Abstract
Aim
The aim of this report was to present the laparoscopic portoenterostomy (LapPE) procedure developed by the Department of Pediatric General and Urogenital Surgery, Juntendo University School of Medicine (JLapPE). We also attempted to obtain an understanding of the current status of laparoscopic portoenterostomy in the world as reported in the English literature to compare with our experience.
Methods
There were 22 BA patients who had JLapPE between 2009 and 2016. BA classification was type III (n = 19) and type II (n = 3). There was 1 case of syndromic BA and 1 case was positive for cytomegalovirus. A systematic search in PubMed of all BA patients treated by LapPE in the English literature was conducted. Jaundice clearance (JC) and survival with the native liver (SNL) were compared.
Results
Mean age at JLapPE was 67.1 days (range 29–119). Mean postoperative follow-up was 4.6 years (1.3–8.3). Mean operative time was 514 min (240–662) and mean blood loss was 13.4 g (3–21). Postoperative JC (Total bilirubin ≤ 1.5 mg/dL) was 77.3% (17/22) at 3 months and 90.9% (20/22) at 6 months. SNL at 6 months of age was 90.9% (20/22); at 1 year of age was 77.3% (17/22), at 2 years of age was 73.7% (14/19); and at 3 years of age was 81.3% (13/16).
Conclusions
Despite recent reports that outcome of LapPE for BA may be unfavorable compared with the conventional open portoenterostomy, our results would suggest that JLapPE can be performed successfully, because it is performed exactingly according to a standard protocol. JLapPE will continue to be our procedure of choice for treating BA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biliary atresia (BA) is a progressive inflammatory disease that causes deterioration and obliteration of the intra- and extrahepatic biliary ducts [1]. After Kasai [2] reported his original portoenterostomy (KPE) procedure for BA, the number of cases achieving jaundice clearance (JC; total bilirubin ≤ 1.5 mg/dL) and long-term survival with the native liver (SNL) increased to such an extent that KPE came to be regarded as the procedure of choice for treating BA. Unfortunately, as KPE spread around the world, it began to be modified by surgeons in the hope of achieving better JC and SNL with the result that dissection was extended laterally with a wider anastomosis and the valuable concepts of KPE devised to treat BA were largely lost [3].
Minimally invasive surgery (MIS) has evolved significantly in the past few decades and has already replaced open surgery for many routine procedures because of less surgical stress and better wound cosmesis, faster postoperative recovery, and shorter hospital stay, appealing to parents with children requiring surgery. However, some procedures have been considered to be too complicated to be performed laparoscopically. Hepato-biliary surgery is one such field because of the complexity of hepato-biliary-pancreatic anatomy with numerous anomalous variations that render orientation and performance extremely difficult because of the limited surgical field. Laparoscopic intervention at the porta hepatis thus requires technical expertise to work in a limited space and mastery of normal and variant anatomy to proceed safely with limited visualization.
The first laparoscopic portoenterostomy (LapPE) was performed by Esteves et al. in Brazil in 2002 [4]. Thereafter, reports from San Francisco and Buenos Aires confirmed that the procedure was feasible and safe [5, 6]. However, skeptical scientific belief that JC and SNL could actually be improved by LapPE, with some centers reporting worse outcome than the conventional open portoenterostomy (COPE) [7], and a misconception that LapPE was more a demonstration of technical prowess than a therapeutically superior procedure led the International Pediatric Endosurgery Group (IPEG) to discourage LapPE as a viable option for treating BA in 2007 [8].
