Abstract
Background
Hypertension is prevalent in children on dialysis and associated with cardiovascular disease. We studied the blood pressure (BP) trends and the evolution of BP over 1 year in children on conventional hemodialysis (HD) vs. hemodiafiltration (HDF).
Methods
This is a post hoc analysis of the “3H – HDF-Hearts-Height” dataset, a multicenter, parallel-arm observational study. Seventy-eight children on HD and 55 on HDF who had three 24-h ambulatory BP monitoring (ABPM) measures over 1 year were included. Mean arterial pressure (MAP) was calculated and hypertension defined as 24-h MAP standard deviation score (SDS) ≥95th percentile.
Results
Poor agreement between pre-dialysis systolic BP-SDS and 24-h MAP was found (mean difference − 0.6; 95% limits of agreement −4.9–3.8). At baseline, 82% on HD and 44% on HDF were hypertensive, with uncontrolled hypertension in 88% vs. 25% respectively; p < 0.001. At 12 months, children on HDF had consistently lower MAP-SDS compared to those on HD (p < 0.001). Over 1-year follow-up, the HD group had mean MAP-SDS increase of +0.98 (95%CI 0.77–1.20; p < 0.0001), whereas the HDF group had a non-significant increase of +0.15 (95%CI −0.10–0.40; p = 0.23). Significant predictors of MAP-SDS were dialysis modality (β = +0.83 [95%CI +0.51 − +1.15] HD vs. HDF, p < 0.0001) and higher inter-dialytic-weight-gain (IDWG)% (β = 0.13 [95%CI 0.06–0.19]; p = 0.0003).
Conclusions
Children on HD had a significant and sustained increase in BP over 1 year compared to a stable BP in those on HDF, despite an equivalent dialysis dose. Higher IDWG% was associated with higher 24-h MAP-SDS in both groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension (HTN) is common among children on dialysis [1], is often inadequately controlled [2], and is causally associated with adverse cardiovascular outcomes [3, 4]. HTN can cause left ventricular hypertrophy (LVH) and vascular stiffness [5], which are key pathogenic mechanisms for early cardiovascular events [6, 7]. Appropriate management of blood pressure (BP) in children on dialysis is important to prevent cardiovascular morbidity and reduce mortality [8]. Two large, prospective multicenter studies in children with chronic kidney disease (CKD) have shown that HTN is highly prevalent and associated with LVH and vasculopathy even in early CKD [9, 10] with an increase in prevalence of HTN as CKD progresses.
Previous studies have not identified therapeutic modifications to control BP adequately and around 30% of children with CKD on anti-hypertensive treatment have uncontrolled hypertension [2]. The “3H – HDF-Hearts-Height” study, a multicenter, longitudinal study in children receiving hemodiafiltration (HDF) compared to conventional hemodialysis (HD), showed that subclinical cardiovascular disease is prevalent in children on dialysis, with attenuated progression of vascular changes in children receiving HDF compared to conventional HD [11, 12]. HTN was significantly more common in HD compared to HDF patients, but the risk factors for HTN and effects of different dialysis modalities in controlling BP were not explored. In addition, 3H is one of the only studies in adults or children on HDF that has utilized 24-h ambulatory BP monitoring (ABPM) to characterize HTN, uniquely allowing an in-depth analysis of the BP profile, daytime and nocturnal HTN, as well as comparing ABPM with the routinely used pre-dialysis systolic and diastolic BP measurements.
In this study, we perform a post hoc analysis of the 3H data in order to determine the risk factors associated with the evolution of BP over a 1-year follow-up. Our hypothesis is that HDF achieves and maintains a normal BP in children without the need for anti-hypertensive medications.
Methods
Data collection
This is a post hoc analysis of the “3H – HDF-Hearts-Height” dataset. 3H was a multicenter, non-randomized, parallel-arm intervention study that was performed across 28 pediatric dialysis centers in 10 countries, following children receiving kidney replacement therapy with either HD or HDF for 1 year. Standardized procedures for HDF and HD were provided to all centers, but in order to keep the study as “real life” as possible individualized changes to the dialysis prescription were left to the treating physician. Inclusion criteria were incident and prevalent patients between 5 and 20 years of age undergoing post-dilution HDF or HD on a 4 h per session, three times per week schedule. A minimum follow-up of 12 months was required. Children in whom a living donor kidney transplant was planned, those on predilution HDF, and prevalent patients on HD in whom the single pool Kt/V was <1.2 in the month preceding recruitment were excluded. In order to determine the effect of convective clearance on outcome, only prevalent HD patients with single pool Kt/V > 1.2 in the preceding month were enrolled. Given that recent randomized controlled trials (RCTs) in adults have shown that all-cause and cardiovascular mortality are lower when higher convection volumes are used [13,14,15], we aimed for a comparable target convection volume of 12–15 L/m2 body surface area in children. Ultrapure dialysis fluid (defined as containing <0.1 colony-forming unit/ml (CFU/ml) and < 0.03 endotoxin unit/ml (EU/ml)) was used as per international standards and depending on availability in each unit. HD was performed with high-flux membranes using a similar blood flow rate and dialysate composition as on HDF. Full details are described in the publications on study design [11] and primary outcomes [12]. Here we focus on BP control, the evolution of BP over the 12 months follow-up, risk factors for HTN including dialysis-related parameters, and effect of anti-hypertensive medications on BP control. The study is registered with ClinicalTrials.gov (NCT02063776).
