Abstract
Purpose
To investigate the clinical characteristics of cystic biliary atresia (CBA) and evaluate the midterm follow-up outcomes after laparoscopic treatment.
Methods
We analyzed and compared data retrospectively on CBA patients (group A) and nonsyndromic type III biliary atresia (BA) patients (group B), who underwent laparoscopic Kasai portoenterostomy (LKPE) during the same period.
Results
There were no significant differences in operative time, conversion rate, or the incidence of any postoperative complications between groups A and B (P > 0.05). The mean age at surgery (P < 0.01), rates of clearance of jaundice (CJ), cholangitis (P < 0.05), and 5-year survival with a native liver (SNL) were significantly lower in group A than in group B. Among the 35 patients with CBA, the CJ and 5-year SNL rates were significantly better in those with type I (n = 27) than in those with type IIId (n = 8) (P < 0.05).
Conclusions
LKPE is a feasible and safe procedure for CBA. The 5-year SNL after LKPE was better in patients with CBA than in those with nonsyndromic type III BA. The 5-year SNL after LKPE for type I CBA was better than that for type IIId CBA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biliary atresia (BA) is a neonatal disease characterized by progressive extrahepatic bile duct occlusion and inflammation. Cystic biliary atresia (CBA) is defined as cystic changes in an otherwise obliterated biliary tract. Although CBA is a specific subgroup of BA, this cystic biliary malformation is a feature associated with an excellent outcome in young infants treated with timely portoenterostomy, especially assisted by laparoscopy [1]. However, there are few reports on the laparoscopic treatment of CBA. We reviewed retrospectively our experience of performing laparoscopic Kasai portoenterostomy (LKPE) for CBA at our hospital.
Methods
Design and study population
This study was a retrospective single-center cohort study performed in China, based on the data from May, 2009 to January, 2017. The Ethical Committee of the West China Hospital of Sichuan University approved the study (HX-2017–12). Infants with CBA and type III isolated BA were suitable for inclusion, but patients with BA splenic malformation and other type I or type II BA were excluded. The parents or guardians of all patients gave written and informed consent. The demographic perioperative data and clearance of jaundice (CJ), cholangitis, and survival with the native liver (SNL) rates were compared between CBA patients (group A) and nonsyndromic type III biliary atresia (BA) patients (group B) and between patients with type I and those with type IIId CBA.
Operative technique
LKPE procedure for type III BA
The procedure of LKPE for nonsyndromic type III BA was performed as described previously [2]. Briefly, the patient was placed supine on the operative table. A 5-mm trocar with a camera was inserted through the umbilicus for 30° laparoscopy using the open Hasson technique. Two or three 5-mm trocars were inserted in the left and right upper quadrants. The carbon dioxide pressure was maintained at between 6 and 8 mmHg with a flow rate of 3–6 L/min. A percutaneous suture was used to snare the round and falciform ligament and retract the liver. Other percutaneous transhepatic sutures were introduced into the left or right lobe simultaneously for better hilar exposure. If the condition of the gall bladder was suitable, the diagnosis of BA was confirmed using cholecystocholangiography. Briefly, a small needle was advanced into the gallbladder with laparoscopic guidance. Under radiographic guidance, iohexol (Omnipaque) was injected slowly and multiple images were obtained. The gallbladder and cystic duct were dissected free from the gall bladder fossa, and the distal part of the extrahepatic duct was divided behind the duodenum. The portal plate over the bifurcation of the main portal vein was found along the proximal end of the common hepatic duct. Once the base of the fibrous cone was reached, the fibrous cord was dissected using laparoscopic scissors. The level of fibrous cone resection depended on the abundant bile-like juice found over the fibrous stump. Bleeding from the fibrous remains of the portal plate was controlled by direct pressure with moist gauze. The ligament of Treitz was identified, and the jejunum 15 cm distal to the ligament was exteriorized through the umbilical port site. A 25- to 40-cm-long Roux-en-Y limb was fashioned, then returned into the peritoneal cavity. After re-establishing the pneumoperitoneum, the Roux loop was delivered to the hilum via a retro-colic path. One layer of end-to-side portoenterostomy (anastomotic diameter, 1.0–1.5 cm) was performed using interrupted 5–0 absorbable sutures. The posterior border was the connective tissue around the stump of the hilar fibrous cone. A drain was inserted into the foramen of Winslow.
