Abstract
Background
The aim is to evaluate safety and efficacy of near infra-red (NIR) indocyanine green (ICG) fluorescence structural imaging during laparoscopic cholecystectomy (LC) (Group A) and to compare perioperative data, including operative time, with a series of patients who underwent LC with routine traditional intraoperative cholangiography (IOC) (Group B).
Methods
Forty-four patients with acute or chronic cholecystitis underwent NIR-ICG fluorescent cholangiography during LC. ICG was administered intravenously at different time intervals or by direct gallbladder injection during surgery. Fluorescence intensity and anatomy identification were scored according to a visual analogue scale between 1 (least accurate) and 5 (most accurate). Group B patients (n = 44) were chosen from a prospectively maintained database of patients who underwent LC with routine IOC, matched for age, sex, body mass index, and diagnosis with group A patients.
Results
No adverse reactions were recorded. In group A, mean time between intravenous administration of ICG and surgery was 10.7 ± 8.2 (range 2–52) h. Administered doses ranged from 3.5 to 13.5 mg. Fluorescence was present in all cases, scoring ≥ 3 in 41 patients. Mean operative time was 86.9 ± 36.9 (30–180) min in group A and 117.9 ± 43.4 (40–220) min in group B (p = 0.0006). No conversion to open surgery nor bile duct injuries were observed in either group.
Conclusions
LC with NIR-ICG fluorescent cholangiography is safe and effective for early recognition of anatomical landmarks, reducing operative time as compared to LC with IOC, even when residents were the main operator. NIR-ICG fluorescent cholangiography was effective in patients with acute cholecystitis and in the obese. Data collection into large registries on the results of NIR-ICG fluorescent cholangiography during LC should be encouraged to establish whether this technique might set a new safety standard for LC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Laparoscopic cholecystectomy (LC) is one of the most commonly performed procedures in digestive surgery [1], with more than one million cholecystectomies being performed in the United States per year [2]. In spite of several safety measures and recommendations [1, 3,4,5,6,7,8,9,10,11,12,13], the rate of iatrogenic bile duct injury (BDI) during LC is still relatively high, ranging from 0.2 to 1.5% in individual reports [3, 5,6,7,8,9], much higher than initial reports [9, 10], associated with significant morbidity and mortality, lower quality of life and increased costs, related to additional health care measures, loss of work days, and insurance claims [1,2,3,4, 12]. The pattern of clinical presentation of BDI due to cholecystectomy has changed since the advent of LC, with lesions being more proximal, more frequently revealed by bile leakage and sepsis, followed by jaundice from bile duct obstruction [13]. When vascular injury is associated, the management of BDI may be so complex that it may require an orthotopic liver transplantation [14].
Routine intraoperative cholangiography (IOC) has been reported to reduce the risk of BDI [3, 7, 8, 12, 14, 15], but is performed selectively or rarely in most centers, due to increased operative time, need for dedicated radiologic instrumentation and manpower, which may not be always available, and increased costs [14, 15]. The critical view of safety (CVS) technique, introduced in 1995 [16], has been widely acclaimed to reduce the BDI rate during LC, but has not yet been formally shown to reduce the incidence of BDI, most likely because it has not been adopted universally and above all, is often poorly performed [17, 18].
One of the reasons why the risk of BDI has not been definitely eliminated might be the lack of an objective method of positive recognition of the biliary anatomy before any dissection takes place.
Recently, intraoperative indocyanine green (ICG) fluorescence imaging was introduced in minimal access surgery to improve the anatomical definition of the extrahepatic biliary tree and to provide dynamic real time intraoperative evaluation of biliary anatomy and of its blood supply [19,20,21]. Once injected intravenously, ICG concentrates in the bile and becomes fluorescent when illuminated by near infra-red (NIR) light thus highlighting the extrahepatic biliary tree. NIR-ICG fluorescence imaging during LC has been reported to improve the visualization and identification of the biliary anatomy, possibly reducing the risk of BDI [19, 22,23,24].
The aim of this study was to evaluate the safety and efficacy of NIR-ICG fluorescence imaging in defining the biliary anatomy during LC and to compare the perioperative data, including the operative time, with a matched series of patients who underwent LC with routine traditional IOC.
Materials and methods
This is a prospective study. Institutional review board approval and informed consent from all participants were obtained. Every patient undergoing LC with NIR-ICG fluorescence imaging signed a specific informed consent form for ICG administration prior to surgery, together with the informed consent form for data registration in the European-Fluorescence Imaging-Guided Surgery (EURO-FIGS) registry [24], in partnership with the European Association for Endoscopic Surgery (EAES).
Patients
From April 2016 to October 2018, 44 consecutive patients with acute or chronic gallbladder disease scheduled to undergo LC with NIR-ICG fluorescence imaging in our surgical department were enrolled in the study. Patients with preoperatively suspected choledocholithiasis underwent preoperative cholangio-magnetic resonance imaging and, if this was positive, IOC prior to laparoscopic management of common bile duct stones. These patients formed Group A.
