Abstract
Background
Controlled radial expansion (CRE) balloon dilators are traditionally used to dilate esophageal strictures during an esophagogastroduodenoscopy (EGD). EndoFLIP is a diagnostic tool used during an EGD to measure important parameters of the gastrointestinal lumen, capable of assessing treatment before and after dilation. EsoFLIP is a related device that combines a balloon dilator with high-resolution impedance planimetry to provide some of the luminal parameters in real time during dilation. We sought to compare procedure time, fluoroscopy time, and safety profile of esophageal dilation using either CRE balloon dilation combined with EndoFLIP (E + CRE) versus EsoFLIP alone.
Methods
A single-center retrospective review was performed to identify patients ≤ 21 years of age who underwent an EGD with biopsy and esophageal stricture dilation using E + CRE or EsoFLIP between October 2017 and May 2022.
Results
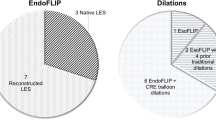
Twenty-nine EGDs with esophageal stricture dilation were performed in 23 patients (19 E + CRE and 10 EsoFLIP). The two groups did not differ in age, gender, race, chief complaint, type of esophageal stricture, or history of prior gastrointestinal procedures (all p > 0.05). The most common medical history in the E + CRE and EsoFLIP groups were eosinophilic esophagitis and epidermolysis bullosa, respectively.
Median procedures times were shorter in the EsoFLIP cohort compared to E + CRE balloon dilation (40.5 min [IQR 23–57 min] for the EsoFLIP group; 64 min [IQR 51–77 min] for the E + CRE group; p < 0.01). Median fluoroscopy times were also shorter for patients who underwent EsoFLIP (0.16 min [IQR 0–0.30 min] for EsoFLIP dilation; 0.30 min [IQR 0.23–0.55] for the E + CRE group; p = 0.003). There were no complications or unplanned hospitalizations in either group.
Conclusion
EsoFLIP dilation of esophageal strictures was faster and required less fluoroscopy than CRE balloon dilation combined with EndoFLIP in children, while being equally as safe. Prospective studies are needed to further compare the two modalities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
An esophageal stricture (ES), defined as a fixed narrowing of the esophagus, is relatively uncommon in the pediatric population, but is associated with significant morbidities such as failure to thrive, dysphagia, and aspiration. Etiologies for esophageal strictures vary and generally fall into three major categories: congenital, acquired, or functional causes. Congenital esophageal stenosis (CES) can be further classified according to the pathohistological type: tracheobronchial remnants, fibromuscular stenosis, and membranous webbing or esophageal membrane. An estimated 10–15% of esophageal stenosis in children are congenital, with the three most common causes being congenital webs, tracheobronchial remnants, and idiopathic muscular hypertrophy [1,2,3,4,5]. Surgical intervention during infancy is often required to treat CES [2]. Acquired esophageal stenosis (AES) in children most often occurs secondary to caustic ingestion (50–60%), with other causes including postsurgical anastomotic stenosis (10–20%), gastroesophageal reflux disease (3–4%), inflammatory disorders (< 1%), tumors (< 0.1%), eosinophilic esophagitis, and epidermolysis bullosa. AES is usually managed with endoscopic balloon dilation, but refractory cases may require surgical intervention [1,2,3,4, 6,7,8,9,10,11,12]. Achalasia is one of the most common functional causes of ES in children [1, 2].
Balloon dilation of an ES occurs during an esophagogastroduodenoscopy (EGD), and mostly involves the use of controlled radial expansion (CRE) balloon dilators, which are widely used in both children and adults with a reported success rate of 76–100%. The ease, safety, and effectiveness of balloon dilation in the management of pediatric ES has been demonstrated in several studies [5, 13,14,15,16,17,18,19,20,21,22,23,24]. Adjunctive fluoroscopy is often used intraoperatively to check for a perforation. The use of fluoroscopy comes with dose- and time-dependent exposure to ionizing radiation for the patient and medical staff, which can have both immediate and long-lasting effects [25,26,27]. Data have shown that even low doses of radiation, such as those during diagnostic imaging, may be associated with increased risk for cancer mortality [27, 28]. The International Commission on Radiological Protection (ICRP) recommends limiting medical radiation exposure to as low as reasonably achievable (ALARA) [29].