LKPE procedure for CBA
LKPE was performed in all CBA patients. The placement of trocars, hilar exposure, and the value of the pneumoperitoneum maintained was the same as in LKPE for nonsyndromic type III BA. The following additional procedures of LKPE for CBA were used. The diagnosis of CBA was based on the cholecystocholangiography in all patients. The hilar cyst was removed completely up to the level of the hilar fibrous cone. All of the other LKPE procedures for CBA were the same as those for nonsyndromic type III BA. A liver biopsy was taken from all patients with BA.
Postoperative management algorithm
All of the patients received postoperative medical treatment using the same protocol. Antibiotic therapy was continued intravenously for 25–30 days in the hospital. Methylprednisolone was administered intravenously for 5 days postoperatively at a dose of 5 mg/kg/day initially and reduced by 1 mg/kg/day every 3 days for 2 weeks or longer until a normal total bilirubin value was reached. Sulfamethoxazole and cephalosporin antibiotics were given orally on alternate weeks until the patient was 1 year of age. Ursodeoxycholic acid and compound glycyrrhizin tablets were given until they were 3 years of age. Postoperative outcomes were followed up until the end of the present study.
Definitions
The operative duration (OD) was calculated as the length of time between skin incision and closure. Perioperative complications (APOCs) were defined as complications that occurred during the perioperative period, including wound infection, umbilical hernia, peritoneal drainage displacement, omental prolapse through the trocar wound, respiratory infection, intestinal anastomotic fistula, and adhesive intestinal obstruction. An atretic gallbladder was defined as a gallbladder < 1.5 cm in length. The degree of liver fibrosis was scored according to the new Inuyama classification as F0 (no fibrosis), F1 (fibrous portal expansion), F2 (bridging fibrosis), F3 (bridging fibrosis with architectural distortion), or F4 (liver cirrhosis). CJ was defined as a total bilirubin level of < 1.2 mg/dL within 6 months postoperatively. Cholangitis was defined as an elevated serum bilirubin level (> 2.5 mg/dL), leukocytosis with a left shift, and normal to acholic stools in a febrile patient (> 38.0 ℃).
Statistical analysis
The software used for statistical calculation was IBM SPSS 22.0 for Windows 10.0 (SPSS, Inc., Chicago, IL, USA). The SNL rate was analyzed using the Kaplan–Meier method, with endpoints of death and liver transplantation, and the results were compared using the log-rank test. Demographic comparisons were performed using Student’s t test or the nonparametric Mann–Whitney U test for continuous variables, where appropriate, and Fisher’s exact test or the chi-squared test was used for categorical variables. A P value < 0.05 was considered statistically significant.
Results
A total of 288 patients with nonsyndromic BA who underwent LKPE were enrolled in this study, 35 of whom with CBA were assigned to group A and 253 with nonsyndromic type III BA were assigned to group B. Among the 35 CBA patients, 27 had type I and 8 had type IIId CBA and were further assigned to the type I and type IIId groups, respectively. A total of 48.6% of cases of CBA (15/27 type I, 2/8 type IIId) were discovered using antenatal sonography.
Comparison of patient data between groups A and B
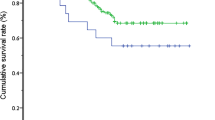
Table 1 shows the perioperative and follow-up demographic data of patients with BA. The mean weight, OD, postoperative resumption of oral intake (PROI), and follow-up were not significantly different between groups A and B (P > 0.05). The sex ratio (female/male) and intraoperative blood transfusion (IOBT), conversion, and APOC rates were also not significantly different between these two groups (P > 0.05). However, the mean age at surgery was significantly younger in group A than in group B (66.4 days vs. 84.9 days, respectively (P < 0.01). The rates of CJ and cholangitis were 91.4 and 28.6% in group A, which were significantly better than those in group B (P < 0.05). The 5-year SNL rate was significantly better in group A than in group B (71.4 vs. 51.4%, respectively (P < 0.05) (Fig. 1).
Comparison of patient data between types I and IIId CBA
35 patients with CBA were further divided into those with type I (n = 27) and those with type IIId (n = 8). Table 2 shows the perioperative parameters and follow-up data of all patients with CBA in the type I and type IIId groups. There were no differences in the mean age, sex ratio (female/male), weight, cyst diameter, occurrence of atretic gallbladder, antenatal examination, OD, PROI, degree of liver fibrosis, occurrence of IOBT, conversion, APOCs, or follow-up time between the type I and type IIId groups (P > 0.05). Among the 27 patients with type I, the bile duct appeared cloudy (Fig. 2a) in 6, treelike (Fig. 2b) in 8, and mixed (Fig. 2c) in 13. Among the 8 patients with type IIId, the isolated and static films of the hilar cysts were observed without any contrast agent tracing in the liver or duodenum (Fig. 2d).