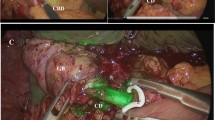
Perioperative results in group A were compared with those of a series of 44 patients matched for age, sex, body mass index (BMI), and diagnosis, who underwent LC with routine traditional IOC (Fig. 1), recorded in our prospectively maintained database (Group B).
Laparoscopic equipment
For group A, the Karl Storz Image 1S D-Light system (Karl Storz Endoscope GmbH & C. K., Tuttlingen Germany) was employed with a 30° forward oblique vision laparoscope. Adjunctive accessory filters (Spectra A) were used to improve image resolution and quality. For better fluorescence visualization, narrowing of the visual operative field was obtained (zooming effect), to focalize the anatomic structures of interest and to improve fluorescence imaging definition. For group B, the radiologic instrumentation and the surgical technique have been previously reported [25].
Indocyanine green
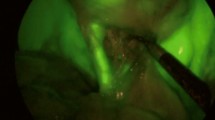
Indocyanine green, 25 mg (Pulsion Medical Systems SE, Feldkirchen, Germany) diluted in 10 cc of sterile water, was employed for fluorescence imaging in group A. Premedication included intravenous Dexamethasone 40 mg and Chlorpheniramine maleate 10 mg administered in every case, administered 30 min before ICG injection. In elective or delayed emergency cases, ICG was injected intravenously before surgery at different time intervals prior to the operation, to determine the best administration timing for adequate fluorescence. Fluorescence imaging was scored according to a visual analogue scale (VAS) from 1 to 5 (1 = least accurate, 5 = most accurate), based on the imaging quality of the visualized biliary structures, as perceived by the surgical team, as described in the EURO-FIGS registry [24]. In cases where the patient was hospitalized on the day of surgery, ICG was injected intraoperatively directly inside the gallbladder with a Veress needle (4 cc of 0.5 mg/ml). The injection site on the gallbladder wall was closed with suture material [26]. A standard dose of 0.1 mg/kg was employed for intravenous administration to avoid the quenching effect, while a total dose of 0.2 mg was employed for direct injection inside the gallbladder. As recommended by the EURO-FIGS registry, dilution, dose and route of administration were registered in the prospective database [24]. Visualization of the cystic duct (CD), cystic duct-CBD junction (J), common bile duct (CBD)/hepatic duct (CHD) was scored before and after dissection of the elements of the triangle of Calot. ICG was not administered to patients with renal and/or liver failure, history of adverse reaction to iodine solution, or thyroid disease uncontrolled by medical treatment, who were excluded from the study.
Surgical technique
In both groups, surgery was performed with the patient supine using the standard four trocars technique (so called “American technique”) with surgeon and cameraman standing on the left side of the patient [27, 28].
Study design
The following data were recorded: age, gender, BMI, American Society of Anesthesiologists (ASA) classification, diagnosis, severity of acute cholecystitis classified according to the Tokyo guidelines criteria [29], symptoms (biliary colic, pancreatitis, dyspepsia), operative time, intraoperative complications, conversion to open surgery, postoperative complications graded according to the Clavien–Dindo classification [30], BDIs described according to the ATOM classification [13], hospital stay, and the operating surgeon.
Biliary colic was defined as: intense pain in the right hypochondrium. Pancreatitis was defined as: rise in pancreatic enzymes. Dyspepsia was defined as: episodic or persistent symptoms that include abdominal pain or discomfort and which are referable to the upper gastrointestinal tract.
Statistical analysis
Data are presented as mean ± standard deviation (SD) for continuous variables and percentages for categorical variables. The Student t test and Fisher’s exact test were employed to evaluate the differences between groups. A p value lower than 0.05 was considered statistically significant. Statistical analyses were carried out with SPSS software 19.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Patient characteristics for groups A and B are reported in Table 1. No statistically significant demographic differences were observed between the two groups.
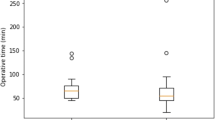
In group A, ICG was administered intravenously in 42 of 44 patients, and twice directly into the gallbladder. No adverse reactions were recorded. Adequate fluorescence was obtained during each procedure and the mean ICG dose that was administered was 0.1 ± 0.1 mg/kg (range 0.03–0.15). The mean time between intravenous administration of ICG and surgery was 10.7 ± 8.2 (range 2–52) h (Fig. 2).
In group A, fluorescence-guided visualization of biliary structures was achieved in 100% of cases, with adequate fluorescence quality (score ≥ 3) in 38 (of 44) patients before dissection, increasing to 41 (of 44) patients after partial dissection. Intraoperative findings during NIR-ICG fluorescence-guided LC are shown in Table 2. Out of 21 group A patients presenting with acute cholecystitis, five underwent surgery within 72 h from the onset of symptoms (therapeutic window). In the remaining 16 patients with acute cholecystitis, LC was performed beyond 72 h after the onset of symptoms because of delayed patient referral to our unit. Intraoperative gallbladder puncture and decompression was required in four cases due to gallbladder thickening and empyema that made gallbladder grasping and/or manipulation difficult. Accidental rupture of an inflamed gallbladder wall occurred in another four patients. A sub-hepatic drain was positioned in 31 patients, removed within 24 h. Five patients underwent laparoscopic transcystic common bile duct exploration during LC, as previously described [27, 28], due to a diagnosis of choledocholithiasis at preoperative cholangio-magnetic resonance imaging, confirmed by intraoperative imaging (IOC) in four. Stone extraction was successfully completed in two patients, while no stones were found at laparoscopic transcystic choledochoscopy in the other three. Completion IOC was carried out to confirm the absence of residual stones in these two patients.