After IPEG’s decision, which was a major blow to supporters of LapPE, a video of KPE performed by Kasai himself [9] was reviewed exhaustively by one of the co-authors (AY) to establish how the various COPE procedures, including KPE, and modified KPE, varied by comparing them directly with techniques recorded on video. AY was moved to reproduce the original KPE procedure as performed by Kasai as a laparoscopic procedure that would combine the benefits of MIS with the physiologic and anatomic concepts that Kasai devised to treat BA directly to ensure good outcome [10]. In that video [9], important techniques were identified, such as shallow transection of the biliary remnant, preservation of the fibrotic connective tissues connecting the right and left biliary remnants, because they could conceivably contain micro bile ducts, and using continuous sutures in the edges of the transected biliary remnant except at the 2 and 10 o’clock positions where the original right and left bile ducts would have been, where sutures appeared to be deliberately placed shallowly, that is, only to the connective tissues at the porta hepatis, not to the edges of the transected biliary remnant. In addition, when the Department of Pediatric General and Urogenital Surgery at Juntendo University Medical School undertook the task of resurrecting KPE as a laparoscopic procedure in 2009, technology and instrumentation had evolved to the stage where more delicate suturing could be performed laparoscopically than previously with the result that AY was confident that KPE could be performed laparoscopically according to the principles of MIS. Such diligent perseverance based on the belief of the brilliance of KPE enabled Juntendo to perfect JLapPE, a modified LapPE procedure, with encouragingly good outcome [10,11,12,13]. Recently, Uchida et al. in Japan also reported good outcomes by not transecting too deeply, that is, by reverting to the original KPE technique rather than persevering with extended lateral dissection [14].
Methods
The 22 BA patients treated by JLapPE between 2009 and 2016 were compared for age at JLapPE, operative time, JC, SNL over time, and incidence of complications including cholangitis. JC and SNL were used as indicators of success.
For the literature review, an unrestricted search was performed on PubMed until April 2017 using the following keywords: biliary atresia, Kasai, portoenterostomy, laparoscopy, and laparoscopic Kasai. Case series, case reports, and reports (both abstracts and papers) of outcome of LapPE in the English language were chosen for assessment, while reports including COPE, robotic cases, or not involving LapPE were excluded. The reference lists from each article were also reviewed for thoroughness.
JLapPE
Preoperative technical considerations
Most patients with BA have abnormal liver function test results and vitamin K deficiency by the time of diagnosis. All infants should have parenteral vitamin K2 (menatetrenone 2.0 mg/day) supplementation, usually given for several days before surgery. Bowel preparation should commence with oral kanamycin and glycerin enemas to decrease abundant colon microbiota to minimize intestinal gas that could hinder visualization during JLapPE. Patients should be nil by mouth for 24 h prior to surgery. Parenteral broad-spectrum antibiotics should be administered preoperatively just before inserting the initial trocar. Preoperative blood tests should include complete blood count, coagulation profile, and liver function tests.
Patient/port positioning and initial preparation
Operating room setup is important for saving time and ensuring smooth progress of surgery. The patient is positioned at the foot of the operating table; the surgeon at the patient’s feet, a laparoscope assistant stands on the left side of the operating surgeon, and another assistant on either the left or right sides of the operating table. A monitor is placed at the head of the operating table facing the surgeon. Surgery is performed under general anesthesia. Vascular access is obtained, an orogastric tube is placed, and a urinary catheter is inserted. A 30° 5-mm or 10-mm laparoscope is inserted through a 10-mm trocar placed supraumbilically using the open Hasson technique. Pneumoperitoneum is created with CO2 pressure at 8 mmHg, and increased to 10 mmHg if required, using CO2 gas at a flow rate of 0.5–1.0 L min.
Three additional ports are placed under direct vision, two 5-mm ports are placed on both sides of the supraumbilical port, for the surgeon’s right and left hands, slightly above the umbilicus, just lateral to the rectus abdominis; another 5 mm is placed between the supraumbilical port and the left upper quadrant port, slightly below the umbilicus (para-umbilical port) (Fig. 1). Stay sutures and rubber O-rings are used to stabilize the trocars and prevent displacement. The use of a Nathanson retractor (Teleflex Medical, High Wycombe, UK) inserted directly through the abdominal wall in the epigastrium, and an additional trocar placed in the epigastrium is crucial for exposing the porta hepatis at the time of anastomosis.
Percutaneous stay sutures are used to improve exposure and elevate the liver; one just below the xiphoid process to snare the falciform ligament and one each to the right and left lobes of the liver.
Dissection of the porta hepatis and biliary remnant
Meticulous dissection of the cystic duct and the mid-to-distal biliary remnant is performed with hook diathermy, tissue forceps, and a Ligasure® device (Valley lab, Boulder, CO, USA); the latter is used through the existing para-umbilical port. Special attention is required for identifying all anatomic features and avoids unnecessary dissection around the biliary remnant. The fibrotic distal remnant is clipped and transected at the level of the duodenum, elevated and dissection proceeds from the hepatic artery and portal vein to the porta hepatis; in other words, the fibrous biliary remnant cone is dissected free.