Endoluminal functional lumen imaging probe (EndoFLIP) (Medtronic, Minneapolis, Minnesota) is a relatively new endoscopic tool that can measure the mechanical properties of the gastrointestinal (GI) lumen during an EGD. Through high-resolution impedance planimetry and volume-controlled distension, several luminal parameters (i.e., diameter, cross-sectional area (CSA), compliance, distensibility index (DI), and pressure) can be obtained in real time. EsoFLIP is both a diagnostic and therapeutic tool that, like EndoFLIP, uses high-resolution impedance planimetry to measure esophageal luminal parameters, but it is housed within a rigid balloon, allowing for therapeutic dilation. EndoFLIP is approved by the Food and Drug Administration (FDA) for patients 5 years of age and over, while EsoFLIP is approved for patients 18 years of age and over. Both modalities are available in select pediatric centers. EndoFLIP and EsoFLIP have both been used to evaluate several pediatric esophageal disease processes, including esophageal stenosis, esophageal atresia, reflux esophagitis, eosinophilic esophagitis, esophageal duplication cysts, and achalasia [30,31,32,33,34,35,36,37,38,39,40,41]. However, there are no studies that have compared EsoFLIP with CRE balloon dilation in the pediatric population. In this study, we sought to compare the procedure time, fluoroscopy time, and safety profile of esophageal dilation using CRE balloon dilation combined with EndoFLIP (E + CRE) versus EsoFLIP.
Materials and methods
Study design
A single-center retrospective chart review was performed to identify all patients ≤ 21 years of age who underwent an EGD with biopsy and dilation of an esophageal stricture with either E + CRE or EsoFLIP between October 2017 and May 2022. The study was approved by the Johns Hopkins University Institutional Review Board.
Data assessment
Electronic medical records were reviewed. Demographic, clinical, and procedural data were extracted from the record. Procedural data included esophageal luminal parameters (i.e., diameter, CSA, compliance, DI, and pressure). Procedural data also included the total length of the procedure based on time from scope-in to scope-out, total fluoroscopy exposure time, and any associated complications. All families had a follow-up phone call from the hospital the day after the procedure to screen for post-procedural complications.
Endoscopy
All procedures were performed in the endoscopy unit at Johns Hopkins Children’s Center by one pediatric gastroenterologist (KN). Each case was completed under general anesthesia and sedation managed by a pediatric anesthesiologist. No paralytic agents were used. EGDs were performed using an Olympus gastroscope (model GIF-H180 or GIF-H190, Tokyo, Japan). Biopsies were taken at the discretion of the endoscopist.
EndoFLIP procedure
FLIP catheter size was determined by the height of the patient. An 8 cm catheter was used for children under 42 inches in height, and a 16 cm catheter for children 42 inches or taller. Pre-study catheter calibration was performed by the pediatric endoscopy nurse using EndoFLIP software per the manufacturer’s guidelines (Medtronic, Minneapolis, Minnesota). EndoFLIP analysis was completed before and after CRE dilation with placement of the FLIP catheter at the site of stenosis under direct visualization using the gastroscope. The catheter was inserted into the oral cavity and advanced alongside the gastroscope. The balloon catheter’s position was centered at the stricture after inflation to 15 mL using a sodium chloride-based (0.30%) solution supplied by the manufacturer in each kit. The gastroscope tip was positioned above the top of the balloon catheter. Serial measurements were then recorded at 20 mL, 30 mL, 40 mL, and 50 mL at the discretion of the endoscopist. Value sets in which an intra-bag pressure ≥ 15 mmHg was not achieved were excluded based on manufacturer recommendations from adult data [42]. Balloon inflation was stopped if the balloon pressures exceeded 60 mmHg per the manufacturer’s guidelines. The DI value was calculated by the computer (CSA (mm2) divided by the intra-bag pressure (mmHg) needed to maintain the select area) [43]. At the end of the procedure, the EndoFLIP balloon was deflated and removed.
CRE dilation
The size of the CRE dilation balloon selected (CRE PRO Wireguided Esophageal Balloon Dilatation Catheter; Boston Scientific, Marlborough, Massachusetts) was determined by the stricture size measured by EndoFLIP (pre-dilation). During the CRE dilation, the middle of the balloon catheter was centered at the esophageal stenosis under direct visualization using the gastroscope. At the endoscopist’s discretion, spot-film fluoroscopy was used during catheter placement, if needed. The CRE balloon was first inflated to the nearest millimeter from the measured value on EndoFLIP. Next, the balloon was inflated incrementally (holding each time for ~ 60 s). The goal dilation diameter was approximately 3–4 mm from the measured value by EndoFLIP. After dilation, water-soluble contrast was administered into the esophagus and fluoroscopic images were taken to assess for esophageal perforation.