Intraoperative bleeding from the liver parenchyma occurred in one group A patient with acute cholecystitis and dense adhesions, managed laparoscopically by monopolar coagulation. This patient developed a postoperative sub-hepatic abscess, treated with antibiotics and by leaving the sub-hepatic drain in place for 5 days (Clavien–Dindo grade II). Mean hospital stay was 2.2 ± 1.2 (range 1–7) days (Table 3). Thirty-day readmission rate was 0% and mortality was nil. Whenever satisfactory landmarks were obtained and after decision by the tutor, residents performed the operation in 23 out of 44 group A patients, compared to none in group B.
Duration of operative time in group A was 86.9 ± 36.9 (range 30–180) min versus 117.9 ± 43.4 (40–220) min in group B. This difference was statistically significant (p = 0.0006). Two postoperative complications were observed. One postoperative sub-hepatic abscess (mentioned above) treated by antibiotics and prolonged drainage and one postoperative mild pancreatitis that resolved with medical therapy alone (both Clavien–Dindo grade II). Operative results are reported in Table 3.
Discussion
In this case-matched series, the duration of operation was statistically significantly shorter in patients undergoing cholecystectomy with NIR-ICG fluorescent cholangiography than in patients undergoing cholecystectomy with traditional IOC, due to earlier recognition of the anatomical landmarks by NIR-ICG fluorescent cholangiography and prolonged operative time when using IOC. The number of procedures entrusted to and performed by residents (23 out of 44 procedures) was also statistically significantly higher in group A (vs. B) due to greater confidence on the part of the tutors, that the essential anatomical landmarks were easy to recognize, allowing the residents to handle the case even in more difficult settings such as acute cholecystitis and empyema.
NIR-ICG fluorescent cholangiography imaging of biliary structures was achieved in 100% of cases, with adequate fluorescence quality (score ≥ 3) in 38 patients before dissection increasing to 41 of 44 patients after partial dissection. The dose of ICG did not affect the outcome: it was never necessary to inject more than 0.1 mg/kg of ICG to obtain adequate fluorescence imaging.
In the occasional patient when early ICG administration was not possible, direct ICG injection inside the gallbladder with a Veress needle allowed to obtain good fluorescence in one patient with acute cholecystitis while imaging remained poor in the other patient with empyema. However, in our opinion, ICG intravenous administration is the best option, because in case of Veress technique ICG may spread in the abdominal cavity affecting the outcomes related to biliary tree visualization. Moreover, when a gallstone is impacted at the infundibulum, ICG may not reach the common bile duct affecting its visualization. Notwithstanding, this procedure may be a good alternative when the patient undergoes surgery on the same day of admission, as shown by Graves et al. who reported adequate fluorescence in a series of 11 patients [26].
In our study, shorter duration of operation was partly attributed to earlier and better recognition of the anatomical structures of the extrahepatic biliary tree as obtained by the use of NIR-ICG fluorescent cholangiography. While there is no formal evidence that a causal effect is involved, and in agreement with others [15, 31], common sense would dictate that earlier and better vision could shorten the duration of operation. This is why the authors of the Falcon randomized trial chose the time necessary to obtain the critical view of safety as their main endpoint [20]. That LC could be entrusted to and safely performed by residents in 23 out of 44 patients in some complex cases was obviously a subject decision on the part of the senior surgeon, but does lead to believe that being sure of the underlying structures may have contributed to letting the resident perform the operation, similar to the experience of Roy et al. [32]. The improvement in fluorescence imaging that was observed in the latter part of the series was due to better selection of the dose and timing of ICG administration as well as a better understanding of the importance of the zooming effect, and it may be related to a learning curve effect.
As anatomical misinterpretation of extrahepatic bile ducts during LC is one of the most widely recognized causes of BDI [13, 33, 34], objective and positive identification of the biliary anatomy during LC may effectively reduce the risk of BDI. The three most widely used techniques to reduce or avoid BDI today include IOC, intraoperative ultrasound, and obtaining the critical view of safety. All have advantages and disadvantages, and NIR-ICG fluorescence cholangiography can solve most of the latter.