Of 177 children recruited, 133 children (78 [74%] on HD and 55 [77%] on HDF) completed 12 months follow-up and were included in this post-hoc analysis. Of the 44 children excluded, 35 (80%) progressed to transplantation and 9 moved center or were lost to follow-up. At baseline the HD and HDF patients were comparable for age, sex, race, underlying kidney disease, time on dialysis before start of the 3H study, previous transplantation, type of vascular access and residual kidney function [12]. At baseline, 26 (33%) on HD and 27 (49%) on HDF were on dialysis, with a median dialysis vintage of 24.5 (18–52) and 29.5 (17–53.3) months respectively (p = 0.91). As with all dialysis studies, incident patients were allowed a period of stability on dialysis before inclusion in the 3H study and had a median dialysis vintage of 1.03 (0.2–1.7) and 1.4 (0.61–1.9) months in the HD and HDF groups, respectively (p = 0.69). As previously described [12], within-center comparisons on incident patients on HD and HDF in the five largest centers, contributing 28 (36%) patients on HD and 18 (33%) on HDF, showed no difference in patient demographics or MAP-SD score between patients on HD and HDF (p > 0.05 for all).
Measurements
Children underwent ABPM using the Spacelab ABPM portable device (Spacelabs 90,207–2Q) as previously described [11]. ABPM and all BP measures were recorded in the mid-week dialysis period, with BP recordings starting immediately post-dialysis and continuing for a 24-h period. All patients had three ABPM measurements (baseline, 6 and 12 months). The 24-h BP measurements were obtained every 15 min during the day and every 30 min at night. For further analysis, ABPM profiles were divided into daytime (08:00 to 20:00 h) and nighttime periods (24:00 to 06:00 h). Routine measurements of systolic and diastolic BP measured by auscultation with a standard sphygmomanometer before the start of dialysis were collected, and the mean over the previous 4 weeks was used for analysis. Pre-dialysis (auscultatory) and ambulatory BP measurements were normalized for age, sex, and height and expressed as standard deviation scores (SDS) [16]. Systolic and diastolic BP SDS were derived from the National High Blood Pressure Education Program Working Group (NHBPEP) Fourth report [17].
Definition of variables
The time-averaged 24-h MAP was used for primary analyses and HTN defined as 24-h time-integrated MAP exceeding the 95th percentile [16]. Patients on anti-hypertensive medication were referred to as having controlled or uncontrolled HTN if their 24-h MAP was below or above the 95th percentile respectively. HTN was also defined based on pre-dialysis auscultatory systolic and diastolic BP SDS according to the European Society of Hypertension guidelines [18] that define HTN as a persistently elevated systolic or diastolic BP above the 95th percentile for gender, age and height measured on at least three separate occasions. The cut-off age considered in these guidelines is 16 years, beyond which the absolute values for defining HTN in adults must be used [18]. More recently, the American Heart Association (AHA) guidelines have moved this age cut-off to 13 years [19, 20]. The European Society of Hypertension guidelines have been used throughout this document, but comparison made with the AHA guidelines. As for 24-h MAP, uncontrolled HTN was defined as presence of HTN (above the 95th percentile) when the patient was on anti-hypertensive therapy. Masked HTN was defined as normal pre-dialysis systolic BP SDS but elevated 24-h MAP SDS, white coat HTN as elevated pre-dialysis BP SDS, but without MAP-hypertension.
SDS for height, weight, and BMI were calculated, using the Centers for Disease Control and Prevention growth charts [21]. Interdialytic weight gain percentile (IDWG%), ultrafiltration volume per session, and dialysate sodium levels, all expressed as the mean of the previous four mid-week dialysis sessions were recorded at baseline, 6 months and 12 months of follow-up. The 24-h urine output measured in the inter-dialytic period at the same time intervals of 0, 6, and 12 months was recorded.
Statistical analysis
MAP-SDS and pre-dialysis systolic and diastolic BP-SDS at 0 and 12 months are presented using box plots, stratified by age and dialysis modality. Univariable linear regression analysis was used to screen for parameters potentially associated with changes in the MAP-SDS. Parameters with p value <0.15 in univariable analysis were selected for a multivariable analysis. Ultrafiltration rate was excluded a priori from all multivariable analyses due to its interdependency with IDWG. Predictors of the dependent variable (24-h MAP-SDS) were indicated by the magnitude and sign of the beta estimates. Analyses were repeated considering daytime MAP, nighttime MAP, and pre-dialysis systolic and diastolic BP. However, as pre-dialysis systolic BP SDS was not normally distributed, we instead considered the presence/absence of HTN (binary analyses) using logistic regression, considering systolic and diastolic BP ≥ 95th percentile as our outcome.
The evolution of MAP-SDS over the 1-year study period was examined considering all measurements taken at 0, 6 and 12 months. This was first examined descriptively, considering the exact time since baseline that the 6- and 12-month MAP measurements were taken and divided into five groups (5–7 months, 8–10 months, 11–13 months, and 14–18 months plus baseline values) and plotted using a box plot, stratified by dialysis modality. Next, a multi-level linear regression model was conducted with an outcome of 24-h MAP-SDS, using all available measurements from participants over the study period. An unstructured correlation matrix was used to account for repeated observations on individuals, with a random intercept and time since baseline. Changes over time were investigated and found to follow an approximately linear relationship, so was fit as a continuous variable, with an interaction to account for any differences in rate of change over time according to dialysis modality. The robustness of these results was examined in a sensitivity analysis, by fitting a linear regression model with generalized estimating equations (GEE) and found to be consistent. Daytime and nighttime MAP and pre-dialysis (auscultatory) BP measurements were available at baseline and 12 months. Therefore, evolution of these measures was evaluated by considering the 12-month values using standard linear regression techniques. The agreement of MAP-SDS with pre-dialysis systolic BP SDS both at baseline and month 12 was assessed using a Bland–Altman analysis. Statistical analysis was performed using the SPSS software version 25.0 (SPSS, Chicago, IL) and SDS version 9.3 (SAS Institute Inc., Cary, NC). Differences indicated by a two-sided p value of <0.05 were considered statistically significant.
