Abstract
Background
Intraoperative incisionless fluorescent cholangiogram (IOIFC) has been demonstrated to be a useful tool to increase the visualization of Calot’s triangle. This study evaluates the identification of extrahepatic biliary structures with IOIFC by medical students and surgery residents.
Methods
Two pictures were taken, one with xenon light and one with near-infrared (NIR) light, at the same stage during dissection of Calot’s triangle in ten different cases of laparoscopic cholecystectomy (LC). All twenty pictures were organized in a random fashion to remove any imagery bias. Twenty students and twenty residents were asked to identify the biliary anatomy.
Results
Medical students were able to accurately identify the cystic duct on an average 33.8 % under the xenon light versus 86 % under NIR light (p = 0.0001), the common hepatic duct (CHD) on an average 19 % under the xenon light versus 88.5 % under NIR light (p = 0.0001), and the junction on an average 24 % under xenon light versus 80.5 % under NIR light (p = 0.0001). Surgery residents were able to accurately identify the cystic duct on an average 40 % under the xenon light versus 99 % under NIR light (p = 0.0001), the CHD on an average 35 % under the xenon light versus 96 % under NIR light (p = 0.0001), and the junction on an average 24 % under the xenon light versus 95.5 % under NIR light (p = 0.0001).
Conclusions
IOIFC increases the visualization of Calot’s triangle structures when compared to xenon light. IOIFC may be a useful teaching tool in residency programs to teach LC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Laparoscopic cholecystectomy (LC) remains the most commonly performed procedure by a general surgery resident [1, 2]. Despite the fact that LC has proven to be a safe procedure, the rate of common bile duct (CBD) injury still remains unacceptably high at between 0.2 and 0.4 % even in the hands of minimally invasive trained surgeons [3]. The dissection around Calot’s triangle during LC can be difficult sometimes, especially in a case of acute cholecystitis [4]. This can make it challenging for the attending surgeon to supervise the resident. Technical factors leading to biliary injury are often caused by errors of perception during dissection of Calot’s triangle, including misidentification of anatomy, and failure to recognize injuries when they occur (5).
Laparoscopic cholecystectomy gained popularity among general surgeons in 1990s. During the initial learning curve, some surgeons were not able to identify the anatomy clearly during LC. Some considered the traditional classical stepwise progression in surgical training and teaching, of slowly advancing from camera operator to first assistant and finally to the operating surgeon, as safe and efficient [6]. Over the last two decades, different strategies have been designed and pursued in residency programs in order to improve residents’ skills and efficiency in performing LC. With the rise of the virtual and mental training concepts, there is a shift toward training in the laboratory on simulators before coming to the operating room, but these methods come with their own limitations, including cost and time [7]. While advanced simulators can be helpful in improving surgical skills, they cannot help train on the understanding of the biliary anatomy in real time.
Recently, we described the routine use of intraoperative incisionless fluorescent cholangiogram (IOIFC) as an alternative method to identify the biliary anatomy during LC [8]. The attending can direct the trainee more accurately with IOIFC by showing glowing pictures instead of referring to random directions. We found IOIFC to be an effective communication platform with which an attending surgeon can better guide both medical students and surgery residents due to its efficiency in identifying biliary structures. The aim of this study was to evaluate the effectiveness of fluorescent cholangiography in identifying biliary anatomy from a series of still images shown to students and surgery residents.