Our adequate visualization rates of biliary structures with NIR-ICG fluorescence cholangiography fall in line with the overall results of a 2017 meta-analysis including 19 studies, although not all were analyzed for the same parameters and methods of NIR imaging with ICG; in particular, dosage and timing of injection varied from one study to another [35]. In sum, visualization of the cystic duct (CD) was obtained in 86.5% (95% CI 71.2–96.6) of patients prior to dissection of Calot’s triangle, rising to 96.5% (95% CI 93.9–98.4) after dissection. No details were available, however, to define to what degree extra dissection was required. Results did not differ statistically significantly according to whether the patients received a fixed dosage of 2.5 mg (nine studies) or according to bodyweight 0.05 mg/kg body weight (6 studies). The CD seemed to be visualized more frequently with ICG (vs. IOC, RR 1.16; 95% CI 1.00–1.35); however, this difference was not statistically significant [35]. Conversely, the quality of evidence in favor of ICG over IOC for the visualization of the CBD and CHD was moderate and low, respectively. The authors concluded that NIR imaging with ICG had the potential to replace IOC for biliary mapping [35]. Our results are similar to those observed by others [23, 31, 32, 36]. The results of the randomized trial by Dip et al. [37] have recently been released. The CD was visualized by NIR-ICG twice as often as under white light before dissection (66.6% vs. 36.2%) (p = < 0.001), but the difference was not statistically significantly different after dissection. The right hepatic duct, CHD and CBD were visualized by NIR-ICG (vs. white light), statistically significantly both before (9.1% vs. 2.2%, 28.9% vs. 10.9% and 49.4% vs. 20.6%) and after (22.1% vs. 6.9%, 52.3% vs. 30.5% and 75.7% vs. 50.0%) dissection, respectively.
Liberal use of IOC has been reported to reduce the risk of BDI [2, 7, 8, 14]. However, intraoperative imaging of the biliary tree by IOC is often used selectively because it prolongs the operative time, involves radiation exposure, and additional resources are required, including the C-arm fluoroscope and a technician. Moreover, to perform IOC it is necessary to incise what is presumed to be the CD, which in itself may be the cause of a lateral injury of the CBD (or CHD) in case of mistaken anatomy [29]. Last, only bile duct structures in connection with the point of injection, and sometimes, only by hyper-pressure [38] are visible with IOC, whereas NIR-ICG fluorescent cholangiography lights up every single structure with bile in it. In the present study, no BDI was observed in either group.
Intraoperative laparoscopic ultrasound (LUS) has also been proposed [39, 40] because of its 84% diagnostic accuracy before dissection and 90–100% after initial dissection [39]. This technique is less invasive (no incision on the bile duct required) and cheaper than IOC, can be employed by the surgeon without the need for a technician, does not expose the patient or surgical team to radiation, and it can be repeated during any phase of the surgical procedure. However, LUS has not been widely adopted because operator-dependent and requiring a lengthy learning curve [40], and the fact that the instrumentation is available only in dedicated hepato-biliary centers. Although recommended by major surgical societies [29, 41, 42], the CVS technique [16] has never been shown formally to decrease the rate of BDI in any prospective comparative study [9, 11, 17, 18, 43] and the level of evidence proving that it prevents BDI is very low, even in the words of its author and advocates [44]. CVS, as IOC, require some dissection before the interpretation of anatomy, thus an injury to other biliary structures can occur during the preliminary stage of the identification procedure. Moreover, in case of inflamed gallbladder it may not be possible to achieve the CVS. In these cases, performing IOC or LUS is recommended prior to a fundus-first dissection cholecystectomy or a subtotal cholecystectomy or conversion to open surgery [41].
NIR-ICG fluorescent cholangiography may solve most of the disadvantages reported above concerning IOC, LUS, and CVS as an aid in the correct identification of the biliary anatomy aimed at prevention of BDI during LC [19, 22, 23]. Little, if any, dissection is required before adequate images are obtained. NIR-ICG fluorescent cholangiography can be repeated safely without concerns of safety (exposure to radiation) and the learning curve is short [15, 19, 31, 32]. Although specific equipment is required, it has been shown to be cheaper than IOC [15].
There is no widely admitted consensus today on several factors that influence the quality of the fluorescence imaging, including the dose and timing of ICG injection, and the presence of thickened tissues from acute disease. With regard to the exact dose and concentration of ICG to be administered to the patient, many authors employ a dose of 0.1–0.5 mg/kg body weight [19, 23]. Boni et al. report the administration of 5 ml of 0.3–0.4 mg/ml/kg to provide an adequate ICG concentration in the bile and good visualization of the biliary tree (100% in a series of 52 patients) [19]. The time interval between ICG injection and detection of biliary fluorescence has also been a subject of debate [22, 26]. More than 95% of ICG is captured by hepatocytes within 15 min after intravenous injection and then is excreted into the bile. This time interval is related to liver function: organs with poor liver function (e.g. cirrhosis [45]) take longer to extract ICG from the blood to the bile. In our experience, the fluorescence of the extrahepatic bile ducts was poor when ICG was administered immediately prior to surgery because of background hepatic fluorescence. Moreover, although the best timing for cholecystectomy in patients with acute cholecystitis seems to be the therapeutic window within which the risk of bile duct injury is minimal [46], many centers still opt for deferring surgical treatment after resolution of the acute episode, which is managed conservatively. Indeed, the Tokyo guidelines are not clear on this issue [29]. This policy, however, requires two admissions with increased costs [47] and patients are at risk of recurrent biliary complications during the time interval between the two admissions. In accordance with others [46, 48, 49], early administration of ICG, 5-7 h before surgery, improved the quality of extrahepatic biliary tree visualization, persisting up to 52 h (Table 2, Fig. 2), with no imaging impairment from liver parenchyma fluorescence. This may also be related to higher biliary concentration as compared to the initial, mainly hepatocellular, ICG concentration. The lowest fluorescence score was observed in patients with gallbladder empyema and acute cholecystitis. For empyema, reduced ICG fluorescence might have been related to elevated cellular concentration in the fluid. In acute cholecystitis, fluorescence improved after partial dissection of adhesions and of the peritoneum covering the gallbladder infundibulum.