Results
Demographics, blood results, and medication use in the study cohort have been previously described [12]. Calcium channel blockers were the most commonly used drugs (52 children; 39%), followed by angiotensin converting enzyme inhibitors or angiotensin II receptor blockers (36 children; 18.8%), beta blockers (22; 11.5%), and diuretics (5; 3.8%). There was no difference in the anti-hypertensive medication classes used in HD and HDF patients.
ABPM at baseline
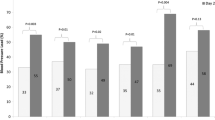
The 24-h MAP-SDS was higher in patients on HD compared to those on HDF in all age groups (Fig. 1a). At baseline, age, IDWG%, baseline ultrafiltration volume, and urine output were significantly associated with a higher 24-h MAP-SDS on univariable analysis (Table 1), with higher baseline IDWG% (0.25; [95%CI 0.04–0.28]; p = 0.03) significantly associated with a higher 24-h MAP-SDS on multivariable analysis. Overall, 64 (82%) children on HD and 24 (44%) patients on HDF had 24-h MAP-SDS > 95th percentile at baseline (Supplemental Table 1). In sub-group analyses of incident vs. prevalent dialysis patients, no risk factors for HTN were found in the incident cohort, but in prevalent dialysis patients, the IDWG% was a significant and independent risk factor for a higher 24-h MAP (0.47; [95%CI 0.15–0.50]; p = 0.001) on multivariable analysis.
Twenty-four-hour mean arterial BP (MAP) SDS by treatment modality and age groups at baseline (a) and 12 months (b). MAP-SDS is consistently lower in HDF compared to HD patients in all age categories both at baseline and 12 months. When compared by treatment modality, there is no difference in MAP-SDS in HD or HDF patients in the three age groups (baseline p = 0.19 and p = 0.17 respectively and 12 months p = 0.07 and p = 0.13 respectively). Box plots show the median, the 25th and 75th percentile within the shaded box area. The 5th and 95th percentile are shown as extremes of the whisker plots. The dotted line shows the SDS ≥ 1.65
Pre-dialysis auscultatory systolic and diastolic BP at baseline
In children on HD, the pre-dialysis systolic BP-SDS was significantly higher in the 5–10-year age group compared to HDF (p = 0.02). In the overall cohort, no difference was seen in systolic BP-SDS between HD and HDF cohorts (ANOVA; p = 0.56; Supplemental Fig. 1A). There was no difference in pre-dialysis diastolic BP-SDS between HD and HDF patients in any age category (Supplemental Fig. 2A). HTN using systolic and diastolic BP-SDS was more common for those aged 5 to 10 years compared to >15 years (OR 6.07; 95%CI 2.04–18; p = 0.001). Higher ultrafiltration volume was a risk factor for higher HTN defined by systolic and diastolic BP-SDS (OR 1.07; 95%CI 1.02–1.13; p = 0.01).
Prevalence of hypertension at 12 months
Sixty-nine patients (88%) on HD and 23 (42%) on HDF had MAP-SDS > 95th percentile at 12 months (Supplemental Table 1). The MAP-SDS was higher in patients on HD compared to those on HDF in all age groups (Fig. 1b). As previously published, both incident and prevalent patients on HD increased their MAP-SDS from baseline to 12 months (p = 0.007 and p = 0.004, respectively), whereas there was no change in incident or prevalent patients on HDF (p = 0.38 and p = 0.11, respectively) [12]. At 12 months, the only independent risk factor for a higher 24-h MAP-SDS was the HD modality (0.46; [95%CI 0.30–0.61]; p < 0.0001; Supplemental Table 2). When the same analysis was performed using HTN based on systolic and diastolic BP-SDS as outcome, HD modality (OR 4.92 vs. HDF; 95% CI 2–12.1; p = 0.001) and female gender (OR 3.47; 95% CI 1.5–7.7; p = 0.002) were independent risk factors.
Evolution of BP status
Twenty-four-hour MAP-SDS increased in both HD and HDF patients over the 12-month study period (adjusting for baseline MAP-SDS in both groups); there was a significant increase in HD (mean + 0.98 [95%CI 0.77–1.20] SDS; p < 0.0001) but no statistically significant increase in HDF patients (mean + 0.15 [95%CI −0.10 – +0.40] SDS; p = 0.23) (Fig. 2). In all patients irrespective of HD or HDF modality, higher 24-h MAP-SDS was associated with higher IDWG% (0.13 [95%CI 0.06–0.19]; p = 0.0003) on multivariable analysis (Table 2).
Changes in MAP-SDS over time calculated with the mixed-model. We measured the p values for the difference between all time points in HD and HDF children. For HD (baseline—6 months p = 0.015; 6 months—9 months p = 0.89; 9 months—12 months p = 0.006; 12 months—15 months p = 0.77). For HDF (baseline—6 months p = 0.069; 6 months—9 months p = 0.9; 9 months—12 months p = 0.058; 12 months—15 months p = 0.79)
Daytime and night-time MAP-SDS
On multivariable analysis, IDWG% was the only independent risk factor for a higher daytime and night-time MAP-SDS at baseline (0.22 [95%CI 0.04–0.4]; p = 0.01 and 0.26 [95%CI 0.09–0.43]; p = 0.003 respectively) whereas at 12 months HD modality (0.25 [95%CI 0.13–0.36]; p < 0.0001 and 0.47 [95%CI 0.32–0.63]; p < 0.0001) and age > 15 years compared to 5–10 years (0.26 [95%CI 0.06–0.45]; p = 0.01 and 0.25 [95%CI −0.07–0.42]; p = 0.01) were associated with a higher daytime and night-time MAP-SDS at 12 months.