EsoFLIP
Pre-study catheter calibration was performed by the pediatric endoscopy nurse using FLIP software per the manufacturer’s guidelines (Medtronic, Minneapolis, Minnesota). A 20 mm EsoFLIP balloon was chosen for all cases based on the physiologic diameter of the pediatric esophagus. The EsoFLIP catheter was advanced from the mouth alongside the gastroscope and then positioned at the esophageal stenosis. With the gastroscope tip positioned above the top of the balloon portion, the EsoFLIP catheter was inflated to 20 mL and the diameter of the stenotic area was recorded. Next, the catheter was inflated in 2 mL increments until there was either complete effacement of the esophageal lumen or an increase of ~ 3 mm in the diameter of the esophagus. Once this desired change was seen and/or complete effacement was achieved, the inflated balloon was left in place for 60 s before deflation and removal. Luminal parameters were monitored in real time throughout the procedure and were recorded by the operating room staff. If there were any concerns for injury beyond the mucosa, fluoroscopy, using water-soluble contrast in the esophagus, was performed at the discretion of the endoscopist prior to procedural completion.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS version 25.0, Armonk, New York). Categorical data were evaluated by either the Chi-square test or the Fisher’s exact test. Independent continuous variables were compared using the Mann–Whitney U test. Two-sided p values < 0.05 were considered statistically significant.
Results
Patient characteristics
Twenty-nine EGDs with esophageal stricture dilation were performed on 23 patients (Table 1). Of these, 19 procedures were completed using E + CRE, and 10 were completed using EsoFLIP. The two groups did not differ in age, gender, race, chief complaint, type of esophageal stricture (congenital versus acquired), or prior GI-related procedures (all p > 0.05). Eosinophilic esophagitis was more common in the E + CRE group, while epidermolysis bullosa was more common in the EsoFLIP group. The median age of patients was 14.2 years (IQR 6.8–16.2) in the E + CRE group and 9.2 years (IQR 6.15–15.75) in the EsoFLIP group. Most patients were male and Caucasian (62% and 70%, respectively). The most common chief complaint in both groups was dysphagia (69–70% in each group). All patients had an esophageal stricture at the time of the procedure.
Procedural data
The median scope-in to scope-out procedural time was shorter in the EsoFLIP group compared to the E + CRE group (40.5 min [IQR 23–57], n = 19; 64 min (IQR 51–77], n = 9; p < 0.01) (Table 2). The median fluoroscopy exposure time was also shorter in the EsoFLIP group than in the E + CRE group (0.16 min [IQR 0–0.3], n = 9; 0.3 min [IQR 0.23–0.55], n = 19; p = 0.03) (Table 2). There were no unplanned hospitalizations or serious adverse events in either group, including esophageal perforation, bleeding, infections, pneumonia, cardiac arrest, or death.
Discussion
This is the first study to compare EsoFLIP and CRE balloon dilation. We showed that dilation of pediatric esophageal strictures with EsoFLIP was faster and required less fluoroscopy when compared to CRE balloon dilation combined with EndoFLIP in our smaller cohort. Despite FLIP technology being relatively new, especially in the pediatric population, we found both EsoFLIP and E + CRE to be relatively safe. These findings support our previously published work comparing the use of EndoFLIP in children less than and older than five years of age, where we found EndoFLIP to be safe and effective in both age groups [44].
EsoFLIP is a variant of EndoFLIP that combines the function of a balloon dilator with features of EndoFLIP. It yields important luminal parameters (i.e., diameter and CSA) in real time as the endoscopist dilates the gastrointestinal lumen. Currently, there is limited data on the use of EsoFLIP, especially in the pediatric population. In children, EsoFLIP has been successfully used to manage esophageal strictures, including those associated with epidermolysis bullosa and eosinophilic esophagitis [30, 45]. In adults, EsoFLIP appears to be utilized more often, and has been used to treat esophageal strictures [46], achalasia [47,48,49,50,51,52], esophagogastric junction outflow obstruction (EGJOO) [51], and refractory gastroparesis [53, 54]. Collectively, the available literature suggests that EsoFLIP is easy to use, effective, and safe. Proposed advantages of using EsoFLIP over traditional dilation modalities include the ability to control the exact size of dilation through volumetric expansion of the balloon in real time without the need of fluoroscopy [46, 50]. This allows the endoscopist to better understand the impact of the intervention. Limitations for using EsoFLIP include inability to pass the balloon catheter through the scope, time required to fill and empty the balloon, inability to provide pressure values without an optional external pressure monitoring tool, and a current lack of robust data on its effectiveness [46].
