Abstract
Background
Recent studies have shown the potential benefit of indocyanine green fluorescence imaging (ICG-FI) in lowering the anastomotic leakage (AL) rates by changing the surgical plan. The aim of this study was to evaluate the effect of ICG-FI on the AL rates in laparoscopic low anterior resection (LAR) for rectal cancer.
Methods
From September 2014 to December 2017, data from patients who underwent laparoscopic LAR for rectal cancer were collected and analyzed. The primary endpoint was the AL rate within 30 days after surgery. The incidence of AL in patients who underwent ICG (ICG-FI group) was compared with that in patients who did not undergo ICG (non-ICG-FI group) using propensity score matching.
Results
Data from 550 patients were collected from 3 institutions. A total of 211 patients were matched in both groups by the propensity score. ICG-FI shifted the point of the proximal colon transection line toward the oral side in 12 patients (5.7%). The AL rates of Clavien–Dindo (CD) grade ≥ II and ≥ III were 10.4% (22/211) and 9.5% (20/211) in the non-ICG-FI group and 4.7% (10/211) and 2.8% (6/211) in the ICG-FI group, respectively. ICG-FI significantly reduced the AL rate of CD grade ≥ II and ≥ III (odds ratio (OR) 0.427; 95% confidence interval (CI) 0.197–0.926; p = 0.042 and OR 0.280; CI 0.110–0.711; p = 0.007, respectively). The rate of reoperation was significantly lower (OR 0.192; CI 0.042–0.889; p = 0.036) and the postoperative hospital stay significantly shorter (mean difference 2.62 days; CI 0.96–4.28; p = 0.002) in the ICG-FI group than in the non-ICG-FI group.
Conclusions
ICG-FI was associated with significantly lower odds of AL in laparoscopic LAR for rectal cancer.
Clinical trial
The study was registered with the Japanese Clinical Trials Registry as UMIN000032654.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In colorectal surgery, anastomotic leakage (AL) is one of the most critical complications, occurring in 1%–20% of patients [1,2,3,4], and can worsen not only the short-term outcomes, such as the rate of reoperation and duration of hospitalization, but also the long-term outcomes, such as the rate of local recurrence and concurrent cancer-specific survival [5]. The risk factors for AL are a low-level anastomosis, male gender and preoperative chemoradiotherapy [1]. These factors are beyond the influence of the surgeon. Regarding the surgery-related factors affecting AL, three elements have been implicated: an incomplete anastomosis [6], anastomotic tension [7], and the anastomotic vascular perfusion [8,9,10,11].
Generally, the vascular perfusion to the anastomotic region is intraoperatively assessed by the surgeon. The criteria assessed are parameters such as active bleeding from the resection margin, palpable pulsation in the mesentery and a lack of discoloration [12]. However, these measures are subjective and highly unreliable, as demonstrated by Karliczek et al. [13]. Laser Doppler flowmetry and laser fluorescence angiography have been used as reliable intraoperative methods to assess the vascular perfusion [10, 12, 14, 15]. Near-infrared (NIR) fluorescence technology provides another objective and reliable method of evaluating the perfusion of the proximal and distal margin of resection [16]. This technique can be used to perform a real-time angiography during surgery to evaluate the perfusion of the anastomosis. The recent literature shows the potential benefit of fluorescence imaging with indocyanine green (ICG) in lowering AL rates by changing the surgical plan [17,18,19,20,21,22,23,24,25,26]. To our knowledge, however, there have been no randomized controlled trials or large-scale case-matched studies on this matter. Further research is needed to validate its efficacy in reducing the AL rate.
The aim of this study was to evaluate the effect of ICG fluorescence imaging (ICG-FI) on AL rates during laparoscopic low anterior resection (LAR) for rectal cancer compared to a propensity score-matched series of laparoscopic LAR performed without ICG-FI.
Materials and methods
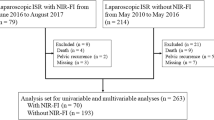
This retrospective multi-institutional study was conducted to evaluate the AL rates after laparoscopic LAR for rectal cancer at three institutions of the Yokohama Clinical Oncology Group in Japan from September 2014 to December 2017. The study protocol was approved by the Ethical Advisory Committee of Yokohama City University Medical Center and the institutional review board of each participating hospital before the study was initiated. The study was registered with the Japanese Clinical Trials Registry as UMIN000032654 (http://www.umin.ac.jp/ctr/index.htm). Patient data were collected from clinical report forms. Eligibility criteria were (1) rectal cancer located within 15 cm from the anal verge with histologically proven adenocarcinoma or signet-ring cell carcinoma and (2) having undergone laparoscopic LAR. The exclusion criteria were (1) multiple primary cancers, (2) a history of treatment for other pelvic malignancy, (3) open or robotic surgery cases and (4) emergent cases.
