was not protective in our cohort of patients.
The main strength of this study is the extensive cardiovascular evaluation in a cohort of hemodialyzed children. The main limitation is the small size of our study, which precluded assessment of independent risk factors of vasculopathy via multivariate analysis to adjust for known risk factors and the inclusion of previously transplanted patients with prior hemodialysis vintage. Given the low incidence of ESRD and hemodialysis in children, multicenter studies are needed to confirm our results.
In conclusion, this is the first pediatric study to report an association between high IDWG and increased cIMT in hemodialyzed children despite well-controlled BP. Strategies to reduce IDWG in those patients may prevent arteriopathy and long-term cardiovascular complications.
References
Mitsnefes MM (2012) Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 23:578–585
Covic A, Mardare N, Gusbeth-Tatomir P, Brumaru O, Gavrilovici C, Munteanu M, Prisada O, Goldsmith DJ (2006) Increased arterial stiffness in children on haemodialysis. Nephrol Dial Transplant 21:729–735
Litwin M, Wühl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P, Tröger J, Mehls O, Schaefer F (2005) Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol 16:1494–1500
Litwin M, Wühl E, Jourdan C (2008) Evolution of large-vessel arteriopathy in paediatric patients with chronic kidney disease. Nephrol Dial Transplant 23:2552–2557
Shroff R, Dégi A, Kerti A, Kis E, Cseprekál O, Tory K, Szabó AJ, Reusz GS (2013) Cardiovascular risk assessment in children with chronic kidney disease. Pediatr Nephrol 28:875–884
Shroff R, Quinlan C, Mitsnefes M (2011) Uraemic vasculopathy in children with chronic kidney disease: prevention or damage limitation? Pediatr Nephrol 26:853–865
Schaefer F, Doyon A, Azukaitis K, Bayazit A, Canpolat N, Duzova A, Niemirska A, Sözeri B, Thurn D, Anarat A, Ranchin B, Litwin M, Caliskan S, Candan C, Baskin E, Yilmaz E, Mir S, Kirchner M, Sander A, Haffner D, Melk A, Wühl E, Shroff R, Querfeld U, 4C Study Consortium (2017) Cardiovascular phenotypes in children with CKD: the 4C study. Clin J Am Soc Nephrol 12:19–28
Chavarria LA, Aguilar-Kitsu A, Rosas P, , Fajardo A, Mendoza-Guevara L, Sanchez L, Zepeda C, Ibarra P, Luna A, Lindholm B, García-López E (2012) Intima media thickness in children undergoing dialysis. Pediatr Nephrol 27:1557–1564
Schmidt BMW, Sugianto RI, Thurn D, Azukaitis K, Bayazit AK, Canpolat N, Eroglu AG, Caliskan S, Doyon A, Duzova A, Karagoz T, Anarat A, Deveci M, Mir S, Ranchin B, Shroff R, Baskin E, Litwin M, Özcakar ZB, Büscher R, Soylemezoglu O, Dusek J, Kemper MJ, Matteucci MC, Habbig S, Laube G, Wühl E, Querfeld U, Sander A, Schaefer F, Melk A, 4C Study Consortium (2018) Early effects of renal replacement therapy on cardiovascular comorbidity in children with end-stage kidney disease. Transplantation 102:484–492
Lee MJ, Doh FM, Kim CH, Koo HM, Oh HJ, Park JT, Han SH, Yoo TH, Kim YL, Kim YS, Yang CW, Kim NH, Kang SW (2014) Interdialytic weight gain and cardiovascular outcome in incident hemodialysis patients. Am J Nephrol 39:427–435
Czyżewski Ł, Wyzgał J, Czyżewska E, Sierdziński J, Szarpak Ł (2017) Contribution of volume overload to the arterial stiffness of hemodialysis patients. Ren Fail 39:333–339
Paglialonga F, Consolo S, Galli MA, Testa S, Edefonti A (2015) Interdialytic weight gain in oligoanuric children and adolescents on chronic hemodialysis. Pediatr Nephrol 30:999–1005
Paglialonga F, Consolo S, Edefonti A, Montini G (2018) Blood pressure management in children on dialysis. Pediatr Nephrol 33:239–250
Marsenic O, Anderson M, Couloures KG (2016) Relationship between interdialytic weight gain and blood pressure in pediatric patients on chronic hemodialysis. Biomed Res Int. https://doi.org/10.1155/2016/5972930
Fischbach M, Zaloszyc A, Shroff R (2015) The interdialytic weight gain: a simple marker of left ventricular hypertrophy in children on chronic haemodialysis. Pediatr Nephrol 30:859–863
Khoury PR, Mitsnefes M, Daniels SR, Kimball TR (2009) Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 22:709–714
Reusz GS, Cseprekal O, Temmar M, Kis E, Cherif AB, Thaleb A, Fekete A, Szabó AJ, Benetos A, Salvi P (2010) Reference values of pulse wave velocity in healthy children and teenagers. Hypertension 56:217–224
Doyon A, Kracht D, Bayazit AK, Deveci M, Duzova A, Krmar RT, Litwin M, Niemirska A, Oguz B, Schmidt BM, Sözeri B, Querfeld U, Melk A, Schaefer F, Wühl E, 4C Study Consortium (2013) Carotid artery intima-media thickness and distensibility in children and adolescents: reference values and role of body dimensions. Hypertension 62:550–556