The additional 5 mm trocar placed in the epigastrium is also used for the Ligasure® device, while dissecting the proximal biliary remnant and to ensure that only the tips of the device make contact with the portal vein branches at the porta hepatis draining into the caudate lobe; the device can be inserted into the abdomen almost vertically (Fig. 2). We are unique in that we do not use monopolar hook diathermy to divide these branches, because we believe that diathermy causes extensive lateral thermal injury that could extend as far as the fibrotic biliary cone and damage any viable microscopic-sized bile ducts that may be present. Instead, we use the Ligasure® device, because it generates far less lateral thermal energy, and to prevent any risk of complications secondary to direct pressure and heat on the right or left portal veins, such as portal vein thrombosis. In its counterpart during COPE, these branches are usually ligated and transected.
Using our approach, then the level of transection of the biliary remnant is more akin to that of KPE [15] (Fig. 3) not the extended dissection previously described as part of the extended COPE that would actually not be technically possible to perform using laparoscopy.
Extracorporeal transumbilical jejunal Roux-en-Y
The ligament of Treitz is identified and the jejunum 15-cm distal of ligament is exteriorized through the umbilical port site to create the Roux-en-Y jejunal loop extracorporeally. Pneumoperitoneum is paused and the jejunum is divided and the length of the Roux limb is determined by bringing it up to above the xiphoid process on the anterior abdominal wall. All our Roux limbs are customized and we never predetermine a Roux limb to be 30, 40, or 50 cm in length. A jejunojejunostomy is performed extracorporeally. This customized Roux limb is approximated to the native jejunum for 8 cm cranially to prevent intestinal contents of the native jejunum refluxing into the Roux limb (Fig. 4). The jejunojejunostomy should fit naturally into the splenic flexure after anastomosis [11, 16]. Finally, a 10-mm-long antimesentric enterotomy is made near the closed end of the Roux limb and the jejunum returned to the abdominal cavity, the pneumoperitoneum is reestablished, and the jejunal limb is passed through a retrocolic window to lie without tension at the porta hepatis. For the enterotomy, a scalpel should be used for creating the enterotomy in the jejunum to prevent burning of the jejunal wall that will be used for the portoenterostomy anastomosis; we never use diathermy for the enterotomy, since it is associated with burning and can cause scarring along the anastomotic line of the portoenterostomy.
The length of the Roux-en-Y limb is determined by placing the jejunal loop at the umbilicus and bringing the distal end (E) to be 3 cm above the xiphoid process. The jejunojejunostomy (arrowheads) will then fit naturally into the splenic flexure after anastomosis. Arrows show approximating the Roux-Y limb to the native jejunum for 8 cm cranially to streamline flow into the distal jejunum and eliminate reflux into the Roux-Y limb preventing biliary stasis
Portoenterostomy
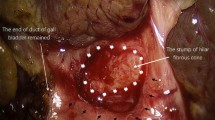
An extracorporeal surgical knot or Roeder knot-tying method and anastomotic interrupted sutures (5/0 or 6/0 PDS: Ethicon, Inc., Somerville, NJ, USA) are placed between the enterotomy and liver parenchyma around the margins of the transected portal plate. The posterior margin is performed first and sutures should be placed deep enough to prevent leakage, but they should be shallow enough to prevent injury to any remnant ductules that may be present. At the 2 and 10 o’clock positions, where the original left and right micro bile ducts should be, no sutures are placed or if there is a risk for leakage, shallow sutures are placed in the connective tissue over the left and right hepatic arteries (Fig. 5). The mesocolic window around the Roux loop is closed, avoiding any kinking or strangulation. A tube drain is inserted into the pouch of Winslow and ports are withdrawn under direct vision; wounds are closed conventionally with infiltration of 0.25% bupivacaine.