The primary endpoint of this study was the rate of AL within 30 days after surgery. Secondary endpoints were the operative time, blood loss, postoperative complications, reoperation within 30 days after surgery, length of hospital stays, oncological clearance and the rate of changing the surgical plan.
The sample size was determined based on the chi-squared test with a significance level of 0.05 (2-sided) and a power of 0.80. Prior published data indicated that the AL rate among controls was 13% [27]. If the AL rate for the ICG-FI group was 5%, we estimated that 200 cases needed to be enrolled in each matched group.
Surgical procedure
During laparoscopic surgery, five ports are generally inserted: a 12-mm port in the umbilical region; 5-mm ports in the upper-right, left and lower-left quadrants; and a 12-mm port in the lower-right quadrant. A 12-mm umbilical trocar is used as a camera port for a rigid scope. Vessel ligation and colon or rectum mobilization are performed laparoscopically. To avoid contamination when specimen is removed, a wound protector is attached to the umbilical region in every case. We used an Endo GIA™ Universal (Medtronic, Minnesota, USA) or an Echelon™ 60 (Ethicon Endo-Surgery, OH, USA) stapler for distal colon transection. The anastomosis was performed with a double-stapling technique (DST) using an ILS™ device (Ethicon Endo-Surgery).
ICG-FI
The laparoscopic NIR camera system was provided by Karl Storz (D-Light P; Tuttlingen, Germany) and the Stryker Corporation (1588 AIM Platform; Michigan, USA). ICG was injected intravenously just before fluorescence observation. The dose of ICG administered was 0.25 mg/kg.
First, central side lymph node dissection with high tie vascular ligation of the inferior mesenteric artery was performed. After transection of the anal-side of the rectum, the specimen was extracted through the umbilical port, which was extended to about 3–5 cm. Just before proximal bowel resection, we injected ICG intravenously after the division of the mesentery at the level of planned transection and observed the vascular perfusion using the NIR camera system. If vascular perfusion via ICG-FI was well visualized within 60 s, it was judged to be good, and anastomosis was performed with the planned transection line (Fig. 1A, B). No vascular perfusion or perfusion times > 60 s was considered to indicate poor perfusion. In such cases, the transection line of proximal bowel was changed to a site with good vascular perfusion, and anastomosis was performed with the changed transection line (Fig. 1C, D).
After transection of the anal side of the rectum, the specimen was extracted through the umbilical incision. A Just before proximal bowel resection, we injected ICG intravenously after the division of the mesentery at the level of planned transection (white arrow) and B observed the vascular perfusion using the NIR camera system. If vascular perfusion via ICG-FI was well visualized within 60 s, it was judged to be good, and anastomosis was performed with the planned transection line (white arrow). C Another case. The white arrow indicates the planned transection line. D No vascular perfusion was observed at the level of planned transection (white arrow). In this case, the transection line of proximal bowel was changed to a site with good vascular perfusion (yellow arrow), and anastomosis was performed with the changed transection line. (Color figure online)
Perioperative care
The duration of preoperative fasting was 2 h for liquids and 6–8 h for solids. During induction to anesthesia, one dose of prophylactic intravenous antibiotics (flomoxef; Shionogi, Osaka, Japan) was administered, and an additional dose was administered every 3 h during surgery. Postoperative oral liquid intake was usually authorized from the day following the surgery, and a normal diet was resumed on postoperative day 3. The intravenous catheter was removed when enteral feeding was adequate without nausea or vomiting.
Statistical analyses
Case matching was performed using the propensity score of five factors: age, sex, body mass index (BMI), tumor distance from anal verge and lateral pelvic lymph node dissection as described in the protocol. Nearest neighbor-matching without replacement within a caliper was used. According to the suggestion of Austin, the size of the caliper was set as 0.20 of the standard deviation of the logit of the estimated propensity score [28].