Though fluoroscopy is not required with EsoFLIP, one may elect to use fluoroscopy after EsoFLIP dilation to ensure esophageal perforation has not occurred, especially in cases with concern for injury beyond the mucosa. Fluoroscopy may also be used both during catheter placement and after CRE balloon dilation [55, 56], though some adult studies have shown that luminal dilation can still be safe and effective without post-procedural fluoroscopy [57,58,59,60]. Non-fluoroscopic CRE balloon dilation has not yet been universally adopted in the pediatric population. We believe that the decreased fluoroscopy exposure with the use of EsoFLIP in our study may have been due to lower concerns for perforation in the EsoFLIP cohort, as we were able to monitor esophageal parameters during dilation.
This study was a retrospective review of children who underwent esophageal dilation at our center using either E + CRE or EsoFLIP. When decisions were made regarding the choice of dilation modality, several factors were considered, including equipment availability, provider preference at the time of the procedure, and past medical history. We elected to use EsoFLIP for most cases of esophageal strictures in epidermolysis bullosa as data suggests that patients with epidermolysis bullosa respond well to EsoFLIP dilation [30]. EsoFLIP was also selected in this group of patients to minimize mucosal trauma from instrumentation. In most patients with eosinophilic esophagitis, E + CRE was selected because the DI value, measured by EndoFLIP but not EsoFLIP, has been described as an important metric for eosinophilic esophagitis [33]. The EndoFLIP diameter measurement also helped with CRE balloon selection. Cost may be another consideration, which can vary between centers. At our institution, EsoFLIP catheters were more expensive than CRE catheters, but cheaper than the two catheters required for E + CRE. The approximate balloon dilation equipment costs were $313 to $403 per EsoFLIP catheter ($1566 to $2015 for a box of 5 depending on the balloon diameter), $167 to $213 per CRE balloon catheter, and $427 per EndoFLIP balloon ($2135 for a box of 5). The estimated total balloon dilation equipment cost of each E + CRE procedure ranged from $594 to $640.
There were some limitations to our study. First, this was a single-center, single-operator, retrospective study that was limited by sample size, which may impact the generalizability and power of our results. We observed no complications or unplanned hospitalizations in either group, though complications from dilation of pediatric strictures are very rare with an estimated rate of 1–1.5% [23, 61,62,63]. Another limitation was that we did not compare the procedure times for EsoFLIP and CRE balloon dilation alone. Comparing these two procedures alone was considered. However, we compared EsoFLIP and E + CRE as we believed that this comparison would provide more information on diagnostic yield and therapeutic capabilities. For example, EndoFLIP and EsoFLIP, but not CRE balloon dilation alone, allow for the measurement of luminal parameters and provide objective data for direct comparisons of post-dilation changes. Furthermore, our institutional practice is to use EndoFLIP pre- and post-CRE balloon dilation in part to help identify the correct CRE balloon dilator, reducing complication risk. Although EndoFLIP did increase the overall procedure time in the E + CRE cohort, we estimate that this accounted for less than 10 min for each case. We also believe that data acquisition variability was minimized as all procedures were performed by one single endoscopist (KN).
Conclusion
EsoFLIP is a unique tool capable of both assessing and dilating the GI lumen in real time. EsoFLIP dilation was faster and required less fluoroscopy than CRE balloon dilation combined with EndoFLIP for pediatric esophageal strictures in our small cohort. Further larger-scale prospective studies are needed to compare these two modalities.