Cholangiographic findings in patients with cystic biliary atresia (CBA). a Cloudy bile duct on cholangiography in type I CBA. b Treelike bile duct on cholangiography in type I CBA. c Mixed bile duct on cholangiography in type I CBA. d Isolated and static image of the hilar cyst without any contrast agent tracing in the liver or duodenum on cholangiography in type IIId CBA
The cholangitis rate was 22.2% in the type I group and 50.0% in the type IIId group, but this difference was not significant. The rate of CJ was significantly better in the type I group than in the type IIId group (P < 0.05). The 5-year SNL rate was significantly better in the type I group than the in the IIId group (81.5% vs. 37.5%, respectively; P < 0.01) (Fig. 3).
Discussion
CBA is an uncommon variant of BA, which has been classified by the Japanese Biliary Atresia Society as type I or type IIId [1, 3,4,5,6,7]. Type I CBA is further classified into two proximal subtypes, subtype α, characterized by a patent hepatic duct with a diameter > 1 mm; and subtype β, characterized by a hepatic duct diameter < 1 mm [8]. Although the incidence of CBA is low, the widespread use of ultrasonography has resulted in an increase in the prenatal diagnosis of CBA, with more than 40% of CBA cases now detected on sonography before birth [9], most of which are type I [10]. In the present series, we identified 35 cases (12.2%; male/female, 14:21) of CBA among 288 nonsyndromic BA patients.
Notably, the mean age of CBA patients at surgery is significantly younger than that of otherwise comparable infants with noncystic and nonsyndromic BA, regardless of whether the abnormalities were detected antenatally [11, 12]. In the present study, the mean age of CBA patients at surgery was 66.4 days, which was significantly younger than that of the type III nonsyndromic BA patients (P < 0.01). However, our patients were older than those reported by Hasegawa (mean 46 days; 14–147 days) [10]. The reason for this discrepancy may be that some of our CBA patients were misdiagnosed with a congenital choledochal cyst (CC) before referral, which delayed treatment. Similar to other variants of BA, earlier surgical intervention for CBA results in a better outcome as determined by CJ and SNL [13]. Any delay in portoenterostomy or hepaticoenterostomy for CBA beyond 70 days of age is associated with a poor long-term SNL rate [14].
CBA and CC are two entities with dramatically different management approaches and prognoses [15]. However, the similarities between these conditions and their complexities in young infants make preoperative misdiagnosis common. Sonography is very helpful for the preoperative differential diagnosis of CBA and CC. The ultrasonography findings of gallbladder abnormalities; namely, the triangular cord sign and dilatation of the hepatic artery are useful in identifying CBA. However, other ultrasonography findings are also useful in discerning CC, such as intrahepatic bile duct dilatation, a normal gallbladder, and cystic sludge. Some studies found that the hilar cysts in fetuses and neonates differed between those with CBA and those with CC [4, 9, 16, 17]. Small cysts in the hepatic hilum are highly suspicious for CBA, but there are exceptions to the rules, and it is difficult to draw a clear line between a large cyst and a small cyst. The definitive diagnosis of CBA is confirmed only by intraoperative cholangiography and pathological specimen analysis.
The optimal surgical procedure for CBA remains controversial. Lilly et al. [5] recommended Kasai portoenterostomy instead of hepaticoenterostomy for patients with the correctable type of CBA. Other studies also found that hepatic portoenterostomy was effective after unsuccessful hepaticojejunostomy for CBA [13, 18, 19]. In contrast, some investigators [20] reported excellent long-term outcomes after hepaticojejunostomy for CBA. Nio [8] used hepaticoenterostomy only in patients with subtype α without stenotic segments in the extrahepatic ducts; however, it is difficult to determine the exact diameter of the common hepatic duct during surgery for CBA, even after cholangiography. The common hepatic duct may be extremely narrow in type I CBA, which is easily misdiagnosed as type IIId CBA. Therefore, we perform LKPE for all CBA patients at our hospital, regardless of CBA type.
The treatment of BA via laparoscopy is controversial. An argument against laparoscopy is the difficulty in overcoming the learning curve of the technique due to the rarity of its application in the treatment of BA [21, 22]. Poor portal exposure may also lead to inferior results after laparoscopic Kasai surgery. However, we think that the LKPE procedure is feasible and safe based on more than 10 years of experience with the laparoscopic treatment of various types of BA at our hospital. Avoiding large muscle incisions results in quicker recovery after surgery and a shorter hospital stay. The 3-year and 5-year SNL rates after LKPE were comparable or better than those after open Kasai portoenterostomy [2, 23, 24]. A recent meta-analysis provided further evidence of LKPE as a feasible option for patients with BA [25]. As a subtype of BA, CBA is more suitable for laparoscopic resection than other variants of BA, although only a few reports of laparoscopic surgical treatment for CBA have been published [13].