Materials and methods
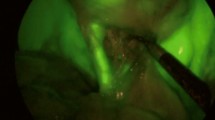
After internal review board (IRB) approval and following HIPAA guidelines, two pictures were taken, one with xenon light and one with near-infrared (NIR) light, at the same stage during dissection of Calot’s triangle in ten different cases of laparoscopic cholecystectomy (LC) after preoperative intravenous injection of 0.05 mg/kg indocyanine green (ICG). While IOIFC can useful in difficult cases or to identify aberrant anatomy, the goal of this paper was to see whether it could be used as a teaching tool to identify biliary anatomy regardless of case difficulty level. Hence the cases were chosen randomly and their difficulty level was not assessed. The first five cases (type I) represented symptomatic cholelithiasis (Fig. 1A), and the last five cases (type II) represented acute cholecystitis (Fig. 1B). All twenty pictures were organized in a random fashion to remove any imagery bias. The Web site randomization.com (http://www.randomization.com) was used to generate the randomization scheme. Once a random permutation of all integers was generated, it was checked to ensure that xenon light and NIR light pictures from the same case did not appear simultaneously. Twenty students and 20 residents were asked to identify the extrahepatic biliary anatomy, including the cystic duct, common hepatic duct (CHD), and their junction, in both xenon and NIR light pictures. None of the medical students had any experience with IOIFC. All twenty residents utilized IOIFC between zero and five cases. Residents were divided into two groups depending upon the number of LC cases performed, with group 1 performing less than 30 cases and group 2 performing greater than 30 cases. The cutoff number was chosen in order to divide junior (first 2 years) from senior (last year) residents.
A Pictures taken during dissection of Calot’s triangle in five different cases of laparoscopic cholecystectomy with xenon and near-infrared light. These five cases presented with symptomatic cholelithiasis. B. Pictures taken during dissection of Calot’s triangle in five different cases of laparoscopic cholecystectomy with xenon and near-infrared light (NIR). These five cases presented with acute cholecystitis CD cystic duct, CHD common hepatic duct, junction cystic duct & common hepatic duct junction
The data were exported from an Excel workbook to a comma-separated value (CSV) file in preparation for an analysis. The binary outcome indicating accuracy of identification was modeled using generalized linear mixed models, with a crossed random effects structure for cases and readers, and fixed effects for reader level, the structure being identified, and the lighting condition. All analyses were performed using the R software version 1.1–7 (Vienna, Austria 2014), and models were fit using the “lme4 package” (linear mixed-effects models using Eigen and S4, 2014).
Results
Medical students were able to accurately identify the cystic duct on an average 33.8 % under the xenon light versus 86 % under the NIR light (p = 0.0001), the CHD on an average 19 % under the xenon light versus 88.5 % under the NIR light (p = 0.0001), and the junction on an average 24 % under xenon light versus 80.5 % under the NIR light (p = 0.0001) (Fig. 2). Surgery residents were able to accurately identify the cystic duct on average 40 % of the time under xenon light versus 99 % under NIR light (p = 0.0001), the CHD on an average 35 % under the xenon light versus 96 % under the NIR light (p = 0.0001), and the junction on an average 24 % under the xenon light versus 95.5 % under the NIR light (p = 0.0001) (Fig. 3).
The odds ratio of a student accurately identifying the cystic duct under xenon light was 0.36 (95 % CI 0.27–0.48). An estimate of the odds ratio of accurate identification of biliary structures with NIR light by students and residents is shown in Table 1. The odds ratio of accurate identification of a given structure in NIR light by a resident is 2.09 compared to by a student. There is a very large and significant improvement in identification accuracy under NIR versus under xenon light (OR 33.44, 95 % CI 25.16–45.20, P < 0.001) for a given reader level and structure. There appears to be no difference between type I and type II cases for a given reader level and structure. In Table 2, we see estimates of a model of accurate identification of biliary structures by residents only, based on the lighting condition, number of LC cases performed (greater or less than 30), and case types (symptomatic cholelithiasis vs acute cholecystitis). Based on this model, neither of the associations between case types or number of previous LC cases performed by residents is significant when done under NIR light, after controlling for the other variables.
Discussion
Accuracy of IOIFC has been evaluated in previous studies with a sensitivity between 71.4 and 100 % in detecting the cystic duct, and between 76 and 100 % in detecting the CBD, in patients with cholelithiasis and cholecystitis [9]. Similarly, cost analysis of IOIFC has been published [10]. Accuracy of this novel technique has been proven in the obese population [11]. To our knowledge, this is the first study that analyzes surgery residents’ and medical students’ understanding of the extrahepatic bile duct structures with fluorescent cholangiography.
