Abstract
Background
Robotic-assisted Kasai portoenterostomy (RAKPE) has been utilized to treat biliary atresia (BA). However, RAKPE is not widely performed and its efficacy remains unknown. We summarized the experience of RAKPE for BA and determined its efficacy.
Materials and methods
We retrospectively analyzed 25 consecutive infants with non-syndromic type III BA who received RAKPE in our center from January 2020 to July 2021. RAKPE is a three-arm setup and four-trocar operation. Bipolar coagulation was used to dissect the small blood vessels at the hepatic portal. The fibrous cone was shallowly transected with bending electric scissors, followed by gelatin sponge compression to staunch the hemorrhage. Finally, a wide anastomosis was accurately constructed. Demographics and outcomes were recorded.
Results
The mean operative time was 211.64 ± 18.93 min. No conversion to laparotomy or intraoperative complications occurred. The mean estimated blood loss was 7.64 ± 2.43 mL. Enteral feeding was resumed after 3.44 ± 1.23 days. All patients achieved bile excretion postoperatively, and dark green bile-stained stools were passed 1.50 days (range 1.00–3.00 days) after surgery. The average postoperative length of hospital stay was 10.32 ± 2.59 days. The jaundice clearance (JC) rate was 76.00% within 6 months after surgery and the incidence of cholangitis was 48.00% within 1 year following surgery. The survival with native liver (SNL) rate was 80.00% at 1 year and 66.67% at 2 years.
Conclusion
RAKPE can be regarded as a treatment option for patients with BA due to the good outcomes reported. However, long-term studies comparing open or laparoscopic approaches are needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Since 1959, open Kasai portoenterostomy (OKPE) has been introduced to restore bile drainage for patients with BA and become the gold standard [1]. Esteves et al. reported laparoscopic Kasai portoenterostomy (LKPE) for BA in 2002 [2], but its efficacy remains controversial compared with OKPE [3,4,5]. Several centers have revealed positive results with modified LKPE procedures [6,7,8]. Nonetheless, LKPE is still a complex and challenging procedure with difficulties in fiber block dissection and anastomosis, resulting in a long learning curve. With merits of articulating wrists, 3D imaging field of vision and filter tremor, robotic surgery has been gradually applied to hepatobiliary disorders in children [9]. Theoretically, robotic-assisted Kasai portoenterostomy (RAKPE) may overcome the difficulties of LKPE in fiber block dissection and anastomosis, thereby becoming a better option for BA. Currently, reports of RAKPE in infants with BA are limited to small case series [10, 11], and its effectiveness remains controversial. Herewith, we present the results of 25 BA patients treated with RAKPE and discuss the technical details.
Materials and methods
Patients
We conducted a retrospective single-center analysis of 25 consecutive patients with type III BA who received RAKPE from January 2020 to July 2021. The study was approved by the Ethical Committee of Tongji Medical College, Huazhong University of Science and Technology (2016-LSZ-S180). Informed consent was obtained from parents or guardians.
The gender, age, body weight, serum bilirubin, liver enzyme, serum matrix metalloproteinase-7 (MMP-7) levels and abdominal ultrasonography findings of the patients before the operation were reviewed. Routine hepatobiliary iminodiacetic acid (HIDA) scan and magnetic resonance cholangiopancreatography (MRCP) examinations were not performed before surgery. All RAKPE procedures were performed by the same surgical team using the da Vinci Surgical System Si (Intuitive Surgical, Sunnyvale, CA).
The intraoperative complications, jaundice clearance (JC), frequency of cholangitis episodes, and 1- and 2-year survival with native liver (SNL) were recorded. JC was defined as serum total bilirubin level ≤ 20 μmol/L (or ≤ 1.2 mg/dL) within 6 months after the Kasai operation. JC within 6 months after surgery is widely used as the accepted measure of successful KPE [12,13,14]. Cholangitis was defined as having more than two clinical presentations [fever (> 38 °C) or stool color change or increased/increasing jaundice] and two laboratory tests [elevated inflammatory parameters or increased/increasing transaminases or increased/increasing gamma-glutamyl transferase (GGT)/bilirubin]. Indications for postoperative liver transplantation included failed Kasai operation, liver cirrhosis, liver failure, severe malnutrition, recurrent cholangitis and bleeding due to portal hypertension.