Agreement between 24-h MAP-SDS and pre-dialysis (auscultatory) systolic BP-SDS
In both HD and HDF treatment modalities, the agreement between systolic BP-SDS and 24-h MAP-SDS was investigated by a Bland–Altman analysis. The systolic (auscultatory) BP-SDS systematically underestimated the 24-h MAP-SDS (mean difference = −0.6), with poor agreement and wide variability between the two measures (95% Limits of Agreement (95% LoA) −4.9–3.8; Fig. 3a). Consistent results were found for the 12-month values (mean difference − 1.42; 95% LoA −6.28–3.44; Fig. 3b), suggesting poor agreement between the two measures. When the definition of HTN was considered as per the new AHA guidelines [20], only three children diagnosed as hypertensive with the European definition [18] were high-normal by AHA guidelines.
When comparing HTN determined by ABPM and pre-dialysis BP, we found that of the children with ambulatory HTN, only 57% on HD and 26% on HDF were identified by pre-dialysis systolic BP.
Anti-hypertensive treatment
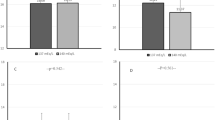
At baseline, 43 (55%) of HD and 24 (44%) of HDF patients were on anti-hypertensive medications, but uncontrolled HTN was present in 38 (88%) of HD and 6 (25%) of HDF patients (Fig. 4a). At 12 months, 45 (58%) on HD and 21 (38%) on HDF received anti-hypertensive medications; despite these, uncontrolled HTN was highly prevalent and present in 42/45 (93.3%) on HD and 8/21 (38%) on HDF (p < 0.0001; Fig. 4b). During the study period, 47 (80%) children who were not on anti-hypertensive therapy at baseline remained off anti-hypertensives; of these 27/33 (82%) on HD and 15/33 (45%) on HDF were hypertensive. Of the children taking one or more anti-hypertensives at baseline, 13 (19.4%) stopped all medications (2 on HD and 11 on HDF) and 10 (7.3%; 3 on HD and 7 patients on HDF) reduced the number of anti-hypertensive medications at 12 months.
Distribution of hypertension in HD and HDF patients based on MAP-SDS at baseline (a) and 12 months (b). The Y-axis indicates the percentage of patients in each category. The X-axis indicates the number of anti-hypertensive medications. Striped columns represent BP < 95th percentile and filled columns represent BP > 95th percentile
Discussion
This is the first prospective study in children on dialysis showing that HTN is significantly more common and increases more rapidly in children on conventional HD compared to a matched cohort on HDF, despite an equivalent dialysis dose. Over a 1-year follow-up, the MAP-SDS increased by 0.98 SDS in HD patients while there was an attenuated and non-significant increase of 0.15 SDS in HDF patients. Significant and independent risk factors that correlated with change in the MAP-SDS were the dialysis modality and the IDWG%, suggesting that effective volume control is the key to managing HTN in children on dialysis. Uncontrolled HTN was significantly more common in HD compared to HDF patients despite a greater use of anti-hypertensive medications, challenging the effectiveness of anti-hypertensives without effective volume control in dialysis patients. Pre-dialysis BP measurements showed a poor correlation with ABPM and cannot be relied on in dialysis patients. This study compliments our original publication [12] and includes detailed analysis of potential risk factors (age, sex, ethnicity, underlying kidney diagnosis, previous transplant, BMI, ultrafiltration volume, dialysate sodium, IDWG, urine output) related to a higher MAP-SDS at baseline, 12 months and through 1 year of follow-up. The same risk factors were analyzed also in relation to systolic and diastolic BP-SDS at baseline and 12 months. Correlation and agreement between MAP-SDS and SBP-SDS have been investigated. The effect of medications on ABPM is presented and the daytime and nocturnal BP analyzed separately.
Although HTN and its causal effects on LVH and cardiovascular disease are widely prevalent in dialysis patients [22], few studies have addressed the risk factors for HTN in this unique cohort of pediatric dialysis patients, nor examined interventions to attenuate its progression. The high prevalence of HTN in our cohort confirms previous studies showing that there is little improvement in the diagnosis and management of HTN in children with CKD over the past decade despite recent guidelines [17, 18] and a better understanding of the risks of HTN-related cardiovascular disease [1, 16, 23]. The 3H study is the first multicenter, prospective, parallel-arm observational study in children that studies the evolution of HTN and associated risk factors in children on HD and HDF.
Routine office BP measurements, the current cornerstone of HTN management, do not reflect the true BP load recorded by the “gold standard” method of ABPM [24]. Of note, as it is the SDS and not the absolute values for systolic or diastolic BP, a comparison of their SDS vs. that of MAP-SDS should not differ if both measures are truly the same. The fact that there is a difference between measures itself suggests that both measurement techniques are not identical and that the “gold standard” of MAP-SDS should be used in hemodialysis patients. Given that the circadian BP rhythms are markedly impaired and that the burden of nocturnal HTN is high among patients with CKD [24, 25], it is to be expected that ABPM provides a more accurate estimate of HTN. As ABPM facilitates the identification of specific BP phenotypes (such as masked, white coat and isolated nocturnal HTN), the wider adoption of this technique may also improve the management of HTN, particularly in children on dialysis. Twenty-four-hour ambulatory BP samples the patient over a range of extracellular fluid volumes and uremic states, and therefore has a greater prognostic significance, and correlates better with end-organ damage, including LVH, than a single pre-dialysis BP measurement [22, 26]. In the 3H study, we measured the 24-h mean ambulatory BP at three key study points, whereas all other randomized trials and most cohort studies on HDF in adults have relied on a single pre-dialysis BP reading to define HTN. This becomes even more important as, in children, masked HTN predisposes to the development of sustained HTN and LVH [27]. Indeed, two recent multicenter prospective studies in children with CKD have shown a similarly high proportion of masked HTN (15–35%) [5, 24], stressing the importance of performing regular ABPM measurements. The Cardiovascular Comorbidity in Children with CKD (4C) study has shown that approximately 20% of children with CKD3–5 have masked HTN [5], with similar rates of 37% with masked HTN in the Chronic Kidney Disease in Childhood (CKiD) study [24]. However, in a sub-group analysis of the CKiD study, the same authors report that in a cohort of CKD patients not on dialysis, the clinic BP taken in a protocol-driven setting were not inferior to ABPM in the discrimination of BP-related adverse outcomes of LVH or progression of CKD [28].