LC is one of the most common surgical procedures performed during surgical residency, and it has been increasingly performed by residents [2]. During an open surgery, it is easier for an attending surgeon to guide the trainee with accurate demonstration of the anatomy, as he or she has the added ability to guide the trainee by holding his or her hand if needed. Attending surgeons lose this ability during laparoscopic surgery, where an attending surgeon has to rely more on his or her communication skills to direct the trainee to dissect in correct planes and explain the anatomy [12]. With laparoscopic surgery, there is also loss of haptic input and stereoscopic depth perception, which predisposes to the misperception that leads to biliary injuries [13].
Various methods have been described to identify the structures in Calot’s triangle in order to reduce bile duct injury; these include intraoperative cholangiogram (IOC) by Mirizzi [14] and critical view of safety (CVS) by Strasberg [15]. IOC can be difficult to teach and residents do not gain enough experience with it as not all surgeons perform it routinely. The resident has to dissect around the Calot’s triangle to demonstrate the CVS. It can become challenging for an attending surgeon to supervise the resident to dissect and demonstrate CVS without any visual landmarks. The resident surgeon needs structured surgical training with appropriate instruction by an attending surgeon during the surgical procedure to avoid complications during the learning curve [16].
The correct identification of Calot’s triangle may be challenging for residents, and misidentification of the structures may end in a bile duct injury. The most frequent site of CBD injury is below the bifurcation of the right and left hepatic ducts (65 %), and once anatomic confusion has led the surgeon astray, injury tends to occur in relatively predictable locations along the biliary tree [4]. In our study, the three structures to be identified included the cystic duct, the common hepatic duct, and their junction. This will help in identification of Calot’s triangle and the CHD, which is the most common site of injury. We were able to show that even medical students who had never performed or assisted in LC were able to identify the anatomy with reasonable accuracy under NIR light.
In our study, both students and residents were able to identify all extrahepatic biliary structures in significantly higher percentage with NIR light, which was statistically significant in all cases (p = 0.0001). Residents performed slightly better than the students with both xenon and NIR light because of their previous experience with LC (OR 2.09: 95 % CI 1.62–2.70, P < 0.001).
There is a significant improvement in identification accuracy under NIR versus xenon lighting (OR 33: 44, 95 % CI 25.16–45.20, P < 0.001) for a given reader level and structure, which shows the efficacy of using NIR lighting in trainees. There was no difference in identification of structures in cases with acute cholecystitis versus symptomatic cholelithiasis. This suggests that in the cases that were evaluated in this study, NIR was effective even when there is significant inflammation around Calot’s triangle, though this may be interpreted cautiously because of low power.
In teaching hospitals, overcoming the problem of providing high surgical quality while educating surgical trainees can be challenging [17]. LC cases performed by surgical trainees are not associated with higher operative morbidity [18]. But the length of operative time is significantly increased when compared with that of LC cases performed by attending surgeons, due to difficulties in identifying the anatomical structures [19] [20], and this sometimes leads to an attending surgeon taking away the case from the trainee. This may change in the future if residents are able to better identify the anatomy with IOIFC. There was no difference in identification of biliary structures with NIR light within the resident group, based on the number of LC cases performed (greater or less than 30). This may suggest a quick learning curve with the use of NIR light, but the power is too low to make a conclusion. We did not divide the groups according to the postgraduate year (PGY) level because it was already a small group, and dividing it further into resident level would make any kind of statistical analysis inconclusive. Also, there can be disparity of training among different residency programs the resident PGY level.