The data are presented as the median and variance. Statistical analyses were performed using the SPSS statistical software program (SPSS Inc., Chicago, IL, USA). Differences between categorical variables were tested using Pearson’s Chi squared test. Differences between continuous variables were tested using the Mann–Whitney U test. All p values were two-sided, and values less than 0.05 were considered statistically significant.
Results
Data from 550 patients were collected from three institutions. ICG-FI was performed in 236 cases (ICG-FI group) and not performed in 314 cases (non-ICG-FI group). A total of 211 patients each were matched to the ICG-FI and non-ICG-FI groups using the propensity score (Fig. 1). The patient and tumor characteristics of the overall cohort and matched cases are presented in Table 1. Before matching, the age was older and the tumor height from the anal verge longer in the ICG-FI group than in the non-ICG-FI group. After matching, these results became more balanced with regard to the age and the tumor height from anal verge.
The operative outcomes are summarized in Table 2. The operative time, blood loss, vessel ligation level, lateral lymph node dissection, diverting stoma, length of proximal margin, length of distal margin and number of lymph nodes harvested were similar between the two groups. The median time for perfusion fluorescence after ICG injection was 35 s (range 20–180 s). ICG-FI shifted the point of the proximal colon transection line toward the oral side in 12 patients (5.7%). The median distance from the planned transection line was 6.5 cm (range 2–45 cm). There were 2 anastomotic leaks in the 12 patients in whom a change in the surgical plan occurred based on ICG-FI. Two cases were treated with conservative antibiotics treatment (CD grade 2).
The postoperative complications are shown in Table 3. In the ICG-FI group, the complication rates of Clavien–Dindo (CD) grade ≥ II were significantly lower than in the non-ICG-FI group (p = 0.001). The AL rates of CD grade ≥ II and ≥ III were 10.4% (22/211) and 9.5% (20/211) in the non-ICG-FI group and 4.7% (10/211) and 2.8% (6/211) in the ICG-FI group, respectively. ICG-FI was associated with significantly lower odds of AL of CD grade ≥ II and ≥ III in laparoscopic LAR for rectal cancer. The odds ratio (OR) (95% confidence interval [CI]; p value) was 0.427 (0.197–0.926; p = 0.042) and 0.280 (0.110–0.711; p = 0.007) in the complication rates of CD grade ≥ II and ≥ III, respectively. The rate of reoperation was significantly lower (OR 0.192; CI 0.042–0.889; p = 0.036) and the postoperative hospital stay significantly shorter (mean difference 2.62 days; CI 0.96–4.28; p = 0.002) in the ICG-FI group than in the non-ICG-FI group.
Discussion
This multicenter, propensity score-matched cohort study showed that ICG-FI significantly reduced the rate of AL of CD grade ≥ II and ≥ III and reoperation during laparoscopic LAR for rectal cancer. Recent studies have described the potential utility of ICG-FI in reducing AL rates by changing the surgical plan [25]. Furthermore, ICG-FI was shown to be safe and feasible in colorectal surgery [25]. However, most of these previous studies were retrospective case series and cohort studies without case matching [25]. This is the first study evaluating the effect of ICG-FI on AL rates during laparoscopic LAR for rectal cancer in a large-scale propensity score-matched study.
Regarding the dose of ICG, no standard dosage has yet been established, and dosages ranging from 2.5 to 25 mg have been reported [17,18,19,20,21,22,23,24, 26, 29,30,31,32,33]. In the present study, 0.25 mg/kg (12.5 mg at 50 kg) was used, and fluorescence observation was possible with good contrast in all cases.
Previous studies have been published regarding shifting the planned transection line [17,18,19,20,21,22,23,24,25,26]. Jafari et al. reported that the resection margin was changed in 6.5% of cases in the PILLAR II trial [19], and Wada et al. reported that it was changed in 16% of cases [24]. A recent meta-analysis of five articles on left-sided colorectal surgery reported that the planned anastomotic level was changed in 7.4% of cases (41 of 555 patients in the ICG-FI group) [25]. In the present study, the transection line had to be shifted more proximally to an adequate fluorescent portion in 12 cases (5.7%). We considered additional transection criteria of no vascular perfusion and perfusion times > 60 s via ICG-FI in our study. No standard additional excision criteria by ICG-FI have yet been established. According to previous reports, the perfusion time to fluorescence after ICG injection at the planned transection line is reported to range from 29 to 44 s [17, 24, 34]. In the present study, the perfusion time to fluorescence after ICG injection was 35 s. However, in cases of perfusion times > 60 s, the transection line of the proximal bowel was shifted to a site with good vascular perfusion, and anastomosis was performed with the new transection line, so whether or not AL would definitely have occurred if anastomosis had been performed without changing the transection line of the proximal bowel is unknown.