There is no definitive explanation of the mechanism of type IIId CBA. Previous studies have found that type IIId CBA patients had a connection between the common hepatic duct and the cystic lesion during the perinatal period. Complete obstruction of the common hepatic duct was thought to occur after birth and result in the formation of type IIId CBA [26]. Type I and IIId CBA may be in the same category of the BA classification, but further data are needed before any definitive conclusions about the possible mechanism can be made. Comparisons of the cyst diameter, detection on prenatal ultrasonographic examination, occurrence of atretic gallbladder, and degree of hepatic fibrosis showed no significant differences between the type I and type IIId groups in our study. However, the morphological images on cholangiography and the postoperative results differed between patients with type I and those with type IIId cysts. We observed significantly better CJ in the type I group than in the type IIId group. Moreover, the 5-year SNL rate in the type I group was 81.5%, which was significantly better than that in the type IIId group in our study. This is comparable to the 5-year SNL rate of 83.0% in type I CBA patients after laparoscopic cystojejunostomy reported by Faure et al. [27].
The present study has several limitations. First, it was a retrospective study without long-term follow-up. Second, the number of patients in both CBA subtype groups was insufficient for comparisons with nonsyndromic type III BA patients. Third, the advantages of LKPE for CBA were not fully demonstrated because there was not a control group of patients treated with open Kasai portoenterostomy for CBA. We must continue accumulating CBA cases and extend the follow-up time to obtain more convincing results.
In conclusion, CBA is a rare subtype of BA, which is classified into types I and IIId. Our study demonstrated that LKPE for CBA was feasible and safe with a better 5-year SNL rate than LKPE for nonsyndromic type III BA. Comparisons of demographic perioperative data between the type I and type IIId CBA groups showed no significant differences; however, the 5-year SNL rate was much better after LKPE for type I CBA than for type IIId CBA.
References
Komuro H, Makino SI, Momoya T, Nishi A. Biliary atresia with extrahepatic biliary cysts–cholangiographic patterns influencing the prognosis. J Pediatr Surg. 2000;35(12):1771–4.
Li Y, Xiang B, Wu Y, Wang C, Wang Q, Zhao Y, et al. Medium-term outcome of laparoscopic Kasai portoenterostomy for biliary atresia with 49 cases. J Pediatr Gastroenterol Nutr. 2018;66(6):857–60.
Lobeck IN, Sheridan R, Lovell M, Dupree P, Tiao GM, Bove KE. Cystic biliary atresia and choledochal cysts are distinct histopathologic entities. Am J Surg Pathol. 2017;41(3):354–64.
Lal R, Prasad DK, Krishna P, Sikora SS, Poddar U, Yachha SK, et al. Biliary atresia with a “cyst at porta”: management and outcome as per the cholangiographic anatomy. Pediatr Surg Int. 2007;23(8):773–8.
Lilly JR, Hall RJ, Vasquez-Estevez J, Karrer F, Shikes RH. The surgery of “correctable” biliary atresia. J Pediatr Surg. 1987;22(6):522–5.
Arima E, Fonkalsrud EW, Neerhout RC. Experience in the management of surgically correctable biliary atresia. Surgery. 1974;75(2):228–32.
Nio M. Japanese biliary atresia registry. Pediatr Surg Int. 2017;33(12):1319–25.
Nio M, Wada M, Sasaki H, Tanaka H. Does hepatic hilum morphology influence long-term prognosis in type I/I cyst biliary atresia? Pediatr Surg Int. 2015;31(10):931–6.
Redkar R, Davenport M, Howard ER. Antenatal diagnosis of congenital anomalies of the biliary tract. J Pediatr Surg. 1998;33(5):700–4.
Hasegawa T, Sasaki T, Kimura T, Sawai T, Nose K, Kamata S, et al. Prenatal ultrasonographic appearance of type IIId (uncorrectable type with cystic dilatation) biliary atresia. Pediatr Surg Int. 2002;18(5–6):425–8.
Davenport M, Ville De, de Goyet J, Stringer MD, Mieli-Vergani G, Kelly DA, McClean P, et al. Seamless management of biliary atresia in England and Wales (1999–2002). Lancet. 2004;363(9418):1354–7.
Davenport M, Betalli P, D’Antiga L, Cheeseman P, Mieli-Vergani G, Howard ER. The spectrum of surgical jaundice in infancy. J Pediatr Surg. 2003;38(10):1471–9.