Surgical technique
RAKPE was performed with a three-arm setup and four-trocars. Following general anesthesia, the patients were positioned in a supine position and the body was elevated to approximately 10 cm from the operating table (Fig. 1A), with the right liver slightly raised. The posture was based on our experience with robotic surgery for choledochal cysts. The positions of four trocars are shown in Fig. 1B. The 12 mm trocar was utilized to establish the carbon dioxide pneumoperitoneum at a pressure of 6–10 mmHg and served as a camera port.
Pre‑docking stage
Intraoperative hepatic subcapsular spider-like telangiectasis (HSST) sign and cholangiography were observed to confirm the diagnosis. Laparoscopic exploration of the abdominal cavity revealed the characteristic HSST sign (Fig. 2) in all patients with BA [15, 16]. Cholangiography was performed to diagnose BA when extrahepatic bile ducts were still present and the gallbladder was incomplete atrophy. Otherwise, RAKPE was performed directly when the gallbladder was atresic for punction. Traction sutures to the gallbladder and round ligament were initially applied laparoscopically using 2–0 absorbable sutures to fully expose the hilar region. We found the Treitz ligament and exteriorized the proximal jejunum through the umbilical trocar. The Roux-en-Y jejunojejunostomy reconstruction was fashioned extracorporeally using the Echelon Flex™ powered plus stapler (Ethicon Endo-Surgery, Cincinnati, OH, USA) at the jejunum 18 cm away from the Treitz ligament. The anastomosed jejunum was returned into the abdominal cavity, and the Roux-en-Y limb (30–35 cm) was placed without tension to the portal hepatis via the retrocolic route under direct inspection by the laparoscope.
Docking stage
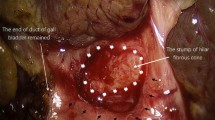
Dissecting forceps and electrocautery were applied to dissociate the gallbladder, and the fibrous cone above the bifurcation of the main portal vein could be found along the proximal section of the bile duct. We divided the tissue surrounding the fibrous cone at portal hepatis by gentle blunt dissection and clearly exposed the hepatic artery and portal vein. Then, all portal vein tributaries that drain into the fibrous cone (typically 3–5 branches) were coagulated by Maryland bipolar coagulation to expose the portal plate for resection (Fig. 3A and B). With electric scissors, the fibrous cone of the hilar region was transected from left to right (the level of transection depends on adequate bile outflow), and the fibrous cone around the left and right bile ducts was clipped (Fig. 4A). This step can clearly distinguish the border between the liver parenchyma and the fibrous mass, thereby preventing damage to the liver. The opening of microbile ducts and abundant bile outflow were clearly visible (Fig. 4B). A minor quantity of bleeding was controlled by direct pressure with a gelatin sponge (pressure for 10–20 min). No electrocautery was used to control the bleeding on the portal plate throughout the operation. Last, an end-to-side hepaticojejunostomy was conducted with one-layer continuous 5–0 PDS sutures posteriorly and anteriorly. The jejunum and right hepatic porta were fixed with a suture and tightened to bring the two ends of the anastomosis closer, facilitating anastomosis of the posterior wall. With careful attention to avoid damaging blood vessels and microbile ducts, the posterior wall of the jejunum was anastomosed with the connective tissue around the fibrous cone transection behind the portal vein (Fig. 5A and B). While the anterior wall was anastomosed with the liver capsule in front of the fibrous cone transection (Fig. 6). Notably, the sutures at the 2 and 10 o'clock positions were only used at the outermost edge of the fibrous cone. The suture was finally knotted at the left hepatic porta, and both the left and right hepatic hilum were located within the anastomosis. After the Roux limb was fixed to the transverse mesocolon, the liver tissue was obtained for pathological biopsy. A drainage tube was left under the liver, and the incision was closed.