References
Ghiselli A, Bizzarri B, Ferrari D, Manzali E, Gaiani F, Fornaroli F, Nouvenne A, Di Mario F, De’Angelis GL (2018) Endoscopic dilation in pediatric esophageal strictures: a literature review. Acta Biomed 89:27–32. https://doi.org/10.23750/abm.v89i8-S.7862
Sag E, Bahadir A, Imamoglu M, Sag S, Reis GP, Erduran E, Cakir M (2020) Acquired noncaustic esophageal strictures in children. Clin Exp Pediatr 63:447–450. https://doi.org/10.3345/cep.2020.00199
Raboei E, Alabdali A, Sayed MH, Yousef Y, Bawazir O, Alsaggaf A, Kattan M, Mustafa L, Algarawi A, Albadawi R, Soofy S, Aldhubiban K (2021) The outcome of pediatric esophageal strictures managed with endoscopic balloon dilation in Saudi Arabia. J Laparoendosc Adv Surg Tech A 31:210–215. https://doi.org/10.1089/lap.2020.0455
Brzački V, Mladenović B, Jeremić L, Živanović D, Govedarović N, Dimić D, Golubović M, Stoičkov V (2019) Congenital esophageal stenosis: a rare malformation of the foregut. Nagoya J Med Sci 81:535–547. https://doi.org/10.18999/nagjms.81.4.535
Cakmak M, Boybeyi O, Gollu G, Kucuk G, Bingol-Kologlu M, Yagmurlu A, Aktug T, Dindar H (2016) Endoscopic balloon dilatation of benign esophageal strictures in childhood: a 15-year experience. Dis Esophagus 29:179–184. https://doi.org/10.1111/dote.12305
Thomson M, Tringali A, Dumonceau J-M, Tavares M, Tabbers MM, Furlano R, Spaander M, Hassan C, Tzvinikos C, Ijsselstijn H, Viala J, Dall’Oglio L, Benninga M, Orel R, Vandenplas Y, Keil R, Romano C, Brownstone E, Hlava Š, Gerner P, Dolak W, Landi R, Huber WD, Everett S, Vecsei A, Aabakken L, Amil-Dias J, Zambelli A (2017) Paediatric gastrointestinal endoscopy: European Society for Paediatric Gastroenterology Hepatology and Nutrition and European Society of Gastrointestinal Endoscopy Guidelines. J Pediatr Gastroenterol Nutr 64:133–153. https://doi.org/10.1097/MPG.0000000000001408
Aprigliano F (1980) Chevalier Jackson Lecture. Esophageal stenosis in children. Ann Otol Rhinol Laryngol 89:391–396. https://doi.org/10.1177/000348948008900501
Dall’Oglio L, Caldaro T, Foschia F, Faraci S, Federici di Abriola G, Rea F, Romeo E, Torroni F, Angelino G, De Angelis P (2016) Endoscopic management of esophageal stenosis in children: new and traditional treatments. World J Gastrointest Endosc 8:212–219. https://doi.org/10.4253/wjge.v8.i4.212
Saleem MM (2009) Acquired oesophageal strictures in children: emphasis on the use of string-guided dilatations. Singapore Med J 50:82–86
Vandenplas Y (2017) Management of benign esophageal strictures in children. Pediatr Gastroenterol Hepatol Nutr 20:211–215. https://doi.org/10.5223/pghn.2017.20.4.211
Pearson EG, Downey EC, Barnhart DC, Scaife ER, Rollins MD, Black RE, Matlak ME, Johnson DG, Meyers RL (2010) Reflux esophageal stricture–a review of 30 years’ experience in children. J Pediatr Surg 45:2356–2360. https://doi.org/10.1016/j.jpedsurg.2010.08.033
Baird R, Laberge J-M, Lévesque D (2013) Anastomotic stricture after esophageal atresia repair: a critical review of recent literature. Eur J Pediatr Surg 23:204–213. https://doi.org/10.1055/s-0033-1347917
Alshammari J, Quesnel S, Pierrot S, Couloigner V (2011) Endoscopic balloon dilatation of esophageal strictures in children. Int J Pediatr Otorhinolaryngol 75:1376–1379. https://doi.org/10.1016/j.ijporl.2011.07.031
Uygun I, Arslan MS, Aydogdu B, Okur MH, Otcu S (2013) Fluoroscopic balloon dilatation for caustic esophageal stricture in children: an 8-year experience. J Pediatr Surg 48:2230–2234. https://doi.org/10.1016/j.jpedsurg.2013.04.005
Dai D-L, Zhang C-X, Zou Y-G, Yang Q-H, Zou Y, Wen F-Q (2020) Predictors of outcomes of endoscopic balloon dilatation in strictures after esophageal atresia repair: a retrospective study. World J Gastroenterol 26:1080–1087. https://doi.org/10.3748/wjg.v26.i10.1080
Chang C-F, Kuo S-P, Lin H-C, Chuang C-C, Tsai T-K, Wu S-F, Chen A-C, Chen W, Peng C-T (2011) Endoscopic balloon dilatation for esophageal strictures in children younger than 6 years: experience in a medical center. Pediatr Neonatol 52:196–202. https://doi.org/10.1016/j.pedneo.2011.05.005