Caponcelli E, Knisely AS, Davenport M. Cystic biliary atresia: an etiologic and prognostic subgroup. J Pediatr Surg. 2008;43(9):1619–24.
Davenport M, Caponcelli E, Livesey E, Hadzic N, Howard E. Surgical outcome in biliary atresia: etiology affects the influence of age at surgery. Ann Surg. 2008;247(4):694–8.
Mackenzie TC, Howell LJ, Flake AW, Adzick NS. The management of prenatally diagnosed choledochal cysts. J Pediatr Surg. 2001;36(8):1241–3.
Tanaka N, Ueno T, Takama Y, Fukuzawa M. Diagnosis and management of biliary cystic malformations in neonates. J Pediatr Surg. 2010;45(11):2119–23.
Huang FC, Hwang KP. Differential diagnosis of infantile choledochal cyst with or without biliary atresia. Acta Paediatr Taiwan. 2006;47(4):175–80.
Obaidah A, Dhende NP, Mane SB, Acharya H. Biliary atresia associated with choledochal cyst. Afr J Paediatr Surg. 2009;6(1):61–2.
Nio M, Sano N, Ishii T, Sasaki H, Hayashi Y, Ohi R. Long-term outcome in type I biliary atresia. J Pediatr Surg. 2006;41(12):1973–5.
Takahashi Y, Matsuura T, Saeki I, Zaizen Y, Taguchi T. Excellent long-term outcome of hepaticojejunostomy for biliary atresia with a hilar cyst. J Pediatr Surg. 2009;44(12):2312–5.
Ji Y, Yang K, Zhang X, Chen S, Xu Z. Learning curve of laparoscopic Kasai portoenterostomy for biliary atresia: report of 100 cases. BMC Surg. 2018;18(1):107.
Wong KK, Chung PH, Chan KL, Fan ST, Tam PK. Should open Kasai portoenterostomy be performed for biliary atresia in the era of laparoscopy? Pediatr Surg Int. 2008;24(8):931–3.
Murase N, Hinoki A, Shirota C, Tomita H, Shimojima N, Sasaki H, et al. Multicenter, retrospective, comparative study of laparoscopic and open Kasai portoenterostomy in children with biliary atresia from Japanese high-volume centers. J Hepatobiliary Pancreat Sci. 2019;26(1):43–50.
Shirota C, Murase N, Tanaka Y, Ogura Y, Nakatochi M, Kamei H, et al. Laparoscopic Kasai portoenterostomy is advantageous over open Kasai portoenterostomy in subsequent liver transplantation. Surg Endosc. 2020;34(8):3375–81.
Li Y, Gan J, Wang C, Xu Z, Zhao Y, Ji Y. Comparison of laparoscopic portoenterostomy and open portoenterostomy for the treatment of biliary atresia. Surg Endosc. 2019;33(10):3143–52.
Masumoto K, Kai H, Oka Y, Otake R, Yoshizato T, Miyamoto S, et al. A case of cystic biliary atresia with an antenatally detected cyst: the possibility of changing from a correctable type with a cystic lesion (I cyst) to an uncorrectable one (IIId). Pediatr Surg Int. 2011;27(1):99–102.
Faure A, Hery G, Colavolpe N, Bevilacqua C, Guys JM, De Lagausie P. Laparoscopic cystojejunostomy for type I cystic biliary atresia in children. J Minim Access Surg. 2015;11(4):263–6.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant No: 81401606 and 81400862), the Key Project in the Science & Technology Program of Sichuan Province (Grant No: 2019YFS0322), the Science Foundation for Excellent Youth Scholars of Sichuan University (Grant No: 2015SCU04A15), and the 1·3·5 Project for Disciplines of Excellence–Clinical Research Incubation Project, West China Hospital of Sichuan University (Grant No: 2019HXFH056 and 2020HXFH048).
Author information
Authors and Affiliations
Contributions
YJ, JYZ, XPZ, SYC, and ZCX were involved in the clinical management of the patients and collected clinical details for this study. JYZ reviewed the literature and drafted the manuscript. YJ reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
We have no conflicts of interest to declare in association with the present study.
Ethical approval and consent to participate
The Ethics Committee of the West China Hospital of Sichuan University approved the study. Written informed consent was obtained from the patients’ parents according to the provisions of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, Y., Zhou, J., Zhang, X. et al. Laparoscopic Kasai portoenterostomy for cystic biliary atresia: midterm follow-up results of 35 patients. Surg Today 51, 1924–1931 (2021). https://doi.org/10.1007/s00595-021-02297-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02297-3