Initial reports of the use of NIR-ICG cholangiography indicated that imaging was suboptimal in patients with inflamed or thickened tissues (acute or chronic cholecystitis) and the obese [23]. However, similar to the experience of Boni et al. [48], imaging was adequate (≥ 3) in 16 of 21 patients with acute cholecystitis or empyema before dissection, improving to 19 of 21 after minimal dissection, while improving from grades 1 to 2 in the two remaining patients.
Another point of concern is the possibility of poorer imaging in the obese. Daskalaki et al. reported that complementary dissection was needed for adequate visualization in patients with BMI over 30 kg/m2 (15/22 cases) [23]. Osayi et al. [49] reported a comparative analysis of biliary tract visualization in a series of 62 patients undergoing both ICG administration and IOC. The rate of biliary tree anatomy identification was lower in patients with BMI > 35 kg/m2. However, CD and CBD identification rates were 95% and 76.8% in the ICG group versus 65% and 75.6% in the IOC group (p = 0.001), respectively. No BDI were observed. According to Dip et al., fluorescent cholangiography is useful in both the obese and non-obese population: obesity does not affect the efficacy of fluorescent cholangiography in detection of the biliary structures [50].
According to these preliminary results and our analysis of the literature, NIR-ICG fluorescent cholangiography during LC is safe and effective, and is associated with a shorter duration of operation as compared to LC with IOC. If NIR-ICG fluorescent cholangiography proves to significantly reduce the risk of BDI even in acute conditions, whatever the time interval from the beginning of symptoms, as suggested by experimental and clinical data [51, 52], this might represent a new safety standard for LC and could lead to abolish the practice of surgical treatment deferral, with cost savings and increased safety. As the current rate of BDI is relatively low, a randomized controlled trial aimed at demonstrating a significant reduction in BDI rate comparing NIR-ICG fluorescent cholangiography with standard LC techniques (i.e., with and without IOC), even in a multicenter setting, would require enrollment of a huge number of patients in each arm [44]. The inherent difficulties in setting up such trial make it highly improbable; instead, an international registry (such as the EURO-FIGS registry, in partnership with the EAES) enrolling a substantial number of patients, although not comparative, might provide an alternative, feasible way of demonstrating a reduction in BDI rate and therefore provide the basis to set up a new safety standard.
Additionally, an extra bolus of intraoperative ICG may be administered intravenously to identify the vascular anatomy at Calot’s triangle, as reported by Shols et al. [21]. This may prove to be of value when the right hepatic artery is also located in the Calot’s triangle [53]. Lastly, the implications of ICG fluorescence imaging during LC as an educational tool for safer resident training should be underscored [32].
The main weaknesses of the present study are the lack of an objective and standardized tool for intraoperative assessment of fluorescence quality, the small sample size of patients in group A, collected in more than two years, and the retrospective nature of group B patients.
References
Pucher PH, Brunt LM, Davies N, Linsk A, Munshi A, Rodriguez HA, Fingerhut A, Fanelli RD, Asbun H, Aggarwal R, SAGES Safe Cholecystectomy Task Force (2018) Outcome trends and safety measures after 30 years of laparoscopic cholecystectomy: a systematic review and pooled data analysis. Surg Endosc 32(5):2175–2183. https://doi.org/10.1007/s00464-017-5974-2
National Center for Health Statistics. Health, United States, 2009. In Brief—medical technology (2010). https://www.cdc.gov/nchs/data/hus/hus09_InBrief_MedicalTech.pdf. Accessed 4 Jan 2019
Flum DR, Dellinger EP, Cheadle A, Chan L, Koepsell T (2003) Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA 289(13):1639–1644
Gartland RM, Bloom JP, Fong ZV, DeRoo C, Dwyer K, Quinn G, Lillemoe K, Mort E (2018) What have we learned from malpractice claims involving the surgical management of benign biliary disease?: A 128 million dollar question. Ann Surg. https://doi.org/10.1097/sla.0000000000003155