Postoperative treatment and management
All patients were routinely given prophylactic intravenous cefoperazone for 2 weeks postoperatively, followed by oral tebipenem pivoxil for 6 months. Oral ursodeoxycholic acid was administered to patients for 1 year once oral intake was resumed. The patients received a decreasing dose regimen of methylprednisone starting on postoperative day 5 at an initial dose of 4 mg/kg/day for 3 months, which was then decreased by 1 mg/kg every 3 days until discontinuation. Postoperative outcomes were routinely followed up by outpatient visits at 1, 3, 6, 12 and 24 months for all patients.
When the patients suffered from cholangitis, they were readmitted with intravenous meropenem and prednisone for 1–2 weeks. Patients with resistant cholangitis who did not respond to conventional therapy were given intravenous immunoglobulin. The patients were discharged after cholangitis subsided and were given oral tebipenem pivoxil and methylprednisone for 6 months. If patients progressed to liver failure or other indicators as listed above, liver transplantation was offered.
Statistical analysis
Data were analyzed by SPSS software version 26.0. Categorical variables were presented as counts (n) and percentages (%). Continuous variables were presented as the mean ± standard deviation (SD) or median (range).
Results
RAKPE was successfully carried out in 25 patients with BA (17 females and 8 males) in our center. Histological study of liver biopsies of all patients was consistent with the diagnosis of BA. The characteristics and preoperative data of all cases are listed in Table 1, and the intraoperative data and postoperative outcomes are shown in Table 2. The mean age at operation was 59.12 ± 6.32 days, and the mean weight was 4.84 ± 0.53 kg. The preoperative total bilirubin, direct bilirubin, alanine transaminase, aspartate transaminase and γ-glutamyltransferase levels were 164.00 ± 31.88 μmol/L, 89.79 ± 21.56 μmol/L, 127.92 ± 81.78 U/L, 168.08 ± 94.98 U/L and 395.42 ± 284.05 U/L, respectively. The serum MMP-7 level was 69.16 ± 20.98 ng/mL. Preoperative ultrasonography revealed a triangular cord sign (> 4 mm) in 22 patients (88.00%), a contracted or invisible gall bladder in 20 patients (80.00%) and invisible bile ducts in 19 patients (76.00%). All patients underwent an uneventful operation without conversion, and there were no intraoperative complications including hepatic artery injury, main portal vein injury, bile duct injury, other organ injury and bleeding requiring blood transfusion. The mean operative time was 211.64 ± 18.93 min, and the mean estimated intraoperative blood loss was 7.64 ± 2.43 mL. The patients resumed enteral feeding in 3.44 ± 1.23 days. The bile excretion rate was 100% and bile-stained stools were passed 1.50 days (range 1.00–3.00 days) after RAKPE. The mean postoperative hospital stay was 10.32 ± 2.59 days, and the median follow-up was 23 months (range 12–27 months). During follow-up, all patients had good recovery with nearly invisible scars. JC was obtained in 19 patients (76.00%). Twelve (48.00%) patients had cholangitis within 1 year postoperatively, including 7 patients with single cholangitis and 5 patients with recurrent cholangitis (≥ 2 times). Unfortunately, 6 patients had persistent jaundice and/or recurrent cholangitis, eventually progressing to liver failure and requiring liver transplantation at 10, 10, 11, 11, 12 and 14 months. To date, there have been no deaths in this study. The SNL rate was 80.00% at 1 year and 66.67% at 2 years.
Discussion
To date, this is the largest case series of BA patients receiving RAKPE. The outcome of the surgery has been optimized by this surgical procedure which is highly effective. We achieved encouraging outcomes (Table 2) that were comparable to the outcomes obtained by LKPE (Table 3) [6, 8, 17,18,19,20], and these were not worse than those of OKPE [8, 21, 22]. It might be related to the younger age of the patients, and the robot's ability to better perform extended shallow dissection and wide portoenterostomy anastomosis. In addition, RAKPE has the advantage of being LKPE and is technically easy. These findings suggest that RAKPE is effective and could be an appropriate choice for BA.