Antoniou D, Soutis M, Christopoulos-Geroulanos G (2010) Anastomotic strictures following esophageal atresia repair: a 20-year experience with endoscopic balloon dilatation. J Pediatr Gastroenterol Nutr 51:464–467. https://doi.org/10.1097/MPG.0b013e3181d682ac
Akarsu C, Unsal MG, Dural AC, Kones O, Kocatas A, Karabulut M, Kankaya B, Ates M, Alis H (2015) Endoscopic balloon dilatation as an effective treatment for lower and upper benign gastrointestinal system anastomotic stenosis. Surg Laparosc Endosc Percutan Tech 25:138–142. https://doi.org/10.1097/SLE.0000000000000090
Allmendinger N, Hallisey MJ, Markowitz SK, Hight D, Weiss R, McGowan G (1996) Balloon dilation of esophageal strictures in children. J Pediatr Surg 31:334–336. https://doi.org/10.1016/s0022-3468(96)90733-2
Geng L, Gong S, Huang H, He W, Ou W, Pan R, Huo X, Chen B (2008) Balloon dilation with gastroscope for esophageal stricture in children. Zhonghua Er Ke Za Zhi 46:895–898
Ikeya T, Ohwada S, Ogawa T, Tanahashi Y, Takeyoshi I, Koyama T, Morishita Y (1999) Endoscopic balloon dilation for benign esophageal anastomotic stricture: factors influencing its effectiveness. Hepatogastroenterology 46:959–966
Kahriman G, Hosgecin C, Herdem N, Dogan A, Altay D, Pehlivan SS (2022) Fluoroscopy-guided balloon dilatation of benign esophageal strictures in children: 11-year experience. Pediatr Radiol 52:977–984. https://doi.org/10.1007/s00247-021-05253-y
Lan LCL, Wong KKY, Lin SCL, Sprigg A, Clarke S, Johnson PRV, Tam PKH (2003) Endoscopic balloon dilatation of esophageal strictures in infants and children: 17 years’ experience and a literature review. J Pediatr Surg 38:1712–1715. https://doi.org/10.1016/j.jpedsurg.2003.08.040
Youn BJ, Kim WS, Cheon J-E, Kim W-Y, Shin S-M, Kim I-O, Yeon KM (2010) Balloon dilatation for corrosive esophageal strictures in children: radiologic and clinical outcomes. Korean J Radiol 11:203–210. https://doi.org/10.3348/kjr.2010.11.2.203
Takenaka M, Hosono M, Hayashi S, Nishida T, Kudo M (2022) How should radiation exposure be handled in fluoroscopy-guided endoscopic procedures in the field of gastroenterology? Dig Endosc 34:890–900. https://doi.org/10.1111/den.14208
Mettler FA, Mahesh M, Bhargavan-Chatfield M, Chambers CE, Elee JG, Frush DP, Miller DL, Royal HD, Milano MT, Spelic DC, Ansari AJ, Bolch WE, Guebert GM, Sherrier RH, Smith JM, Vetter RJ (2020) Patient exposure from radiologic and nuclear medicine procedures in the United States: procedure volume and effective dose for the period 2006–2016. Radiology 295:418–427. https://doi.org/10.1148/radiol.2020192256
Bang JY, Hough M, Hawes RH, Varadarajulu S (2020) Use of artificial intelligence to reduce radiation exposure at fluoroscopy-guided endoscopic procedures. Am J Gastroenterol 115:555–561. https://doi.org/10.14309/ajg.0000000000000565
Richardson DB, Cardis E, Daniels RD, Gillies M, O’Hagan JA, Hamra GB, Haylock R, Laurier D, Leuraud K, Moissonnier M, Schubauer-Berigan MK, Thierry-Chef I, Kesminiene A (2015) Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 351:5359. https://doi.org/10.1136/bmj.h5359
(2007) The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 37:1–332. https://doi.org/10.1016/j.icrp.2007.10.003
Ng K, Mogul D, Hollier J, Khashab MA (2020) Utility of functional lumen imaging probe in esophageal measurements and dilations: a single pediatric center experience. Surg Endosc 34:1294–1299. https://doi.org/10.1007/s00464-019-06898-5
Abreu M, Nunes I, Corujeira S, Tavares M, Trindade E, Dias JA (2016) Caustic esophageal stenosis: a case report of endoscopic dilation with a dynamic stent. GE Port J Gastroenterol 23:218–223. https://doi.org/10.1016/j.jpge.2015.12.006
Taylor JS, Danzer E, Berquist WE, Wall JK (2018) Dilation of esophageal stricture in a pediatric patient using functional lumen imaging probe technology without the use of fluoroscopy. J Pediatr Gastroenterol Nutr 67:e20–e21. https://doi.org/10.1097/MPG.0000000000001936