Postoperative management
Intravenous fluids and nasogastric aspiration are continued until bowel activity can be confirmed (usually 3–4 days). Careful monitoring of blood glucose levels, electrolytes, and coagulation is important in the early postoperative period. Blood tests including complete blood count, liver function tests, and cholinesterase are assessed routinely for the first 7 days postoperatively, then as required. Liver biochemistry (including bilirubin) may well worsen in the first postoperative week whatever the eventual outcome, and should not been seen as discouraging. By about the fourth postoperative week, there should be a definite fall in bilirubin and consistently pigmented stools in those who will do well. During the 4 weeks after portoenterostomy, daily monitoring of stool color and C-reactive protein (CRP) is crucial, since if pigmented stools become pale or CRP increases, the antibiotics being administered may need to be changed. Preventing inflammation in the portoenterostomy in the first 4 week after portoenterostomy is crucial to enable bilioenteric fistulae to develop. Strict attention to nutritional needs is important and all postoperative JLapPE cases require vitamin supplementation that may need to be aggressive in some cases especially with regard to vitamin K. Medium-chain triglyceride (MCT) formula milk such as Caprilon® (SHS, Liverpool, UK) is advocated to maximize calorie input and facilitate lipid absorption, as MCT is processed by the portal vein and is readily available as a source of cellular energy.
Intravenous administration of double-agent broad-spectrum antibiotics (usually a cephalosporin and an aminoglycoside) is commenced at the start of surgery and continued postoperatively until CRP is < 0.3 mg/dL or leukocytosis has resolved. Prophylactic antibiotics of the treating surgeon’s preference are then commenced orally. The role of corticosteroids is controversial, but we strongly believe that they are choleretic and decrease inflammation at the anastomosis site [17, 18]. Ursodeoxycholic acid is used for augmenting bile flow. Juntendo’s standard protocol includes usage of cholagogues and a decreasing dose regimen of corticosteroids [19]. An intravenous cholagogue (usually dehydrocholic acid) is commenced on day 2 after surgery and continued until JC. Oral cholagogues such as ursodeoxycholic acid or aminoethylsulfonic acid are administered once oral feeding is commenced, generally on day 5 after surgery and continued thereafter. Once CRP falls below 1.0 mg/dL a decreasing dose regimen of prednisolone is commenced by intravenous administration. Each dose is given for 3 days, starting with an initial dose of 4 mg/kg/day, then 3, 2, 1, and 0.5 mg/kg/day. This 15-day cycle can be repeated up to four or five times if there is evidence of clinical benefit such as lower serum bilirubin or improved stool color. However, if jaundice persists (total bilirubin > 1.5 mg/dL) without evidence of apparent clinical benefit, only three cycles of corticosteroids are administered and the patient is actively considered for liver transplantation (LTx). An important feature of this protocol is that if stools begin to turn pale, the cycle is either recommenced from the beginning, or the previous dose is readministered, depending on clinical circumstances.
Postoperative cholangitis is a serious setback and can occur in the early postoperative period, especially before bilioenteric fistulae have developed, and should not be undertreated. It is defined as elevated serum bilirubin (> 2.5 mg/dL), leukocytosis, and a change from normal to acholic stools in a febrile patient (> 38.5 °C). It must be treated by intravenous antibiotics according to each center’s protocol. Once resolved, prophylactic antibiotics such as sulfamethoxazole/trimethoprim should be administered orally.
Ethics
This study was approved by the Ethics Committee of Juntendo University School of Medicine and complies with Helsinki Declaration of 1975 (revised 1983).
Results
We reviewed 22 JLapPE cases (19 type III and 3 type II) treated between 2009 and 2016. Female:male ratio was 1.2:1. Mean age at LapPE was 67.1 days (range 29–119), mean weight 4.3 kg (range 3.3–6.2), mean operative time was 514 min (range: 240–662), and blood loss was minimal 13.4 g (range 3–21). One patient had syndromic BA and another was positive for cytomegalovirus. We divided our subjects according to when JC was achieved; ≤ 3 months was 77.3% (17/22) and > 6 months 90.9% (20/22). SNL at 6 months of age was 90.9% (20/22); at 1 year of age was 77.3% (17/22), at 2 years of age was 73.7% (14/19); and at 3 years of age was 81.3% (13/16).
For the literature review, 49 reports were found on PubMed. Of these, 18 reports including two meta-analyses and systematic reviews were excluded, leaving 31 reports for review. Table 1 summarizes published LapPE cases. Initially, 449 cases were identified, but with careful scrutiny, duplications, repetitions, and combined reports from different centers were deleted leaving 274 actual cases (Table 2). Because of varying reporting styles and different criteria for categorization, we focused on SNL at 6 months of age and ≥ 1 year of age postoperatively, JC (T-Bil: ≤ 20 µmol/L or ≤ 2 mg/dL), and SNL ≥ 1 year old without jaundice as predictors of success and incidence of postoperative complications, which included cholangitis to compare reports in the literature.