BA is a disease characterized by progressive obliteration and inflammation of extrahepatic bile ducts that results in the death of untreated children before 3 years of age [23]. OKPE affords good short-term prognosis to patients and delays the need for liver transplantation [1]. Modified OKPE further improves the efficacy by the application of surgical microscopes or surgical loupes [24], dislocation of the liver outside the abdominal cavity for the best exposure of the porta hepatis [25], extensive dissection at the porta hepatis [26, 27], and shallow fibrous mass transection and wide suturing for portoenterostomy [21].
LKPE has been utilized to treat BA with benefits in improved visualization by magnification, less wound pain, better cosmetic appearance and minimal intraabdominal adhesions. However, its efficacy remains controversial and it was once opposed by the International Pediatric Endosurgery Group in 2007 [2, 28, 29]. Concerns about LKPE have mainly focused on the difficulty of fibrous cone resection and thermal injury to the microbile ducts. It takes a great deal of experience for operators to perform LKPE, which is challenging for most surgeons with a long learning curve [30,31,32,33]. However, in the modified procedure, the LigaSure device was used instead of monopole hook diathermy for dividing portal vein branches at the porta hepatis and dissecting the biliary remnant to minimize lateral thermal damage. [34, 35]. Recently, an increasing number of studies have indicated that LKPE has favorable results comparable to those of OKPE [6, 8, 17, 30, 34, 36, 37]. Nevertheless, LKPE is still not a preferred option for BA in many centers and remains challenging (especially in the resection of fibrous cones) that is not as flexible as OKPE.
The application of the Da Vinci system is gradually extending from adults to infants. It has been widely used in children with choledochal cysts and is gaining acceptance in complex operations, such as portoenterostomy. In 2007, Dutta et al. [10] reported the practice of RAKPE in three cases, and Meehan et al. [11] shared the experience of RAKPE in two cases, both of which demonstrated the feasibility of RAKPE. The large size of the robotic system may cause collisions in the limited operating space. Based on our experience with OKPE and LKPE for BA, and robotic-assisted surgery for choledochal cysts in young children (aged ≤ 1 year) [38, 39], we triangulate the position of the ports according to the primary operating region. It could space the ports to avoid intraoperative instrument collisions, which varies from prior reports of RAKPE [10, 11]. Moreover, we adopted a hybrid approach, first exteriorizing the proximal jejunum by laparoscopy and then creating an external end-to-side anastomosis of the jejunum. It was returned to the abdominal cavity and delivered to the porta hepatis under laparoscopic guidance. The da Vinci Surgical System was then applied to perform fibrous block resection and hepaticojejunostomy to reduce the re-docking of robotic system. All patients underwent RAKPE successfully, with a mean operative time of 211.64 ± 18.93 min, which was shorter than RAKPE in two previous reports [10, 11] and similar to that of LKPE (Table 3). This was also related to the shorter anastomosis time and use of the Echelon FlexTM powered plus endoscopic linear cutter stapler to establish a jejunojejunostomy extracorporeally, which shortens the operative time by 30–45 min. No conversion or intraoperative complications were encountered in this series, illustrating the feasibility of the optimized RAKPE.