Menard-Katcher C, Benitez AJ, Pan Z, Ahmed FN, Wilkins BJ, Capocelli KE, Liacouras CA, Verma R, Spergel JM, Furuta GT, Muir AB (2017) Influence of age and eosinophilic esophagitis on esophageal distensibility in a pediatric cohort. Am J Gastroenterol 112:1466–1473. https://doi.org/10.1038/ajg.2017.131
Williamson P, Proudfoot J, Gharibans A, Dohil L, Newbury R, Barsamian J, Hassan M, Rawson R, Katzka D, Kurten R, Dohil R, Mousa H, Aceves S (2020) Plasminogen activator inhibitor-1 as a marker of esophageal functional changes in pediatric eosinophilic esophagitis. Clin Gastroenterol Hepatol S1542–3565(20):31370–31377. https://doi.org/10.1016/j.cgh.2020.09.040
Korotkaya Y, Kunisaki S, Ng K (2020) Use of EndoFLIP to diagnose a duplication cyst in a child with chronic dysphagia. J Pediatr Gastroenterol Nutr 71:e97. https://doi.org/10.1097/MPG.0000000000002738
Petrosyan M, Khalafallah AM, Guzzetta PC, Sandler AD, Darbari A, Kane TD (2016) Surgical management of esophageal achalasia: evolution of an institutional approach to minimally invasive repair. J Pediatr Surg 51:1619–1622. https://doi.org/10.1016/j.jpedsurg.2016.05.015
Yeung F, Wong IYH, Chung PHY, Wong KKY, Law SYK, Tam PKH (2018) Peroral endoscopic myotomy with EndoFLIP and double-endoscope: novel techniques for achalasia in pediatric population. J Laparoendosc Adv Surg Tech A 28:343–347. https://doi.org/10.1089/lap.2017.0268
Wood LS, Chandler JM, Portelli KE, Taylor JS, Kethman WC, Wall JK (2020) Treating children with achalasia using per-oral endoscopic myotomy (POEM): Twenty-one cases in review. J Pediatr Surg 55:1006–1012. https://doi.org/10.1016/j.jpedsurg.2020.02.028
Kethman WC, Thorson CM, Sinclair TJ, Berquist WE, Chao SD, Wall JK (2018) Initial experience with peroral endoscopic myotomy for treatment of achalasia in children. J Pediatr Surg 53:1532–1536. https://doi.org/10.1016/j.jpedsurg.2017.07.023
Rosen R, Garza JM, Nurko S (2020) Functional luminal imaging probe assessment in postfundoplication patients changes management beyond manometry. J Pediatr Gastroenterol Nutr 70:e119–e123. https://doi.org/10.1097/MPG.0000000000002658
Kotilea K, Mahler T, Bontems P, Devière J, Louis H (2018) Management of esophageal motility disorders in children: a review. Acta Gastroenterol Belg 81:295–304
Pandolfino J, Clarke J, Vela M, Gyawali P, Yadlapati R, Khan A, Carlson D (2018) EndoFLIPTM impedance planimetry system protocol and interpretation. Medtronic Review: White Paper
Carlson DA, Kou W, Lin Z, Hinchcliff M, Thakrar A, Falmagne S, Prescott J, Dorian E, Kahrilas PJ, Pandolfino JE (2019) Normal values of esophageal distensibility and distension-induced contractility measured by functional luminal imaging probe panometry. Clin Gastroenterol Hepatol 17:674-681.e1. https://doi.org/10.1016/j.cgh.2018.07.042
Hoskins B, Almazan E, Mogul D, Ng K (2022) endoluminal functional lumen imaging probe is safe in children under five years old. J Pediatr Gastroenterol Nutr 74:e148. https://doi.org/10.1097/MPG.0000000000003430
Lirio RA, Nazarey P, O’Dea J, Lightdale JR (2015) Sa1664 the first case report of Esoflip for dilation of a pediatric esophageal stricture. Gastrointest Endosc 81:299–300. https://doi.org/10.1016/j.gie.2015.03.1424
Baumann AJ, Carlson DA (2020) EsoFLIP for esophageal dilation: proposed advantages. Curr Opin Gastroenterol 36:329–335. https://doi.org/10.1097/MOG.0000000000000639
O’Dea J, Siersema PD (2013) Esophageal dilation with integrated balloon imaging: initial evaluation in a porcine model. Therap Adv Gastroenterol 6:109–114. https://doi.org/10.1177/1756283X12467566
Kappelle WFW, Bogte A, Siersema PD (2015) Hydraulic dilation with a shape-measuring balloon in idiopathic achalasia: a feasibility study. Endoscopy 47:1028–1034. https://doi.org/10.1055/s-0034-1392481
Schnurre L, Murray FR, Schindler V, Runggaldier D, Fischbach L, Bordier V, Pohl D (2020) Short-term outcome after singular hydraulic EsoFLIP dilation in patients with achalasia: a feasibility study. Neurogastroenterol Motil 32:e13864. https://doi.org/10.1111/nmo.13864