We found that a significant risk factor for an increase in 24-h MAP-SDS was a high IDWG%, implying that HTN in dialysis patients is closely related to their volume status. IDWG% is a surrogate for sodium mass removal rate [29], a key factor in the management of patients on chronic HD, both because a high IDWG% leads to a supra-physiological expansion of extracellular water, leading to volume overload, and also because excessive ultrafiltration during HD carries the risk of relative hypovolemia, reduction of myocardial perfusion and myocardial stunning, with negative effects on cardiac status [30]. A lower IDWG% suggests lower ultrafiltration rates per session and greater hemodynamic stability. As shown in the 3H outcomes paper, a lower IDWG% was directly associated with fewer symptoms of headaches, dizziness or cramps, fewer hypotensive episodes, and a shorter post-dialysis recovery time [12], with an associated improvement in school attendance and greater physical activity in children on HDF compared to those on conventional HD. The mechanisms underlying the better hemodynamic control of HDF compared to HD are still not fully understood. One hypothesis is that HDF allows a better fluid balance by providing a reinfusion in pre or post dilution and consequently allowing higher pre-dialysis systolic pressures to be obtained [31]. Secondly, the convective technique allows a more efficient removal of the middle molecules allowing a better and more lasting hemodynamic control. Finally, in HDF, the dialysate is naturally cooled, which in itself may reduce the rate of interdialytic hypotension compared with standard dialysis [32]. In addition to improved fluid removal, greater clearance of middle-molecular-weight uremic toxins by HDF may also play a role in greater hemodynamic stability on HDF. A pediatric study evaluated the inflammatory state and the changes in myocardial function in children on conventional HD after 6 months of switching to HDF, and showed that HDF significantly reduced the high sensitivity-CRP and improved diastolic function, but this did not correlate with improved BP [33]. Fischbach et al. have shown that pre-dilution HDF performed 6 days per week leads to normalization of BP and amelioration of LVH [34] but it is not clear if increased dialysis frequency or HDF per se resulted in improvement in the fluid status. Other authors report that there is no difference in BP control between HDF and HD patients [35, 36]; randomized trials are required to definitively answer this question.
In our cohort, the use of anti-hypertensive medications on extracorporeal dialysis (HD or HDF) did not improve BP control—despite the use of anti-hypertensive medications in nearly 60% of HD and 40% of HDF patients, 93% of children on HD, and 38% on HDF had uncontrolled HTN at 12-month follow-up. Several studies have confirmed that anti-hypertensive medications without adequate volume control are not useful in dialysis patients [37,38,39,40], yet a significant number of dialysis patients in our multicenter study were prescribed anti-hypertensive medications. The key to BP control in dialysis patients is much more related to maintaining a good fluid balance [38, 39]. It is important to avoid an overestimation of the optimal weight that can lead to an inadequate ultrafiltration prescription, resulting in chronic fluid overload and left ventricular strain, an important predictor of cardiovascular morbidity and mortality [41, 42]. On the other hand, underestimation of dry weight puts patients at risk of higher ultrafiltration rates, resulting in intradialytic hypotension symptoms. High ultrafiltration rates in children on HD have been correlated with higher left ventricular mass index (LVMI) [43]; and children with an IDWG% of >4% are at high risk of LVH [29, 43]. Bioimpedance spectroscopy improves the clinical assessment of hydration status in children on dialysis and correlates with established biomarkers such as NT-proBNP as well as peripheral pulse pressure and left ventricular end-diastolic diameter [44].
There are some limitations of our study, partly related to small numbers of pediatric dialysis patients, even though 3H included 40% of the pediatric extracorporeal dialysis cohort in Europe [12]. 3H was a non-randomized study, largely because all centers were not able to offer both HD and HDF modalities. Given the small numbers of children on dialysis, both incident and prevalent patients on dialysis were included; however, the two groups were comparable and on sub-group analysis there were no significant differences in the risk factors for HTN in the two groups. The 3H study was designed to have a short follow-up period of only 1 year as high transplantation rates in children preclude a longer study. The gold standard for evaluating BP in dialysis patients is 44-h ABPM, but many study centers did not have the facilities for this; however, future studies must consider 44-h ABPM measurements in dialysis patients. Data on systolic and diastolic BP and anti-hypertensive therapy were not available for the 6-month follow-up and this limited our analysis. We do not have data on nocturnal dipping from a number of centers, and are unable to report these findings. Cooling of dialysate in the HD cohort was not performed as the original 3H study design aimed to compare HDF with conventional HD; however, this is an area for future study. Body composition monitoring for an objective assessment of fluid overload must be considered in future studies.