Harboe KM, Bardram L (2011) The quality of cholecystectomy in Denmark: outcome and risk factors for 20,307 patients from the national database. Surg Endosc 25(5):1630–1641. https://doi.org/10.1007/s00464-010-1453-8
Navez B, Ungureanu F, Michiels M, Claeys D, Muysoms F, Hubert C, Vanderveken M, Detry O, Detroz B, Closset J, Devos B, Kint M, Navez J, Zech F, Gigot JF, Belgian Group for Endoscopic Surgery (BGES) and the Hepatobiliary and Pancreatic Section (HBPS) of the Royal Belgian Society of Surgery (2012) Surgical management of acute cholecystitis: results of a 2-year prospective multicenter survey in Belgium. Surg Endosc 26(9):2436–2445
Törnqvist B, Strömberg C, Akre O, Enochsson L, Nilsson M (2015) Selective intraoperative cholangiography and risk of bile duct injury during cholecystectomy. Br J Surg 102(8):952–958. https://doi.org/10.1002/bjs.9832
Törnqvist B, Strömberg C, Persson G, Nilsson M (2012) Effect of intended intraoperative cholangiography and early detection of bile duct injury on survival after cholecystectomy: population based cohort study. BMJ 345:e6457. https://doi.org/10.1136/bmj.e6457
Waage A, Nilsson M (2006) Iatrogenic bile duct injury: a population- based study of 152 776 cholecystectomies in the Swedish inpatient registry. Arch Surg 141(12):1207–1213
Dolan JP, Diggs BS, Sheppard BC, Hunter JG (2005) Ten-year trend in the national volume of bile duct injuries requiring operative repair. Surg Endosc 19(7):967–973
Landman MP, Feurer ID, Moore DE, Zaydfudim V, Pinson CW (2013) The long-term effect of bile duct injuries on health-related quality of life: a meta-analysis. HPB (Oxford) 15(4):252–259. https://doi.org/10.1111/j.1477-2574.2012.00586.x
Fletcher DR, Hobbs MS, Tan P, Valinsky LJ, Hockey RL, Pikora TJ, Knuiman MW, Sheiner HJ, Edis A (1999) Complications of cholecystectomy: risks of the laparoscopic approach and protective effects of operative cholangiography: a population-based study. Ann Surg 229(4):449–457
Fingerhut A, Dziri C, Garden OJ, Gouma D, Millat B, Neugebauer E, Paganini A, Targarona E (2013) ATOM, the all-inclusive, nominal EAES classification of bile duct injuries during cholecystectomy. Surg Endosc 27(12):4608–4619. https://doi.org/10.1007/s00464-013-3081-6
Rystedt JML, Tingstedt B, Montgomery F, Montgomery AK (2017) Routine intraoperative cholangiography during cholecystectomy is a cost-effective approach when analysing the cost of iatrogenic bile duct injuries. HPB (Oxford) 19(10):881–888. https://doi.org/10.1016/j.hpb.2017.06.004
Dip FD, Asbun D, Rosales-Velderrain A, Lo Menzo E, Simpfendorfer CH, Szomstein S, Rosenthal RJ (2014) Cost analysis and effectiveness comparing the routine use of intraoperative fluorescent cholangiography with fluoroscopic cholangiogram in patients undergoing laparoscopic cholecystectomy. Surg Endosc 28(6):1838–1843. https://doi.org/10.1007/s00464-013-3394-5
Strasberg SM, Hertl M, Soper NJ (1995) An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 180(1):101–125
Booij KA, de Reuver PR, Nijsse B, Busch OR, van Gulik TM, Gouma DJ (2014) Insufficient safety measures reported in operation notes of complicated laparoscopic cholecystectomies. Surgery 155(3):384–389. https://doi.org/10.1016/j.surg.2013.10.010.epub
Deal SB, Stefanidis D, Telem D, Fanelli RD, McDonald M, Ujiki M, Michael Brunt L, Alseidi AA (2017) Evaluation of crowd-sourced assessment of the critical view of safety in laparoscopic cholecystectomy. Surg Endosc 31(12):5094–5100. https://doi.org/10.1007/s00464-017-5574-1
Boni L, David G, Mangano A, Dionigi G, Rausei S, Spampatti S, Cassinotti E, Fingerhut A (2015) Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc 29(7):2046–2055. https://doi.org/10.1007/s00464-014-3895-x
van den Bos J, Schols RM, Luyer MD, van Dam RM, Vahrmeijer AL, Meijerink WJ, Gobardhan PD, van Dam GM, Bouvy ND, Stassen LP (2016) Near-infrared fluorescence cholangiography assisted laparoscopic cholecystectomy versus conventional laparoscopic cholecystectomy (FALCON trial): study protocol for a multicentre randomised controlled trial. BMJ Open 6(8):e011668. https://doi.org/10.1136/bmjopen-2016-011668
Schols RM, Bouvy ND, van Dam RM, Masclee AA, Dejong CH, Stassen LP (2013) Combined vascular and biliary fluorescence imaging in laparoscopic cholecystectomy. Surg Endosc 27(12):4511–4517. https://doi.org/10.1007/s00464-013-3100-7
Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N (2010) Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg 97(9):1369–1377. https://doi.org/10.1002/bjs.7125