Furthermore, the quantitative definition of adequate or inadequate pre-anastomosis perfusion is poorly defined, as none of the laparoscopic ICG-FI systems available at present are able to quantify the fluorescent signal. Automated analysis software programs have been recently developed for a more precise and objective quantification. Although some experimental studies have suggested that fluorescence quantification can effectively detect the ischemic zones in animal models, the cutoff value necessary to judge ischemia was not guided by quantification in these studies [35, 36].
When measuring the magnitude of the therapeutic effect, the most practical measure is “number needed to treat (NNT)” [37]. In this study, since NNT is 1 / 0.06 = 16.7, it means that we prevent anastomotic leak (AL) of one patient by using ICG in 16.7 patients. The cost for ICG dye is 6$ per patient in Japan. In contrast, AL represents over 20.000$ per patient in the USA [32]. AL also increases the mortality risk and the length of hospital stay [3]. Moreover, AL has been associated with reduced long-term cancer-specific survival and a greater risk of systemic and local recurrence [5, 38]. We think that ICG-FI has enough cost effectiveness and therapeutic effect.
There are several limitations associated with this study. First, although the perfusion of the oral-side bowel of the anastomotic site was assessed, the anal-side rectum was not assessed. Second, the vascular perfusion was observed using two kinds of laparoscopic NIR observation systems. Third, this was a retrospective study and not randomized or controlled, although selection bias was reduced by propensity score matching. The results of this study do not provide definitive proof of the benefits of ICG-FI on AL reduction. Thus, further multi-institution, phase III, randomized studies are needed to confirm whether or not ICG-FI can reduce the AL rate in laparoscopic rectal surgery. However, the authors believe that the findings of the present study will provide a firm foundation for future studies.
Conclusion
In conclusion, ICG-FI was associated with significantly lower odds of AL of CD grade ≥ II and ≥ III, lower rate of reoperation and reduced hospital stay in laparoscopic LAR for rectal cancer according to our multicenter, propensity score-matched cohort study. A phase III, randomized controlled study is planned to further evaluate the true clinical significance of ICG-FI on AL reduction.
References
Gendall KA, Raniga S, Kennedy R, Frizelle FA (2007) The impact of obesity on outcome after major colorectal surgery. Dis Colon Rectum 50:2223–2237
Group ESoCc (2017) The relationship between method of anastomosis and anastomotic failure after right hemicolectomy and ileo-caecal resection: an international snapshot audit. Colorectal Dis 19:e296–e311
McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC (2015) Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 102:462–479
Phitayakorn R, Delaney CP, Reynolds HL, Champagne BJ, Heriot AG, Neary P, Senagore AJ, International Anastomotic Leak Study Group (2008) Standardized algorithms for management of anastomotic leaks and related abdominal and pelvic abscesses after colorectal surgery. World J Surg 32:1147–1156
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P (2011) Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 253:890–899
Ito M, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y, Saito N (2008) Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis 23:703–707
Cui Y, Chen H (2003) The effect of tension on esophagogastric anastomotic wound healing in rats. J Cardiovasc Surg 44:775–778
Attard JA, Raval MJ, Martin GR, Kolb J, Afrouzian M, Buie WD, Sigalet DL (2005) The effects of systemic hypoxia on colon anastomotic healing: an animal model. Dis Colon Rectum 48:1460–1470
Shikata J, Shida T (1986) Effects of tension on local blood flow in experimental intestinal anastomoses. J Surg Res 40:105–111
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V (2000) Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum 43:76–82
Wilker D, Sklarek J, Waldner H, Izbicki JR, Siebeck M (1988) [Early phase of healing of anastomoses with special reference to peritonitis and ischemia]. Langenbecks Arch Chir 373:217–221
Kudszus S, Roesel C, Schachtrupp A, Hoer JJ (2010) Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbeck’s Arch Surg 395:1025–1030
Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM (2009) Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 24:569–576