In conclusion, our study of BP control in children on HD and HDF shows that HTN is prevalent in children on dialysis, but patients on HDF have an attenuated increase in BP compared to those on HD, with an almost 1 SD greater increase in MAP in HD compared to HDF cohorts in 1 year. Improved fluid management as indicated by lower IDWG%, rather than anti-hypertensive medications was associated with normal MAP-SDS. We suggest that HDF may be a superior dialysis modality for BP control compared to conventional HD, but confirmation through randomized trials is required.
References
Shroff R, Weaver DJ, Mitsnefes MM (2011) Cardiovascular complications in children with chronic kidney disease. Nat Rev Nephrol 7:642–649. https://doi.org/10.1038/nrneph.2011.116
Kramer AM, van Stralen KJ, Jager KJ, Schaefer F, Verrina E, Seeman T, Lewis MA, Boehm M, Simonetti GD, Novljan G, Groothoff JW (2011) Demographics of blood pressure and hypertension in children on renal replacement therapy in Europe. Kidney Int 80:1092–1098. https://doi.org/10.1038/ki.2011.232
Shroff R, Long DA, Shanahan C (2013) Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol 24:179–189. https://doi.org/10.1681/ASN.2011121191
de Ferranti SD, Steinberger J, Ameduri R, Baker A, Gooding H, Kelly AS, Mietus-Snyder M, Mitsnefes MM, Peterson AL, St-Pierre J, Urbina EM, Zachariah JP, Zaidi AN (2019) Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association. Circulation 139:e603–e634. https://doi.org/10.1161/CIR.0000000000000618
Urbina EM, Mendizábal B, Becker RC, Daniels SR, Falkner BE, Hamdani G, Hanevold C, Hooper SR, Ingelfinger JR, Lanade M, Martin LJ, Meyers K, Mitsnefes M, Rosner B, Samuels J, Flynn JT (2019) Association of blood pressure level with left ventricular mass in adolescents. Hypertension 74:590–596. https://doi.org/10.1161/HYPERTENSIONAHA.119.13027
Chesnaye NC, Schaefer F, Bonthuis M, Holman R, Baiko S, Baskın E, Bjerre A, Cloarec S, Cornelissen EAM, Espinosa L, Heaf J, Stone R, Shtiza D, Zagozdzon I, Harambat J, Jager KJ, Groothoff JW, van Stralen KJ, ESPN/ERA-EDTA Registry Committee (2017) Mortality risk disparities in children receiving chronic renal replacement therapy for the treatment of end-stage renal disease across Europe: an ESPN-ERA/EDTA registry analysis. Lancet 389:2128–2137. https://doi.org/10.1016/S0140-6736(17)30063-6
Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L (2012) United States renal data system 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 59(A7):e1–e420. https://doi.org/10.1053/j.ajkd.2011.11.015
Paglialonga F, Consolo S, Edefonti A, Montini G (2018) Blood pressure management in children on dialysis. Pediatr Nephrol 33:239–250. https://doi.org/10.1007/s00467-017-3666-8
Schaefer F, Doyon A, Azukaitis K, Bayazit A, Canpolat N, Duzova A, Niemirska A, Sözeri B, Thurn D, Anarat A, Ranchin B, Litwin M, Caliskan S, Candan C, Baskin E, Yilmaz E, Mir S, Kirchner M, Sander A, Haffner D, Melk A, Wühl E, Shroff R, Querfeld U, 4C Study Consortium (2017) Cardiovascular phenotypes in children with CKD: the 4C study. Clin J Am Soc Nephrol 12:19–28. https://doi.org/10.2215/CJN.01090216
Doyon A, Haas P, Erdem S, Ranchin B, Kassai B, Mencarelli F, Lugani F, Harambat J, Matteucci MC, Chinali M, Habbig S, Zaloszyc A, Testa S, Vidal E, Gimpel C, Azukaitis K, Kovacevic A, Querfeld U, Schaefer F (2019) Impaired systolic and diastolic left ventricular function in children with chronic kidney disease - results from the 4C study. Sci Rep 9:11462. https://doi.org/10.1038/s41598-019-46653-3
Shroff R, Bayazit A, Stefanidis CJ, Askiti V, Azukaitis K, Canpolat N, Agbas A, Anarat A, Aoun B, Bakkaloglu S, Bhowruth D, Borzych-Dużałka D, Bulut IK, Büscher R, Dempster C, Duzova A, Habbig S, Hayes W, Hegde S, Krid S, Licht C, Litwin M, Mayes M, Mir S, Nemec R, Obrycki L, Paglialonga F, Picca S, Ranchin B, Samaille C, Shenoy M, Sinha M, Smith C, Spasojevic B, Vidal E, Vondrák K, Yilmaz A, Zaloszyc A, Fischbach M, Schaefer F, Schmitt CP (2018) Effect of haemodiafiltration vs conventional haemodialysis on growth and cardiovascular outcomes in children - the HDF, heart and height (3H) study. BMC Nephrol 19:199. https://doi.org/10.1186/s12882-018-0998-y
Shroff R, Smith C, Ranchin B, Bayazit AK, Stefanidis CJ, Askiti V, Azukaitis K, Canpolat N, Ağbaş A, Aitkenhead H, Anarat A, Aoun B, Aofolaju D, Bakkaloglu SA, Bhowruth D, Borzych-Dużałka D, Bulut IK, Büscher R, Deanfield J, Dempster C, Duzova A, Habbig S, Hayes W, Hegde S, Krid S, Licht C, Litwin M, Mayes M, Mir S, Nemec R, Obrycki L, Paglialonga F, Picca S, Samaille C, Shenoy M, Sinha MD, Spasojevic B, Stronach L, Vidal E, Vondrák K, Yilmaz A, Zaloszyc A, Fischbach M, Schmitt CP, Schaefer F (2019) Effects of hemodiafiltration versus conventional hemodialysis in children with ESKD: the HDF, heart and height study. J Am Soc Nephrol 30:678–691. https://doi.org/10.1681/ASN.2018100990
Peters SA, Bots ML, Canaud B, Davenport A, Grooteman MP, Kircelli F, Locatelli F, Maduell F, Morena M, Nubé MJ, Ok E, Torres F, Woodward M, Blankestijn PJ, HDF Pooling Project Investigators (2016) Haemodiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant 31:978–984. https://doi.org/10.1093/ndt/gfv349