RAKPE is appropriate for BA, especially for the fibrous cone resection which is the most determining step as this technique can combine the advantages of modified OKPE and LKPE. First, we used a bipolar coagulation hook to coagulate portal vein tributaries (usually 3 branches, up to 5 branches) bridging from the portal vein to the fibrous mass. It would restrict the blood supply to the fibrous mass to minimize the risk of bleeding. Following this process, it was easier to control the hemorrhage with gelatin sponge compression. No electrocautery was required to stop the bleeding, which could avoid damage to the liver parenchyma and small bile ducts. Second, the magnified surgical field of RAKPE offers a clearer view than that of surgical loupes. In particular, the clear visual field of the hepatic hilum allows surgeons to perform a smooth operation akin to delivering the liver extracorporeally for dissection in an open manner. This makes operations in the deep abdominal cavity easier and the blood circulation of the liver was not impaired. We used curved scissors to accurately and fully excise the fibrous mass in a manner akin to open surgery, especially the biliary remnant at the left and right sides of the hepatic hilum. A distinct plate between the liver parenchyma and the fibrous mass could be identified during this process to help determine the resection level, making the level and width of the fiber block resection more appropriate. Abundant bile outflow from microbile ducts could be observed vividly, and sufficient patency of microbile ducts is necessary for bile excretion postoperatively and JC during follow-up. Third, another advantage of RAKPE is the ability to use the multiangle articulated instruments to perform tighter and more precise anastomosis than LKPE. We sutured the jejunal limb precisely between the left and right portal veins to construct a wide and shallow anastomosis, ensuring that all microbile ducts on the transverse surface were contained within the jejunal limb lumen. Notably, at 2 o'clock and 10 o'clock points, the suture should be placed at the extreme outer margin of the transected biliary remnant. This maneuver helped minimize injury to the microbile ducts and small blood vessels while guaranteeing bile outflow through the hepaticojejunostomy, which may explain our favorable outcomes (Table 3). No anastomotic leakage occurred in our center. At last, RAKPE provides a pleasant ergonomic feeling that reduces the fatigue experience and takes away the impact of fatigue on surgical standards. Besides, age at surgery has been found in studies to be one of the factors affecting the prognosis of BA patients [40, 41]. Thanks to the high accuracy of serum MMP-7 in the early diagnosis of BA [42, 43], the mean age at surgery in this series was 59.12 ± 6.32 days (eighteen patients aged ≤ 60 days and seven patients aged > 60 days).
Although the study has revealed satisfactory outcomes with a large series of BA patients undergoing RAKPE, the retrospective nature of this study, operation experience of a single surgeon, and the lack of comparative data for open or laparoscopic operation are the major limitations of the study. Therefore, a prospective comparative study involving a larger sample size is needed to assess the role of RAKPE for BA. It was not available for the assessment of long-term outcomes in our present study because RAKPE has been tried for less than 5 years. Next, we plan to conduct a comparative study and evaluate long-term follow-up results over the next few years.