Only one-third of the reports in the literature mentioned the type of BA treated, which was usually type III. All data were not available for all cases. Mean age at surgery in 194 cases was 63.7 days (range 20–98). Mean operative time in 194 cases was 295.2 min (range 169.5–514). Data for conversion rates to open surgery were only available for 177 cases and ranged from 0 to 8.3% per center, with an overall conversion rate of 2.8% (5/177 cases were converted to open).
JC in 210 cases was 54.7% (range 17–100) at 10/16 centers; the other centers either did not provide actual data or just made vague comments such as good bile drainage or colored stools after surgery or disappearance of clinical cholestasis. SNL at 6 months of age (n = 134) was 77.6% (range 33–100); and at ≥ 1 year of age (n = 235) was 65% (range 22–100). SNL ≥ 1 year old and jaundice-free was 47.3% (range 8–71.7) from 57 patients.
Postoperative complications reported from all centers for all 274 cases in decreasing order of incidence included cholangitis (72/274 patients; 26.2%), bleeding (5/274 patients; 1.8%), volvulus (4/274; 1.4%), wound infection (2/274; 0.7%), internal hernia secondary to not suturing between the mesocolon and the Roux loop jejunum during the retrocolic route (2/274; 0.7%), variceal bleeding (1/274; 0.3%), accidental transection of the hepatic artery (1/274; 0.3%), and bile leakage (1/274; 0.3%). There have been no sequelae of CO2 pneumoperitoneum on overall growth/development or liver function in any JLapPE case despite the prolonged operative time [26].
As part of the literature review which we conducted, we also summarized the recommendability of LapPE centers for treating BA based on experience and outcome into three categories; recommended, unspecified, and not recommended (Table 2). Again, data were not available for every center, but some centers were downgraded over time, while the rest maintained their status, which, unfortunately, for some centers, was “not recommended”.
Discussion
We encountered numerous limitations during the literature review, most seriously a lack of definitions to allow direct comparison. This was most problematic with JC. Good flow, disappearance of jaundice, or improved stool color give no indication of bilirubin levels, and where recorded, the discrepancy in definitions was large; for example, JC was defined as T-Bil ≤ 20 µmol/L or T-Bil ≤ 2 mg/dL, but 2 mg/dL is actually 34.2 µmol/L. Bilirubin is critical for JC and predicting prognosis. We suggest bilirubin levels be used to define JC all the time and recommend that JC be defined as T-Bil ≤ 1.5 mg/dL or ≤ 20 µmol/L (SI unit). In addition, not all criteria could be compared between centers, because the emphasis of each report was different, some focusing on demographics, others on operative technique, and others on management issues. Complications could also not be adequately compared, because some centers had only a few patients meaning that any percentage incidence seemed unreasonably high, preventing realistic comparison. Raw data are presented in the tables.
Our efforts to resurrect KPE as a laparoscopic procedure in 2009 hoping to emulate the original procedure so masterfully developed by Kasai and achieve the SNL and JC he achieved safely with his open procedure have been highly encouraging. Despite lengthy operative time, JLapPE would appear to be as effective as COPE based on comparisons with historical reports in the literature, and LapPE series from elsewhere [25]. This is an important issue, because the early diagnosis and appropriate surgical intervention greatly influence outcome of BA. In addition, we believe that limited dissection, performed carefully according to Kasai’s original instructions, is probably associated with better results. We now promote this as the principle reason for the success of JLapPE.
Meticulous postoperative management is mandatory for achieving good outcome and it includes a standardized corticosteroid protocol. This includes continuity of care. In too many countries, the pediatric surgical team performs successful surgery and the child is then managed only or mainly by pediatricians or hepatologists. This may be sufficient after discharge from hospital, but in some countries, management is transferred immediately after surgery for biliary atresia. In Japan, at the majority of centers, pediatric surgical cases continue to be monitored and managed mainly by pediatric surgeons even until adulthood, because the only reason those cases are alive is because of successful surgery. Surgeons are more aware of the physical because of the very nature of surgery itself, so any change in stool color, bilirubin levels, or CRP must be acted upon immediately. The treating surgeon is thus most appropriate for managing the abdomen of a postoperative BA patient and should be encouraged and supported at all centers.