Grooteman MPC, van den Dorpel MA, Bots ML, Penne EL, van der Weerd NC, Mazairac AH, den Hoedt CH, van der Tweel I, Lévesque R, Nubé MJ, ter Wee PM, Blankestijn PJ, CONTRAST Investigators (2012) Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol 23:1087–1096. https://doi.org/10.1681/ASN.2011121140
Ok E, Asci G, Toz H, Ok ES, Kircelli F, Yilmaz M, Hur E, Demirci MS, Demirci C, Duman S, Basci A, Adam SM, Isik IO, Zengin M, Suleymanlar G, Yilmaz ME, Ozkahya M, Turkish Online Haemodiafiltration Study (2013) Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF study. Nephrol Dial Transplant 28:192–202. https://doi.org/10.1093/ndt/gfs407
Wühl E, Witte K, Soergel M, Mehls O, Schaefer F, German Working Group on Pediatric Hypertension (2002) Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens 20:1995–2007. https://doi.org/10.1097/00004872-200210000-00019
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, Invitti C, Litwin M, Mancia G, Pall D, Rascher W, Redon J, Schaefer F, Seeman T, Sinha M, Stabouli S, Webb NJ, Wühl E, Zanchetti A (2016) 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 34:1887–1920. https://doi.org/10.1097/HJH.0000000000001039
Gidding SS, Whelton PK, Carey RM, Flynn J, Kaelber DC, Baker-Smith C (2019) Aligning adult and pediatric blood pressure guidelines. Hypertension 73:938–943. https://doi.org/10.1161/HYPERTENSIONAHA.119.12653
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM, SUBCOMMITTEE ON SCREENING AND MANAGEMENT OF HIGH BLOOD PRESSURE IN CHILDREN (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140:e20171904. https://doi.org/10.1542/peds.2017-1904
(2019) Growth Charts - Clinical Growth Charts. https://www.cdc.gov/growthcharts/clinical_charts.htm. Accessed 5 Apr 2020
Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C (2006) Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension 47:62–68. https://doi.org/10.1161/01.HYP.0000196279.29758.f4
Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA, Chronic Kidney Disease in Children Study Group (2008) Blood pressure in children with chronic kidney disease: a report from the chronic kidney disease in children study. Hypertension 52:631–637. https://doi.org/10.1161/HYPERTENSIONAHA.108.110635
Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, Furth S, Chronic Kidney Disease in Children Study Group (2012) Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension 60:43–50. https://doi.org/10.1161/HYPERTENSIONAHA.111.189266
Düzova A, Karabay Bayazit A, Canpolat N, Niemirska A, Kaplan Bulut I, Azukaitis K, Karagoz T, Oguz B, Erdem S, Anarat A, Ranchin B, Shroff R, Djukic M, Harambat J, Yilmaz A, Yildiz N, Ozcakar B, Büscher A, Lugani F, Wygoda S, Tschumi S, Zaloszyc A, Jankauskiene A, Laube G, Galiano M, Kirchner M, Querfeld U, Melk A, Schaefer F, Wühl E, 4C Study Consortium (2019) Isolated nocturnal and isolated daytime hypertension associate with altered cardiovascular morphology and function in children with chronic kidney disease: findings from the Cardiovascular Comorbidity in Children with Chronic Kidney Disease study. J Hypertens 37:2247–2255. https://doi.org/10.1097/HJH.0000000000002160
Alborzi P, Patel N, Agarwal R (2007) Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol 2:1228–1234. https://doi.org/10.2215/CJN.02250507
Lurbe E, Torro I, Alvarez V, Nawrot T, Paya R, Redon J, Staessen JA (2005) Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension 45:493–498. https://doi.org/10.1161/01.HYP.0000160320.39303.ab
Ku E, McCulloch CE, Warady BA, Furth SL, Grimes BA, Mitsnefes MM (2018) Twenty-four-hour ambulatory blood pressure versus clinic blood pressure measurements and risk of adverse outcomes in children with CKD. Clin J Am Soc Nephrol 13:422–428. https://doi.org/10.2215/CJN.09630917
Paglialonga F, Consolo S, Galli MA, Testa S, Edefonti A (2015) Interdialytic weight gain in oligoanuric children and adolescents on chronic hemodialysis. Pediatr Nephrol 30:999–1005. https://doi.org/10.1007/s00467-014-3005-2
Hothi DK, Rees L, Marek J, Burton J, McIntyre CW (2009) Pediatric myocardial stunning underscores the cardiac toxicity of conventional hemodialysis treatments. Clin J Am Soc Nephrol 4:790–797. https://doi.org/10.2215/CJN.05921108
Locatelli F, Altieri P, Andrulli S, Bolasco P, Sau G, Pedrini LA, Basile C, David S, Feriani M, Montagna G, Di Iorio BR, Memoli B, Cravero R, Battaglia G, Zoccali C (2010) Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J Am Soc Nephrol 21:1798–1807. https://doi.org/10.1681/ASN.2010030280
Mustafa RA, Bdair F, Akl EA, Garg AX, Thiessen-Philbrook H, Salameh H, Kisra S, Nesrallah G, Al-Jaishi A, Patel P, Patel P, Mustafa AA, Schünemann HJ (2016) Effect of lowering the dialysate temperature in chronic hemodialysis: a systematic review and meta-analysis. Clin J Am Soc Nephrol 11:442–457. https://doi.org/10.2215/CJN.04580415
Fadel FI, Makar SH, Zekri H, Ahmed DH, Aon AH (2015) The effect of on-line hemodiafiltration on improving the cardiovascular function parameters in children on regular dialysis. Saudi J Kidney Dis Transpl 26:39–46. https://doi.org/10.4103/1319-2442.148731