Daskalaki D, Fernandes E, Wang X, Bianco FM, Elli EF, Ayloo S, Masrur M, Milone L, Giulianotti PC (2014) Indocyanine green (ICG) fluorescent cholangiography during robotic cholecystectomy: results of 184 consecutive cases in a single institution. Surg Innov 21(6):615–621. https://doi.org/10.1177/1553350614524839
European Fluorescence Imaging-Guided Surgery (EURO-FIGS registry). http://www.euro-figs.eu. Accessed 16 Jan 2019
Lezoche E, Paganini A, Guerrieri M, Carlei F, Lomanto D, Sottili M, Nardovino M (1994) Technique and results of routine dynamic cholangiography during 528 consecutive laparoscopic cholecystectomies. Surg Endosc 8(12):1443–1447
Graves C, Ely S, Idowu O, Newton C, Kim S (2017) Direct gallbladder indocyanine green injection fluorescence cholangiography during laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 27(10):1069–1073. https://doi.org/10.1089/lap.2017.0070
Quaresima S, Balla A, Guerrieri M, Campagnacci R, Lezoche E, Paganini AM (2017) A 23 year experience with laparoscopic common bile duct exploration. HPB (Oxford) 19(1):29–35. https://doi.org/10.1016/j.hpb.2016.10.011
Quaresima S, Balla A, Guerrieri M, Lezoche G, Campagnacci R, D’Ambrosio G, Lezoche E, Paganini AM (2016) Results of medium seventeen years’ follow-up after laparoscopic choledochotomy for ductal stones. Gastroenterol Res Pract 2016:9506406. https://doi.org/10.1155/2016/9506406
Takada T, Strasberg SM, Solomkin JS, Pitt HA, Gomi H, Yoshida M, Mayumi T, Miura F, Gouma DJ, Garden OJ, Büchler MW, Kiriyama S, Yokoe M, Kimura Y, Tsuyuguchi T, Itoi T, Gabata T, Higuchi R, Okamoto K, Hata J, Murata A, Kusachi S, Windsor JA, Supe AN, Lee S, Chen XP, Yamashita Y, Hirata K, Inui K, Sumiyama Y, Committee Tokyo Guidelines Revision (2013) TG13: Updated Tokyo Guidelines for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci 20(1):1–7. https://doi.org/10.1007/s00534-012-0566-y
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2
Diana M, Soler L, Agnus V, D’Urso A, Vix M, Dallemagne B, Faucher V, Roy C, Mutter D, Marescaux J, Pessaux P (2017) Prospective evaluation of precision multimodal gallbladder surgery navigation: virtual reality, near-infrared fluorescence, and X-ray-based intraoperative cholangiography. Ann Surg 266(5):890–897. https://doi.org/10.1097/SLA.0000000000002400
Roy M, Dip F, Nguyen D, Simpfendorfer CH, Menzo EL, Szomstein S, Rosenthal RJ (2017) Fluorescent incisionless cholangiography as a teaching tool for identification of Calot’s triangle. Surg Endosc 31(6):2483–2490. https://doi.org/10.1007/s00464-016-5250-x
Way LW, Stewart L, Gantert W, Liu K, Lee CM, Whang K, Hunter JG (2003) Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg 237(4):460–469
Ford JA, Soop M, Du J, Loveday BP, Rodgers M (2012) Systematic review of intraoperative cholangiography in cholecystectomy. Br J Surg 99(2):160–167. https://doi.org/10.1002/bjs.7809
Vlek SL, van Dam DA, Rubinstein SM, de Lange-de Klerk ESM, Schoonmade LJ, Tuynman JB, Meijerink WJHJ, Ankersmit M (2017) Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: results of a systematic review. Surg Endosc 31(7):2731–2742. https://doi.org/10.1007/s00464-016-5318-7
Zroback C, Chow G, Meneghetti A, Warnock G, Meloche M, Chiu CJ, Panton ON (2016) Fluorescent cholangiography in laparoscopic cholecystectomy: the initial Canadian experience. Am J Surg 211(5):933–937. https://doi.org/10.1016/j.amjsurg.2016.01.013
Dip F, LoMenzo E, Sarotto L, Phillips E, Todeschini H, Nahmod M, Alle L, Schneider S, Kaja L, Boni L, Ferraina P, Carus T, Kokudo N, Ishizawa T, Walsh M, Simpfendorfer C, Mayank R, White K, Rosenthal RJ (2019) Randomized trial of near-infrared incisionless fluorescent cholangiography. Ann Surg. https://doi.org/10.1097/sla.0000000000003178
Millat B, Atger J, Deleuze A, Briandet H, Fingerhut A, Guillon F, Marrel E, De Seguin C, Soulier P (1997) Laparoscopic treatment for choledocholithiasis: a prospective evaluation in 247 consecutive unselected patients. Hepatogastroenterology 44(13):28–34
Tranter SE, Thompson MH (2003) A prospective single-blinded controlled study comparing laparoscopic ultrasound of the common bile duct with operative cholangiography. Surg Endosc 17(2):216–219
Dili A, Bertrand C (2017) Laparoscopic ultrasonography as an alternative to intraoperative cholangiography during laparoscopic cholecystectomy. World J Gastroenterol 23(29):5438–5450. https://doi.org/10.3748/wjg.v23.i29.5438