Brindise E, Khashab MA, El Abiad R (2021) Insights into the endoscopic management of esophageal achalasia. Ther Adv Gastrointest Endosc 14:26317745211014704. https://doi.org/10.1177/26317745211014706
Sloan JA, Triggs JR, Pandolfino JE, Dbouk M, Brewer Gutierrez OI, El Zein M, Quader F, Ichkhanian Y, Gyawali CP, Rubenstein JH, Khashab MA (2020) Treatment experience with a novel 30-mm hydrostatic balloon in esophageal dysmotility: a multicenter retrospective analysis. Gastrointest Endosc 92:1251–1257. https://doi.org/10.1016/j.gie.2020.04.076
Ahuja NK, Clarke JO (2017) The role of impedance planimetry in the evaluation of esophageal disorders. Curr Gastroenterol Rep 19:7. https://doi.org/10.1007/s11894-017-0544-2
Lacy BE, Cangemi D (2021) Pyloric dilation with EsoFLIP: time to “flip” treatment options for refractory gastroparesis? Gastrointest Endosc 94:495–497. https://doi.org/10.1016/j.gie.2021.04.014
Murray FR, Schindler V, Hente JM, Fischbach LM, Schnurre L, Deibel A, Hildenbrand FF, Tatu AM, Pohl D (2021) Pyloric dilation with the esophageal functional lumen imaging probe in gastroparesis improves gastric emptying, pyloric distensibility, and symptoms. Gastrointest Endosc 94:486–494. https://doi.org/10.1016/j.gie.2021.03.022
Standards of Practice Committee, Egan JV, Baron TH, Adler DG, Davila R, Faigel DO, Gan S, Hirota WK, Leighton JA, Lichtenstein D, Qureshi WA, Rajan E, Shen B, Zuckerman MJ, VanGuilder T, Fanelli RD (2006) Esophageal dilation. Gastrointest Endosc 63:755–760. https://doi.org/10.1016/j.gie.2006.02.031
Blount KJ, Lambert DL, Shaffer HA, de Lange EE (2010) Fluoroscopically guided balloon dilation of the esophagus. Semin Intervent Radiol 27:232–240. https://doi.org/10.1055/s-0030-1253519
Wang Y-G, Tio T-L, Soehendra N (2002) Endoscopic dilation of esophageal stricture without fluoroscopy is safe and effective. World J Gastroenterol 8:766–768. https://doi.org/10.3748/wjg.v8.i4.766
Chiu Y-C, Hsu C-C, Chiu K-W, Chuah S-K, Changchien C-S, Wu K-L, Chou Y-P (2004) Factors influencing clinical applications of endoscopic balloon dilation for benign esophageal strictures. Endoscopy 36:595–600. https://doi.org/10.1055/s-2004-814520
Rana SS, Bhasin DK, Chandail VS, Gupta R, Nada R, Kang M, Nagi B, Singh R, Singh K (2011) Endoscopic balloon dilatation without fluoroscopy for treating gastric outlet obstruction because of benign etiologies. Surg Endosc 25:1579–1584. https://doi.org/10.1007/s00464-010-1442-y
Kumar P, Jena A, Birda CL, Singh H, Gupta P, Prasad KK, Dutta U, Sharma V (2022) Safety and efficacy of non-fluoroscopic endoscopic dilatation of gastrointestinal tuberculosis related strictures. BMC Gastroenterol 22:60. https://doi.org/10.1186/s12876-022-02140-0
Shah MD, Berman WF (1993) Endoscopic balloon dilation of esophageal strictures in children. Gastrointest Endosc 39:153–156. https://doi.org/10.1016/s0016-5107(93)70055-3
Hsieh K-H, Soong W-J, Jeng M-J, Lee Y-S, Tsao P-C, Chou Y-L (2018) Flexible endoscopic diagnosis and treatment of esophageal stenosis in children with noninvasive ventilation support. Pediatr Neonatol 59:31–34. https://doi.org/10.1016/j.pedneo.2016.11.003
Alessia G, Barbara B, Daniela F, Elisabetta M, Federica G, Fabiola F, Antonio N, Francesco DM, Gian LD (2018) Endoscopic dilation in pediatric esophageal strictures: a literature review. Acta Biomed 89:27–32. https://doi.org/10.23750/abm.v89i8-S.7862
Acknowledgements
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Brett Hoskins, Mr. Erik Almazan, Ms. Brenna Hohl, and Dr. Kenneth Ng have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoskins, B., Almazan, E., Hohl, B. et al. Esophageal dilation with EsoFLIP is faster than CRE balloon dilation combined with EndoFLIP in children. Surg Endosc 37, 6308–6314 (2023). https://doi.org/10.1007/s00464-023-10129-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10129-3