Nonetheless, RAKPE also has several shortcomings. One of the concerns was the bulky robotic system, which may cause postoperative cosmetic issues. Although the 12 mm camera of the da Vinci Si system we used is larger than the 8 mm camera of the fourth-generation da Vinci Xi system, we believe that the two cameras have a similar cosmetic appearance. Since the camera was inserted through the umbilical incision, the size of the camera has little impact on the appearance. High cost is another limitation of RAKPE. Moreover, the equipment lacks tactile feedback, which will be solved with further experience and technological refinement [44].
Conclusion
Our data suggested that RAKPE is safe and effective as a surgical treatment for BA. It is worthy of recommendation. RAKPE has great advantages in complete resection of fibrous cones and the establishment of an accurately shallow anastomosis with adequate width.
References
Kasai M (1959) A new operation for “noncorrectable” biliary atresia: hepatic portoenterostomy. Shujutsu 13:733–739
Esteves E, Clemente NE, Ottaiano NM, Devanir J Jr, Esteves Pereira R (2002) Laparoscopic Kasai portoenterostomy for biliary atresia. Pediatr Surg Int. https://doi.org/10.1007/s00383-002-0791-6
Lishuang M, Zhen C, Guoliang Q, Zhen Z, Chen W, Long L, Shuli L (2015) Laparoscopic portoenterostomy versus open portoenterostomy for the treatment of biliary atresia: a systematic review and meta-analysis of comparative studies. Pediatr Surg Int. https://doi.org/10.1007/s00383-015-3662-7
Li Y, Gan J, Wang C, Xu Z, Zhao Y, Ji Y (2019) Comparison of laparoscopic portoenterostomy and open portoenterostomy for the treatment of biliary atresia. Surg Endosc. https://doi.org/10.1007/s00464-019-06905-9
Hinojosa-Gonzalez DE, Bueno LC, Roblesgil-Medrano A, Salgado-Garza G, Hurtado-Arellano S, Farias JS, Torres-Martinez M, Escarcega-Bordagaray JA, Salan-Gomez M, Flores-Villalba E (2021) Laparoscopic vs open portoenterostomy in biliary atresia: a systematic review and meta-analysis. Pediatr Surg Int. https://doi.org/10.1007/s00383-021-04964-5
Ji Y, Yang K, Zhang X, Jin S, Jiang X, Chen S, Xu Z (2021) The short-term outcome of modified laparoscopic Kasai portoenterostomy for biliary atresia. Surg Endosc. https://doi.org/10.1007/s00464-020-07530-7
Shirota C, Murase N, Tanaka Y, Ogura Y, Nakatochi M, Kamei H, Kurata N, Hinoki A, Tainaka T, Sumida W, Yokota K, Makita S, Oshima K, Uchida H (2020) Laparoscopic Kasai portoenterostomy is advantageous over open Kasai portoenterostomy in subsequent liver transplantation. Surg Endosc. https://doi.org/10.1007/s00464-019-07108-y
Murase N, Hinoki A, Shirota C, Tomita H, Shimojima N, Sasaki H, Nio M, Tahara K, Kanamori Y, Shinkai M, Yamamoto H, Sugawara Y, Hibi T, Ishimaru T, Kawashima H, Koga H, Yamataka A, Uchida H (2019) Multicenter, retrospective, comparative study of laparoscopic and open Kasai portoenterostomy in children with biliary atresia from Japanese high-volume centers. J Hepatobiliary Pancreat Sci. https://doi.org/10.1002/jhbp.594
Lorincz A, Langenburg S, Klein MD (2003) Robotics and the pediatric surgeon. Curr Opin Pediatr. https://doi.org/10.1097/00008480-200306000-00006
Dutta S, Woo R, Albanese CT (2007) Minimal access portoenterostomy: advantages and disadvantages of standard laparoscopic and robotic techniques. J Laparoendosc Adv Surg Tech A. https://doi.org/10.1089/lap.2006.0112
Meehan JJ, Elliott S, Sandler A (2007) The robotic approach to complex hepatobiliary anomalies in children: preliminary report. J Pediatr Surg. https://doi.org/10.1016/j.jpedsurg.2007.08.040
Kumar R, Lal BB, Sood V, Khanna R, Kumar S, Bharathy KGS, Alam S (2019) Predictors of successful Kasai portoenterostomy and survival with native liver at 2 years in infants with biliary atresia. J Clin Exp Hepatol. https://doi.org/10.1016/j.jceh.2018.09.008
Wildhaber BE, Majno P, Mayr J, Zachariou Z, Hohlfeld J, Schwoebel M, Kistler W, Meuli M, Le Coultre C, Mentha G, Belli D, Chardot C (2008) Biliary atresia: Swiss national study, 1994–2004. J Pediatr Gastroenterol Nutr. https://doi.org/10.1097/MPG.0b013e3181633562