Dip et al. demonstrated the identification of the cystic duct and hepatic duct to be 97.7 and 60 % with NIR light, respectively [8]. Ishizawa et al. demonstrated the identification of the cystic duct and cystic duct–common hepatic duct junction to be 100 and 96 %, respectively, with the same technology [21]. As discussed earlier, the virtual models cannot help in increasing the experience of learning anatomy. Although virtual reality-trained subjects acquire skills on the cadaveric porcine model at a faster rate, leading to a shorter and flatter learning curve than the control group, it must be noted that it is not the simulator but rather the mode of simulation-based training (i.e., stepwise, structured, and proficiency-based) that leads to better results [22]. Also, simulator models have been shown to benefit the junior residents more than the senior residents, as senior residents are already experienced in assisting and performing LC [23]. IOIFC seems to decrease the need for experience to demonstrate anatomy to trainees and, if implemented in a surgical residency program, can enhance a resident’s experience because of its short learning curve, better identification of extrahepatic structures, and ability to act as a tool for the attending surgeon to guide the residents. When surgeons inspect the gallbladder and surrounding structures to identify the cystic duct, the subconscious brain seeks a pattern to match the mental model of the biliary tree stored in long-term memory. During dissection of Calot’s triangle, when enough duct is visible, subconscious decisions are made concerning extrahepatic biliary anatomy [13]. With IOIFC, instead of relying on these subconscious decisions, we can accurately identify the anatomy and supervise the residents appropriately.
While IOC has been proven to be helpful in identifying biliary anatomy, the fact that an incision has to be made in order to carry out the IOC in itself poses a risk of CBD injury. There is the literature to support that an incision made during IOC can cause type D (Strasberg classification) injury of the CBD because of misinterpretation of the anatomy [24]. IOIFC can be carried out as many times as needed with no risk of radiation. It provides the resident and the teaching surgeon with an immediate situational awareness of vital structures. It can act a tool for the teaching surgeon to repeatedly assess the areas of concern during dissection. In essence, the teaching surgeon can supervise the resident with more confidence and in a more methodical fashion. The Society of American Gastrointestinal and Endoscopic Surgeons has recommended liberal use of cholangiography or other methods to image the biliary tree intraoperatively within its recent initiative for a “safe cholecystectomy program” [25]. The safe cholecystectomy taskforce identified a total of six steps to take during every cholecystectomy to reduce bile duct injuries. These additional five steps are the following: routine use of the critical view of safety (CVS), consideration of an intraoperative timeout prior to clipping and transecting any ductal structures, understanding potential aberrant anatomy, recognition of dissection in dangerous areas and avoidance of further progression, and obtaining help from another surgeon when needed.
We recommend using IOIFC on a routine basis in laparoscopic LC in which it can be used as a teaching tool as well.
There are a few challenges to the technique and limitations to this study. This is a small sample size; however, this is a proof of concept study. Currently IOIFC is still considered a novel technique and is available for use in only a limited number of hospitals. An attending surgeon has to be familiar with the technique in order to supervise the residents. Finally, the evaluation of this study was performed on still images and not on real-time surgeries.
Conclusions
IOIFC increases the visualization of Calot’s triangle structures when compared to visualization with xenon light. Medical students and surgery residents were able to identify extrahepatic biliary structures more accurately with the use of IOIFC. IOIFC may be a useful teaching tool in surgery residency programs to teach LC in the future as it can act as a communication platform for the attending surgeon to guide residents.
Abbreviations
- IOIFC:
-
Intraoperative incisionless fluorescent cholangiogram
- NIR:
-
Near infrared
- LC:
-
Laparoscopic cholecystectomy
- ICG:
-
Indocyanine green
- IOC:
-
Intraoperative cholangiogram
- CBD:
-
Common bile duct
- CHD:
-
Common hepatic duct
References