Fischbach M, Terzic J, Menouer S, Dheu C, Seuge L, Zalosczic A (2010) Daily on line haemodiafiltration promotes catch-up growth in children on chronic dialysis. Nephrol Dial Transplant 25:867–873. https://doi.org/10.1093/ndt/gfp565
Beerenhout CH, Luik AJ, Jeuken-Mertens SG, Bekers O, Menheere P, Hover L, Klaassen L, van der Sande FM, Cheriex EC, Meert N, Leunissen KM, Kooman JP (2005) Pre-dilution on-line haemofiltration vs low-flux haemodialysis: a randomized prospective study. Nephrol Dial Transplant 20:1155–1163. https://doi.org/10.1093/ndt/gfh775
Vilar E, Fry AC, Wellsted D, Tattersall JE, Greenwood RN, Farrington K (2009) Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: a comparative analysis. Clin J Am Soc Nephrol 4:1944–1953. https://doi.org/10.2215/CJN.05560809
Chavers BM, Solid CA, Daniels FX, Chen SC, Collins AJ, Frankenfield DL, Herzog CA (2009) Hypertension in pediatric long-term hemodialysis patients in the United States. Clin J Am Soc Nephrol 4:1363–1369. https://doi.org/10.2215/CJN.01440209
Mitsnefes M, Stablein D (2005) Hypertension in pediatric patients on long-term dialysis: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Am J Kidney Dis 45:309–315. https://doi.org/10.1053/j.ajkd.2004.11.006
Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG (2003) Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 115:291–297. https://doi.org/10.1016/s0002-9343(03)00366-8
Mitsnefes MM (2005) Cardiovascular disease in children with chronic kidney disease. Adv Chronic Kidney Dis 12:397–405. https://doi.org/10.1053/j.ackd.2005.07.005
Jotterand Drepper V, Kihm LP, Kälble F, Diekmann C, Seckinger J, Sommerer C, Zeier M, Schwenger V (2016) Overhydration is a strong predictor of mortality in peritoneal dialysis patients - independently of cardiac failure. PLoS One 11:e0158741. https://doi.org/10.1371/journal.pone.0158741
Onofriescu M, Siriopol D, Voroneanu L, Hogas S, Nistor I, Apetrii M, Florea L, Veisa G, Mititiuc I, Kanbay M, Sascau R, Covic A (2015) Overhydration, cardiac function and survival in hemodialysis patients. PLoS One 10:e0135691. https://doi.org/10.1371/journal.pone.0135691
Fischbach M, Zaloszyc A, Shroff R (2015) The interdialytic weight gain: a simple marker of left ventricular hypertrophy in children on chronic haemodialysis. Pediatr Nephrol 30:859–863. https://doi.org/10.1007/s00467-015-3086-6
Eng CSY, Bhowruth D, Mayes M, Stronach L, Blaauw M, Barber A, Rees L, Shroff RC (2018) Assessing the hydration status of children with chronic kidney disease and on dialysis: a comparison of techniques. Nephrol Dial Transplant 33:847–855. https://doi.org/10.1093/ndt/gfx287
Acknowledgments
RS is funded by a National Institute for Health Research (NIHR), Career Development Fellowship for this research project. This publication presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. A part of the work took place in the Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London.
Funding
The 3H study was sponsored by Kidney Research UK. Part sponsorship was obtained from Fresenius Medical Care, who approved the study protocol, but had no role in data collection, data analysis or drafting the manuscript.
Author information
Authors and Affiliations
Contributions
RS is the Principal Investigator and obtained funding. RS, EV, CPS and FS designed the study. RS, FDZ and CS drafted the paper. CS performed the statistical analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
RS has an investigator-initiated study funded by Fresenius Medical Care and has received speaker honoraria from Fresenius Medical Care and Amgen. CPS and FS received funding for investigator-initiated research from Fresenius Medical Care, and CPD also from Amgen.
All authors to declare COI.
Ethics approval
The study was performed according to the principles of the declaration of Helsinki. It has been approved by the NRES (National Research Ethics Service) Committee London—Bloomsbury, a Research Ethics Committee established by the Health Research Authority, England. Approval from local Institutional Review Boards was obtained for each participating site.
Consent to participate
Full written informed consent has been obtained from all parents or caregivers, and assent from children, where applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Supplemental Fig. 1. Pre-dialysis systolic blood pressure SDS by treatment modality and age groups at baseline (1A) and 12 months (1B). Supplemental Fig. 2. Pre-dialysis diastolic blood pressure SDS by treatment modality and age groups at baseline (2A) and 12 months (2B). Supplemental Fig. 3. Distribution of hypertension in HD and HDF patients based on systolic and diastolic blood pressure at baseline (3A) and 12 months (3B). The Y-axis indicates the percentage of patients in each category. Striped columns represent BP < 95th percentile and filled columns represent BP > 95th percentile. (PDF 265 kb)
ESM 2
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
De Zan, F., Smith, C., Duzova, A. et al. Hemodiafiltration maintains a sustained improvement in blood pressure compared to conventional hemodialysis in children—the HDF, heart and height (3H) study. Pediatr Nephrol 36, 2393–2403 (2021). https://doi.org/10.1007/s00467-021-04930-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-04930-2