Lee WS, Chai PF, Lim KS, Lim LH, Looi LM, Ramanujam TM (2009) Outcome of biliary atresia in Malaysia: a single-centre study. J Paediatr Child Health. https://doi.org/10.1111/j.1440-1754.2009.01490.x
Li YB, Rong LY, Tang JF, Niu HZ, Jin Z, Zhou Y, Cao GQ, Zhang X, Chi SQ, Tang ST (2022) Re-evaluation of laparoscopic hepatic subcapsular spider-like telangiectasis sign: a highly accurate method to diagnose biliary atresia in infants. Front Pediatr. https://doi.org/10.3389/fped.2022.850449
Zhou Y, Jiang M, Tang ST, Yang L, Zhang X, Yang DH, Xiong M, Li S, Cao GQ, Wang Y (2017) Laparoscopic finding of a hepatic subcapsular spider-like telangiectasis sign in biliary atresia. World J Gastroenterol. https://doi.org/10.3748/wjg.v23.i39.7119
Sun X, Diao M, Wu X, Cheng W, Ye M, Li L (2016) A prospective study comparing laparoscopic and conventional Kasai portoenterostomy in children with biliary atresia. J Pediatr Surg. https://doi.org/10.1016/j.jpedsurg.2015.10.045
Li Y, Xiang B, Wu Y, Wang C, Wang Q, Zhao Y, Chen S, Ji Y, Xu Z (2018) Medium-term outcome of laparoscopic Kasai portoenterostomy for biliary atresia with 49 cases. J Pediatr Gastroenterol Nutr. https://doi.org/10.1097/MPG.0000000000001934
Ji Y, Zhou J, Zhang X, Chen S, Xu Z (2021) Laparoscopic Kasai portoenterostomy for cystic biliary atresia: midterm follow-up results of 35 patients. Surg Today. https://doi.org/10.1007/s00595-021-02297-3
Shirota C, Hinoki A, Tainaka T, Sumida W, Kinoshita F, Yokota K, Makita S, Amano H, Nakagawa Y, Uchida H (2022) Laparoscopic Kasai portoenterostomy can be a standard surgical procedure for treatment of biliary atresia. World J Gastrointest Surg. https://doi.org/10.4240/wjgs.v14.i1.56
Abe E, Koga H, Nakamura H, Ochi T, Seo S, Lane GJ, Yamataka A (2022) Mid-term outcome of postoperative biliary atresia patients according to level of transection of the biliary remnant and depth of suturing. Pediatr Surg Int. https://doi.org/10.1007/s00383-022-05097-z
Chan KWE, Lee KH, Wong HYV, Tsui SYB, Mou JWC, Tam YHP (2018) Ten-year native liver survival rate after laparoscopic and open Kasai portoenterostomy for biliary atresia. J Laparoendosc Adv Surg Tech A. https://doi.org/10.1089/lap.2018.0350
Siddiqui AI, Ahmad T (2022) Biliary atresia. StatPearls Publishing, Treasure Island, FL
Ueno T, Kodama T, Noguchi Y, Nomura M, Saka R, Takama Y, Tazuke Y, Bessho K, Okuyama H (2021) Effect of microscopy-assisted portoenterostomy (MAPE) for biliary atresia. Pediatr Surg Int. https://doi.org/10.1007/s00383-020-04794-x
Davenport M, Grieve A (2012) Maximizing Kasai portoenterostomy in the treatment of biliary atresia: medical and surgical options. S Afr Med J. https://doi.org/10.7196/samj.6120
Toyosaka A, Okamoto E, Okasora T, Nose K, Tomimoto Y, Seki Y (1994) Extensive dissection at the porta hepatis for biliary atresia. J Pediatr Surg. https://doi.org/10.1016/0022-3468(94)90011-6
Kobayashi H, Yamataka A, Urao M, Okazaki T, Yanai T, Koga H, Lane GJ, Miyano T (2006) Innovative modification of the hepatic portoenterostomy. Our experience of treating biliary atresia. J Pediatr Surg. https://doi.org/10.1016/j.jpedsurg.2005.12.056
Bax N, Georgeson K (2007) Biliary atresia panel session. In: presentation at the 16th annual congress of the International Pediatric Endosurgery Group (IPEG). Buenos Aires. 2007: 6–9
Ure BM, Kuebler JF, Schukfeh N, Engelmann C, Dingemann J, Petersen C (2011) Survival with the native liver after laparoscopic versus conventional Kasai portoenterostomy in infants with biliary atresia: a prospective trial. Ann Surg. https://doi.org/10.1097/SLA.0b013e318211d7d8
Wada M, Nakamura H, Koga H, Miyano G, Lane GJ, Okazaki T, Urao M, Murakami H, Kasahara M, Sakamoto S, Ishizaki Y, Kawasaki S, Yamataka A (2014) Experience of treating biliary atresia with three types of portoenterostomy at a single institution: Extended, modified Kasai, and laparoscopic modified Kasai. Pediatr Surg Int. https://doi.org/10.1007/s00383-014-3551-5