Conclusions
JLapPE is an excellent example of combining state-of-the-art surgery and technology successfully. We believe everyone benefits from using MIS to treat BA; patients from excellent cosmesis, less requirement for analgesia and respiratory support, minimal incidence of postoperative morbidity such as bowel adhesions and incisional hernias, and less adhesions for LTx; parents from shorter hospital stay and less compromise to quality of life; and hospital staff from routine postoperative management, stable recovery, and generally reliable outcome.
References
Hartley JL, Davenport M, Kelly DA (2009) Biliary atresia. Lancet 374(9702):1704–1713. doi:10.1016/S0140-6736(09)60946-6
Kasai MSM (1959) A new operation for non-correctable biliary atresia: hepatic portoenterostomy. Shujutsu 13:733–779
Endo M, Katsumata K, Yokoyama J, Morikawa Y, Ikawa H, Kamagata S, Nakano M, Nirasawa Y, Ueno S (1983) Extended dissection of the portahepatis and creation of an intussuscepted ileocolic conduit for biliary atresia. J Pediatr Surg 18(6):784–793
Esteves E, Clemente Neto E, Ottaiano Neto M, Devanir J Jr, Esteves Pereira R (2002) Laparoscopic Kasai portoenterostomy for biliary atresia. Pediatr Surg Int 18(8):737–740. doi:10.1007/s00383-002-0791-6
Lee H, Hirose S, Bratton B, Farmer D (2004) Initial experience with complex laparoscopic biliary surgery in children: biliary atresia and choledochal cyst. J Pediatr Surg 39(6):804–807 (discussion 804–807)
Martinez-Ferro M, Esteves E, Laje P (2005) Laparoscopic treatment of biliary atresia and choledochal cyst. Semin Pediatr Surg 14(4):206–215. doi:10.1053/j.sempedsurg.2005.06.003
Ure BM, Kuebler JF, Schukfeh N, Engelmann C, Dingemann J, Petersen C (2011) Survival with the native liver after laparoscopic versus conventional kasai portoenterostomy in infants with biliary atresia: a prospective trial. Ann Surg 253(4):826–830. doi:10.1097/SLA.0b013e318211d7d8
Bax NMA, Georgeson K et al (2007) Biliary atresia panel session. In: Paper presented at the 16th Annual Congress of the International Pediatric Endosurgery Group (IPEG), Buenos Aires, Argentina, 6–9 September
Kasai M (1978) Surgery for biliary atresia. Japan Surgical Society Video Library No.78-07
Koga H, Miyano G, Takahashi T, Shimotakahara A, Kato Y, Lane GJ, Okazaki T, Yamataka A (2011) Laparoscopic portoenterostomy for uncorrectable biliary atresia using Kasai’s original technique. J Laparoendosc Adv Surg Tech A 21(3):291–294. doi:10.1089/lap.2010.0162
Yamataka A, Lane GJ, Cazares J (2012) Laparoscopic surgery for biliary atresia and choledochal cyst. Semin Pediatr Surg 21(3):201–210. doi:10.1053/j.sempedsurg.2012.05.011
Yamataka A (2013) Laparoscopic Kasai portoenterostomy for biliary atresia. J Hepatobiliary Pancreat Sci 20(5):481–486. doi:10.1007/s00534-013-0607-1
Yamataka A, Lane GJ, Koga H, Cazares J, Nakamura H (2014) Role of laparoscopy during surgery at the porta hepatis. S Afr Med J 104(11 Pt 2):820–824
Murase N, Uchida H, Ono Y, Tainaka T, Yokota K, Tanano A, Shirota C, Shirotsuki R (2015) A new era of laparoscopic revision of kasai portoenterostomy for the treatment of biliary atresia. Biomed Res Int 2015:173014. doi:10.1155/2015/173014
Wada M, Nakamura H, Koga H, Miyano G, Lane GJ, Okazaki T, Urao M, Murakami H, Kasahara M, Sakamoto S, Ishizaki Y, Kawasaki S, Yamataka A (2014) Experience of treating biliary atresia with three types of portoenterostomy at a single institution: extended, modified Kasai, and laparoscopic modified Kasai. Pediatr Surg Int 30(9):863–870. doi:10.1007/s00383-014-3551-5