Eikermann M, Siegel R, Broeders I, Dziri C, Fingerhut A, Gutt C, Jaschinski T, Nassar A, Paganini AM, Pieper D, Targarona E, Schrewe M, Shamiyeh A, Strik M, Neugebauer EA, European Association for Endoscopic Surgery (2012) Prevention and treatment of bile duct injuries during laparoscopic cholecystectomy: the clinical practice guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc 26(11):3003–3039. https://doi.org/10.1007/s00464-012-2511-1
Pucher PH, Brunt LM, Fanelli RD, Asbun HJ, Aggarwal R (2015) SAGES expert Delphi consensus: critical factors for safe surgical practice in laparoscopic cholecystectomy. Surg Endosc 29(11):3074–3085. https://doi.org/10.1007/s00464-015-4079-z
Balla A, Quaresima S, Corona M, Lucatelli P, Fiocca F, Rossi M, Bezzi M, Catalano C, Salvatori FM, Fingerhut A, Paganini AM (2018) ATOM classification of bile duct injuries during laparoscopic cholecystectomy: analysis of a single institution experience. J Laparoendosc Adv Surg Tech A. https://doi.org/10.1089/lap.2018.0413
Strasberg SM, Brunt LM (2017) The critical view of safety: why it is not the only method of ductal identification within the standard of care in laparoscopic cholecystectomy. Ann Surg 265(3):464–465. https://doi.org/10.1097/SLA.0000000000002054
Kawaguchi Y, Ishizawa T, Miyata Y, Yamashita S, Masuda K, Satou S, Tamura S, Kaneko J, Sakamoto Y, Aoki T, Hasegawa K, Sugawara Y, Kokudo N (2013) Portal uptake function in veno-occlusive regions evaluated by real-time fluorescent imaging using indocyanine green. J Hepatol 58(2):247–253. https://doi.org/10.1016/j.jhep.2012.09.028
Verbeek FP, Schaafsma BE, Tummers QR, van der Vorst JR, van der Made WJ, Baeten CI, Bonsing BA, Frangioni JV, van de Velde CJ, Vahrmeijer AL, Swijnenburg RJ (2014) Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg Endosc 28(4):1076–1082
Gutt CN, Encke J, Köninger J, Harnoss JC, Weigand K, Kipfmüller K, Schunter O, Götze T, Golling MT, Menges M, Klar E, Feilhauer K, Zoller WG, Ridwelski K, Ackmann S, Baron A, Schön MR, Seitz HK, Daniel D, Stremmel W, Büchler MW (2013) Acute cholecystitis: early versus delayed cholecystectomy, a multicenter randomized trial (ACDCstudy, NCT00447304). Ann Surg 258(3):385–393. https://doi.org/10.1097/SLA.0b013e3182a1599b
Boni L, Cassinotti EC, David G, Marzorati AM, Fingerhut A (2015) Indocyanine green-enhanced fluorescence cholangiography in laparoscopic cholecystectomy: experience from 100 cases. J Am Coll Surg 221(4):S71
Osayi SN, Wendling MR, Drosdeck JM, Chaudhry UI, Perry KA, Noria SF, Mikami DJ, Needleman BJ, Muscarella P 2nd, Abdel-Rasoul M, Renton DB, Melvin WS, Hazey JW, Narula VK (2015) Nera-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc 29(2):368–375. https://doi.org/10.1007/s00464-014-3677-5
Dip F, Nguyen D, Montorfano L, Szretter Noste ME, Lo Menzo E, Simpfendorfer C, Szomstein S, Rosenthal R (2016) Accuracy of near infrared-guided surgery in morbidly obese subjects undergiong laparoscopic cholecystectomy. Obes Surg 26(3):525–530. https://doi.org/10.1007/s11695-015-1781-9
Liu YY, Kong SH, Diana M, Lègner A, Wu CC, Kameyama N, Dallemagne B, Marescaux J (2016) Near-infrared cholecysto-cholangiography with indocyanine green may secure cholecystectomy in difficult clinical situations: proof of the concept in a porcine model. Surg Endosc 30(9):4115–4123. https://doi.org/10.1007/s00464-015-4608-9
Ankersmit M, van Dam DA, van Rijswijk AS, van den Heuvel B, Tuynman JB, Meijerink WJHJ (2017) Fluorescent imaging with indocyanine green during laparoscopic cholecystectomy in patients at increased risk of bile duct injury. Surg Innov 24(3):245–252. https://doi.org/10.1177/1553350617690309
Bergamaschi R, Ignjatovic D (2000) More than two structures in Calot’s triangle. A postmortem study. Surg Endosc 14(4):354–357
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Silvia Quaresima, Andrea Balla, Livia Palmieri, Ardit Seitaj, Abe Fingerhut, Pietro Ursi, and Alessandro M. Paganini declare that they have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quaresima, S., Balla, A., Palmieri, L. et al. Routine near infra-red indocyanine green fluorescent cholangiography versus intraoperative cholangiography during laparoscopic cholecystectomy: a case-matched comparison. Surg Endosc 34, 1959–1967 (2020). https://doi.org/10.1007/s00464-019-06970-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06970-0