Ji Y, Yang K, Zhang X, Chen S, Xu Z (2018) Learning curve of laparoscopic Kasai portoenterostomy for biliary atresia: report of 100 cases. BMC Surg. https://doi.org/10.1186/s12893-018-0443-y
Li Z, Ye Y, Wu Z, Wang B (2017) Learning curve analysis of laparoscopic Kasai portoenterostomy. J Laparoendosc Adv Surg Tech A. https://doi.org/10.1089/lap.2016.0204
Wang S, Hu X, Wang J (2022) Learning curve of laparoscopic Kasai portoenterostomy in a tertiary hospital with low caseload of biliary atresia. J Laparoendosc Adv Surg Tech A. https://doi.org/10.1089/lap.2021.0653
Cazares J, Koga H, Murakami H, Nakamura H, Lane G, Yamataka A (2017) Laparoscopic portoenterostomy for biliary atresia: single-center experience and review of literatures. Pediatr Surg Int. https://doi.org/10.1007/s00383-017-4171-7
Yamataka A (2013) Laparoscopic Kasai portoenterostomy for biliary atresia. J Hepatobiliary Pancreat Sci. https://doi.org/10.1007/s00534-013-0607-1
Li B, Chen WB, Huang X, Xia SL, Zhang FN, Wang SQ, Wang YB (2019) Modifications to expose porta hepatis for laparoscopic portoenterostomy easier in biliary atresia. J Surg Res. https://doi.org/10.1016/j.jss.2018.08.013
Huang SY, Yeh CM, Chen HC, Chou CM (2018) Reconsideration of laparoscopic Kasai operation for biliary atresia. J Laparoendosc Adv Surg Tech A. https://doi.org/10.1089/lap.2017.0535
Rong LY, Li YB, Tang JF, Cao GQ, Wan L, Li XY, Zhang X, Chi SQ, Tang ST (2022) Robotic-assisted choledochal cyst excision with Roux-en-Y hepaticojejunostomy in children: does age matter? Surg Endosc. https://doi.org/10.1007/s00464-022-09496-0
Tang ST, Ruan QL, Cao ZQ, Mao YZ, Wang Y, Li SW (2005) Diagnosis and treatment of biliary atresia: a retrospective study. Hepatobiliary Pancreat Dis Int 4:108–112
Song Z, Dong R, Shen Z, Chen G, Yang YF, Zheng S (2017) Surgical outcome and etiologic heterogeneity of infants with biliary atresia who received Kasai operation less than 60 days after birth: a retrospective study. Medicine. https://doi.org/10.1097/MD.0000000000007267
Schreiber RA, Barker CC, Roberts EA, Martin SR, Alvarez F, Smith L, Butzner JD, Wrobel I, Mack D, Moroz S, Rashid M, Persad R, Levesque D, Brill H, Bruce G, Critch J, Res CPH, G, (2007) Biliary atresia: the Canadian experience. J Pediatr. https://doi.org/10.1016/j.jpeds.2007.05.051
Lertudomphonwanit C, Mourya R, Fei L, Zhang Y, Gutta S, Yang L, Bove KE, Shivakumar P, Bezerra JA (2017) Large-scale proteomics identifies MMP-7 as a sentinel of epithelial injury and of biliary atresia. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aan8462
Chi S, Xu P, Yu P, Cao G, Wang H, Ye Y, Li S, Zhou Y, Li X, Zhou Y, Zhang X, Niu H, Xu L, Cai P, Tang S (2022) Dynamic analysis of serum MMP-7 and its relationship with disease progression in biliary atresia: a multicenter prospective study. Hepatol Int. https://doi.org/10.1007/s12072-022-10322-x
Meinzer A, Alkatout I, Krebs TF, Baastrup J, Reischig K, Meiksans R, Bergholz R (2020) Advances and trends in pediatric minimally invasive surgery. J Clin Med. https://doi.org/10.3390/jcm9123999
Acknowledgements
We would like to acknowledge Professor Patrick Chung for writing assistance.
Funding
National Natural Science Foundation of China (Grant Numbers 81873848 and 82071689).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Mengxin Zhang, Guo-qing Cao, Xiangyang Li, Xi Zhang, Yibo Li, Shuiqing Chi, Liying Rong, Shao-Tao Tang have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, M., Cao, G., Li, X. et al. Robotic-assisted Kasai portoenterostomy for biliary atresia. Surg Endosc 37, 3540–3547 (2023). https://doi.org/10.1007/s00464-022-09855-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09855-x