Yamataka A, Kobayashi H, Shimotakahara A, Okada Y, Yanai T, Lane GJ, Urao M, Miyano T (2003) Recommendations for preventing complications related to Roux-en-Y hepatico-jejunostomy performed during excision of choledochal cyst in children. J Pediatr Surg 38(12):1830–1832
Bezerra JA, Spino C, Magee JC, Shneider BL, Rosenthal P, Wang KS, Erlichman J, Haber B, Hertel PM, Karpen SJ, Kerkar N, Loomes KM, Molleston JP, Murray KF, Romero R, Schwarz KB, Shepherd R, Suchy FJ, Turmelle YP, Whitington PF, Moore J, Sherker AH, Robuck PR, Sokol RJ, Childhood Liver Disease R, Education N (2014) Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. JAMA 311(17):1750–1759. doi:10.1001/jama.2014.2623
Tyraskis A, Davenport M (2016) Steroids after the Kasai procedure for biliary atresia: the effect of age at Kasai portoenterostomy. Pediatr Surg Int 32(3):193–200. doi:10.1007/s00383-015-3836-3
Nakamura H, Koga H, Wada M, Miyano G, Dizon R, Kato Y, Lane GJ, Okazaki T, Yamataka A (2012) Reappraising the portoenterostomy procedure according to sound physiologic/anatomic principles enhances postoperative jaundice clearance in biliary atresia. Pediatr Surg Int 28(2):205–209. doi:10.1007/s00383-011-3019-9
Garcia AV, Cowles RA, Kato T, Hardy MA (2012) Morio Kasai: a remarkable impact beyond the Kasai procedure. J Pediatr Surg 47(5):1023–1027. doi:10.1016/j.jpedsurg.2012.01.065
Wong KK, Chung PH, Chan KL, Fan ST, Tam PK (2008) Should open Kasai portoenterostomy be performed for biliary atresia in the era of laparoscopy? Pediatr Surg Int 24(8):931–933. doi:10.1007/s00383-008-2190-0
Chan KW, Lee KH, Wong HY, Tsui SY, Wong YS, Pang KY, Mou JW, Tam YH (2014) From laparoscopic to open Kasai portoenterostomy: the outcome after reintroduction of open Kasai portoenterostomy in infant with biliary atresia. Pediatr Surg Int 30(6):605–608. doi:10.1007/s00383-014-3499-5
Sun X, Diao M, Wu X, Cheng W, Ye M, Li L (2016) A prospective study comparing laparoscopic and conventional Kasai portoenterostomy in children with biliary atresia. J Pediatr Surg 51(3):374–378. doi:10.1016/j.jpedsurg.2015.10.045
Laje P, Clark FH, Friedman JR, Flake AW (2010) Increased susceptibility to liver damage from pneumoperitoneum in a murine model of biliary atresia. J Pediatr Surg 45(9):1791–1796. doi:10.1016/j.jpedsurg.2010.02.117
Hussain MH, Alizai N, Patel B (2017) Outcomes of laparoscopic Kasai portoenterostomy for biliary atresia: a systematic review. J Pediatr Surg 52(2):264–267. doi:10.1016/j.jpedsurg.2016.11.022
Nakamura H, Koga H, Okazaki T, Urao M, Miyano G, Okawada M, Doi T, Watayo H, Ogasawara Y, Lane GJ, Yamataka A (2015) Does pneumoperitoneum adversely affect growth, development and liver function in biliary atresia patients after laparoscopic portoenterostomy? Pediatr Surg Int 31(1):45–51. doi:10.1007/s00383-014-3625-4
Li Z, Ye Y, Wu Z, Wang B (2017) Learning curve analysis of laparoscopic kasai portoenterostomy. J Laparoendosc Adv Surg Tech A 27(9):979–982. doi:10.1089/lap.2016.0204
Nakamura H, Koga H, Miyano G, Doi T, Yamataka A (2017) Does the level of transection of the biliary remnant affect outcome after laparoscopic kasai portoenterostomy for biliary atresia? J Laparoendosc Adv Surg Tech A. doi:10.1089/lap.2016.0202
Nakamura H, Murase N, Koga H, Cazares J, Lane GJ, Uchida H, Yamataka A (2016) Classification of biliary atresia in the laparoscopic era. Pediatr Surg Int 32(12):1209–1212. doi:10.1007/s00383-016-3973-3