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Finn SK, Gidding SS, Goodwin C, Leu MG, Powers E, Rea C, Samuels J, Simasek M, Thacker VV, Urbina EM, Subcommittee on screening and management o high blood pressure in Children (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140:e20171904
Shroff RC, Shanahan CM (2007) The vascular biology of calcification. Semin Dial 20:103–109
Shroff R, Weaver DJ, Mitsnefes MM (2011) Cardiovascular complications in children with chronic kidney disease. Nat Rev Nephrol 7:642–649
He FJ, MacGregor GA (2004) Plasma sodium and hypertension. Kidney Int 66:2454–2466
Safar M (2000) Pressure-independent contribution of sodium to large artery structure and function in hypertension. Cardiovasc Res 46:269–276
Shroff RC, Donald AE, Hiorns MP, Watson A, Feather S, Milford D, Ellins EA, Storry C, Ridout D, Deanfield J, Rees L (2007) Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol 18:2996–3003
Ziolkowska H, Brzewski M, Roszkowska-Blaim M (2008) Determinants of the intima–media thickness in children and adolescents with chronic kidney disease. Pediatr Nephrol 23:805–811
Shroff R, Bayazit AK, Stefanidis CJ, Askiti V, Ažukaitis K, Canpolat N, Çaliskan S, Anon B, Bakkaloglu S, Borzych-dużałka D, Duzova A, Habbig S, Hayes W, Hegde S, Krid S, Licht C, Litwin M, Mayes M, Paglialonga F, Picca S, Ranchin B, Samaille C, Shenoy M, Sinha M, Smith C, Spasojevic B, Vidal E, Vondrák K, Yilmaz A, Fischbach M, Schaefer F, Schmitt C (2017) O-11 effects of haemodiafiltration (HDF) vs conventional haemodialysis (HD) on growth and cardiovascular markers in children – data from the HDF vs HD (3H) study. Abstracts of the 50th anniversary ESPN meeting, Glasgow, September 2017. Pediatr Nephrol 32:1643–1834
Saran R, Bragg-Gresham JL, Rayner HC, Goodkin DA, Keen ML, Van Dijk PC, Kurokawa K, Piera L, Saito A, Fukuhara S, Young EW, Held PJ, Port FK (2003) Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int 64:254–262
Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC (2009) Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 119:671–679
Zoccali C, Moissl U, Chazot C, Mallamaci F, Tripepi G, Arkossy O, Wabel P, Stuard S (2017) Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol 28:2491–2497
Kayikcioglu M, Tumuklu M, Ozkahya M, Ozdogan O, Asci G, Duman S, Toz H, Can LH, Basci A, Ok E (2009) The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant 24:956–962
Ok E, Asci G, Chazot C, Ozkahya M, Mees EJ (2016) Controversies and problems of volume control and hypertension in haemodialysis. Lancet 388:285–293
Tycho Vuurmans JL, Boer WH, Bos WJ, Blankestijn PJ, Koomans HA (2002) Contribution of volume overload and angiotensin II to the increased pulse wave velocity of hemodialysis patients. J Am Soc Nephrol 13:177–183
Di Iorio B, Nazzaro P, Cucciniello E, Bellizzi V (2010) Influence of haemodialysis on variability of pulse wave velocity in chronic haemodialysis patients. Nephrol Dial Transplant 25:1579–1583
Gardner AW, Parker DE (2010) Association between arterial compliance and age in participants 9 to 77 years old. Angiology 61:37–41
Fischbach M, Edefonti A, Schröder C, Schröder C, Watson A, European Pediatric Dialysis Working Group (2005) Hemodialysis in children: general practical guidelines. Pediatr Nephrol 20:1054–1066
Wong MM, McCullough KP, Bieber BA, Bommer J, Hecking M, Levin NW, McClellan WM, Pisoni RL, Saran R, Tentori F, Tomo T, Port FK, Robinson BM (2017) Interdialytic weight gain: trends, predictors, and associated outcomes in the international dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 69:367–379
Zaloszyc A, Schaefer B, Schaefer F, Krid S, Salomon R, Niaudet P, Schmitt CP, Fischbach M (2013) Hydration measurement by bioimpedance spectroscopy and blood pressure management in children on hemodialysis. Pediatr Nephrol 28:2169–2177
Acknowledgments
The authors would like to thank Pr. Larry Greenbaum for his advices and his careful review of the manuscript.
Funding
Vasiliki Karava was supported by The Belgian Kids’ Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Karava, V., Benzouid, C., Kwon, T. et al. Interdialytic weight gain and vasculopathy in children on hemodialysis: a single center study. Pediatr Nephrol 33, 2329–2336 (2018). https://doi.org/10.1007/s00467-018-4026-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-4026-z