Richards MK, McAteer JP, Drake FT, Goldin AB et al (2015) A national review of the frequency of minimally invasive surgery among general surgery residents: assessment of ACGME case logs during 2 decades of general surgery resident training. JAMA Surg 150(2):169–172
Parsa CJ, Organ CH Jr, Barkan H (2000) Changing patterns of resident operative experience from 1990 to 1997. Arch Surg 135(5):570–573 discussion 3-5
Velanovich V, Morton JM, McDonald M et al (2006) Analysis of the SAGES outcomes initiative cholecystectomy registry. Surg Endosc 20(1):43–50
Davidoff AM, Pappas TN, Murray EA et al (1992) Mechanisms of major biliary injury during laparoscopic cholecystectomy. Ann Surg 215(3):196–202
Stewart L, Way LW (1995) Bile duct injuries during laparoscopic cholecystectomy. Factors that influence the results of treatment. Arch Surg 130(10):1123–1128 discussion 9
Elder S, Kunin J, Chouri H et al (1996) Safety of laparoscopic cholecystectomy on a teaching service: a prospective trial. Surg Laparosc Endosc 6(3):218–220
Arora S, Aggarwal R, Sirimanna P et al (2011) Mental practice enhances surgical technical skills: a randomized controlled study. Ann Surg 253(2):265–270
Dip F, Roy M, Lo Menzo E et al (2015) Routine use of fluorescent incisionless cholangiography as a new imaging modality during laparoscopic cholecystectomy. Surg Endosc 29(6):1621–1626
Pesce A, Piccolo G, La Greca G et al (2015) Utility of fluorescent cholangiography during laparoscopic cholecystectomy: a systematic review. World J Gastroenterol WJG 21(25):7877–7883
Dip FD, Asbun D, Rosales-Velderrain A et al (2014) Cost analysis and effectiveness comparing the routine use of intraoperative fluorescent cholangiography with fluoroscopic cholangiogram in patients undergoing laparoscopic cholecystectomy. Surg Endosc 28(6):1838–1843
Dip F, Nguyen D, Montorfano L et al (2016) Accuracy of near infrared-guided surgery in morbidly obese subjects undergoing laparoscopic cholecystectomy. Obes Surg 26(3):525–530
Cherrick GR, Stein SW, Leevy CM, Davidson CS (1960) IOC: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest 39:592–600
Blom EM, Verdaasdonk EG, Stassen LP et al (2007) Analysis of verbal communication during teaching in the operating room and the potentials for surgical training. Surg Endosc 21(9):1560–1566
Way LW, Stewart L, Gantert W et al (2003) Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg 237(4):460–469
Mirizzi PL (1950) Operative cholangiography. Revista espanola de las enfermedades del aparato digestivo y de la nutricion 9(3):306–308
Strasberg SM, Hertl M, Soper NJ (1995) An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 180(1):101–125
Aggarwal R, Darzi A (2006) Training in the operating theatre: is it safe? Thorax 61(4):278–279
Balaa F, Moloo H, Poulin EC et al (2007) Broad-based fellowships: a cornerstone of minimally invasive surgery education and dissemination. Surg Innov 14(3):205–210
Koulas SG, Tsimoyiannis J, Koutsourelakis I et al (2006) Laparoscopic cholecystectomy performed by surgical trainees. JSLS 10(4):484–487
Linn JG, Hungness ES, Clark S et al (2011) General surgery training without laparoscopic surgery fellows: the impact on residents and patients. Surgery 150(4):752–758
Fahrner R, Turina M, Neuhaus V et al (2012) Laparoscopic cholecystectomy as a teaching operation: comparison of outcome between residents and attending surgeons in 1,747 patients. Langenbecks Arch Surg 397(1):103–110
Ishizawa T, Bandai Y, Ijichi M et al (2010) Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg 97(9):1369–1377
Aggarwal R, Ward J, Balasundaram I et al (2007) Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. Ann Surg 246(5):771–779
Gamarra A, Hogle NJ, Azab B et al (2012) Assessing the value of the SimPraxis laparoscopic cholecystectomy trainer. JSLS 16(2):191–194
Pucher PH, Brunt LM, Fanelli RD, Asbun HJ, Aggarwal R (2015) SAGES expert Delphi consensus: critical factors for safe surgical practice in laparoscopic cholecystectomy. Surg Endosc 29(11):3074–3085
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Mayank Roy, Fernando Dip, David Nguyen, Conrad H Simpfendorfer, Emanuele Lo Menzo, Samuel Szomstein and Raul J. Rosenthal have no conflict of interest.
Additional information
Poster presentation at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), Boston, MA, USA March 16-19, 2016.
Rights and permissions
About this article
Cite this article
Roy, M., Dip, F., Nguyen, D. et al. Fluorescent incisionless cholangiography as a teaching tool for identification of Calot’s triangle. Surg Endosc 31, 2483–2490 (2017). https://doi.org/10.1007/s00464-016-5250-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5250-x