Nakamura H, Koga H, Cazares J, Okazaki T, Lane GJ, Miyano G, Okawada M, Doi T, Urao M, Yamataka A (2016) Comprehensive assessment of prognosis after laparoscopic portoenterostomy for biliary atresia. Pediatr Surg Int 32(2):109–112. doi:10.1007/s00383-015-3820-y
Wang B, Feng Q, Ye X, Zeng S (2014) The experience and technique in laparoscopic portoenterostomy for biliary atresia. J Laparoendosc Adv Surg Tech A 24(5):350–353. doi:10.1089/lap.2013.0138
Diao M, Li L, Cheng W (2013) Single-incision laparoscopic hepaticojejunostomy using conventional instruments for neonates with extrahepatic biliary cystic lesions. Surg Innov 20(3):214–218. doi:10.1177/1553350612446355
Chan KW, Lee KH, Tsui SY, Wong YS, Pang KY, Mou JW, Tam YH (2012) Laparoscopic versus open Kasai portoenterostomy in infant with biliary atresia: a retrospective review on the 5-year native liver survival. Pediatr Surg Int 28(11):1109–1113. doi:10.1007/s00383-012-3172-9
Oetzmann von Sochaczewski C, Petersen C, Ure BM, Osthaus A, Schubert KP, Becker T, Lehner F, Kuebler JF (2012) Laparoscopic versus conventional Kasai portoenterostomy does not facilitate subsequent liver transplantation in infants with biliary atresia. J Laparoendosc Adv Surg Tech A 22(4):408–411. doi:10.1089/lap.2012.0077
Chan KW, Lee KH, Mou JW, Cheung ST, Tam YH (2011) The outcome of laparoscopic portoenterostomy for biliary atresia in children. Pediatr Surg Int 27(7):671–674. doi:10.1007/s00383-011-2859-7
Liem NT, Son TN, Quynh TA, Hoa NP (2010) Early outcomes of laparoscopic surgery for biliary atresia. J Pediatr Surg 45(8):1665–1667. doi:10.1016/j.jpedsurg.2010.01.019
Liu SL, Li L, Cheng W, Hou WY, Huang LM, Wang WY, Zhang J (2009) Laparoscopic hepatojejunostomy for biliary atresia. J Laparoendosc Adv Surg Tech A 19(Suppl 1):S31-35. doi:10.1089/lap.2008.0119
Ayuso L, Vila-Carbo JJ, Lluna J, Hernandez E, Marco A (2008) [Laparoscopic Kasai portoenterostom: present and future of biliary atresia treatment]. Cir Pediatr 21(1):23–26
Aspelund G, Ling SC, Ng V, Kim PC (2007) A role for laparoscopic approach in the treatment of biliary atresia and choledochal cysts. J Pediatr Surg 42(5):869–872. doi:10.1016/j.jpedsurg.2006.12.052
Dutta S, Woo R, Albanese CT (2007) Minimal access portoenterostomy: advantages and disadvantages of standard laparoscopic and robotic techniques. J Laparoendosc Adv Surg Tech A 17(2):258–264. doi:10.1089/lap.2006.0112
Lima M, Gargano T, De Biagi L, Ruggeri G, Libri M, Lopci E, Salfi N, Sciutti R, Cecini MT, Landuzzi V (2007) [Video-assisted treatment for biliary atresia]. Pediatr Med Chir 29(4):212–217
Lopez M, Kalfa N, Forgues D, Guibal MP, Galifer RB, Allal H (2007) Early laparoscopic Kasai’s procedure in a low weight newborn. J Minim Access Surg 3(2):66–69. doi:10.4103/0972-9941.33276
Al-Qahtani AR, Almaramhi H (2006) Minimal access surgery in neonates and infants. J Pediatr Surg 41(5):910–913. doi:10.1016/j.jpedsurg.2006.01.009
Martinez M, Questa H, Gutierrez V (2004) Laparoscopic Kasai’s operation. Technical details and preliminary results of a promising technique. Cir Pediatr 17(1):36–39
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Cazares, J., Koga, H., Murakami, H. et al. Laparoscopic portoenterostomy for biliary atresia: single-center experience and review of literatures. Pediatr Surg Int 33, 1341–1354 (2017). https://doi.org/10.1007/s00383-017-4171-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-017-4171-7