Hallbook O, Johansson K, Sjodahl R (1996) Laser Doppler blood flow measurement in rectal resection for carcinoma–comparison between the straight and colonic J pouch reconstruction. Br J Surg 83:389–392
Seike K, Koda K, Saito N, Oda K, Kosugi C, Shimizu K, Miyazaki M (2007) Laser Doppler assessment of the influence of division at the root of the inferior mesenteric artery on anastomotic blood flow in rectosigmoid cancer surgery. Int J Colorectal Dis 22:689–697
Toens C, Krones CJ, Blum U, Fernandez V, Grommes J, Hoelzl F, Stumpf M, Klinge U, Schumpelick V (2006) Validation of IC-VIEW fluorescence videography in a rabbit model of mesenteric ischaemia and reperfusion. Int J Colorectal Dis 21:332–338
Ris F, Liot E, Buchs NC, Kraus R, Ismael G, Belfontali V, Douissard J, Cunningham C, Lindsey I, Guy R, Jones O, George B, Morel P, Mortensen NJ, Hompes R, Cahill RA, Near-Infrared Anastomotic Perfusion Assessment Network V (2018) Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br J Surg 105:1359–1367
Grone J, Koch D, Kreis ME (2015) Impact of intraoperative microperfusion assessment with Pinpoint Perfusion Imaging on surgical management of laparoscopic low rectal and anorectal anastomoses. Colorectal Dis 17(Suppl 3):22–28
Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA, Lee SW, Senagore AJ, Phelan MJ, Stamos MJ (2015) Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg 220:82–92
Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E (2017) Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc 31:1836–1840
Hellan M, Spinoglio G, Pigazzi A, Lagares-Garcia JA (2014) The influence of fluorescence imaging on the location of bowel transection during robotic left-sided colorectal surgery. Surg Endosc 28:1695–1702
Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ, Pigazzi A (2013) The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 27:3003–3008
Kawada K, Hasegawa S, Wada T, Takahashi R, Hisamori S, Hida K, Sakai Y (2017) Evaluation of intestinal perfusion by ICG fluorescence imaging in laparoscopic colorectal surgery with DST anastomosis. Surg Endosc 31:1061–1069
Wada T, Kawada K, Takahashi R, Yoshitomi M, Hida K, Hasegawa S, Sakai Y (2017) ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 31:4184–4193
Blanco-Colino R, Espin-Basany E (2018) Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol 22:15–23
Guraieb-Trueba M, Frering T, Atallah S (2016) Combined endoscopic and laparoscopic real-time intra-operative evaluation of bowel perfusion using fluorescence angiography. Tech Coloproctol 20:883–884
Shiomi A, Ito M, Maeda K, Kinugasa Y, Ota M, Yamaue H, Shiozawa M, Horie H, Kuriu Y, Saito N (2015) Effects of a diverting stoma on symptomatic anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis of 1,014 consecutive patients. J Am Coll Surg 220:186–194
Austin PC (2011) Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 10:150–161
Protyniak B, Dinallo AM, Boyan WP Jr, Dressner RM, Arvanitis ML (2015) Intraoperative indocyanine green fluorescence angiography—an objective evaluation of anastomotic perfusion in colorectal surgery. Am Surg 81:580–584
Nishigori N, Koyama F, Nakagawa T, Nakamura S, Ueda T, Inoue T, Kawasaki K, Obara S, Nakamoto T, Fujii H, Nakajima Y (2016) Visualization of lymph/blood flow in laparoscopic colorectal cancer surgery by ICG fluorescence imaging (Lap-IGFI). Ann Surg Oncol 23(Suppl 2):S266–S274
Sherwinter DA, Gallagher J, Donkar T (2013) Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: a feasibility study. Colorect Dis 15:91–96
Kim JC, Lee JL, Yoon YS, Alotaibi AM, Kim J (2016) Utility of indocyanine-green fluorescent imaging during robot-assisted sphincter-saving surgery on rectal cancer patients. Int J Med Robot Comput Assist Surg MRCAS 12:710–717
Foppa C, Denoya PI, Tarta C, Bergamaschi R (2014) Indocyanine green fluorescent dye during bowel surgery: are the blood supply “guessing days” over? Tech Coloproctol 18:753–758
Kim JC, Lee JL, Park SH (2017) Interpretative guidelines and possible indications for indocyanine green fluorescence imaging in robot-assisted sphincter-saving operations. Dis Colon Rectum 60:376–384
Diana M, Agnus V, Halvax P, Liu YY, Dallemagne B, Schlagowski AI, Geny B, Diemunsch P, Lindner V, Marescaux J (2015) Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg 102:e169–e176
Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V, Soler L, Barry B, Namer IJ, Demartines N, Charles AL, Geny B, Marescaux J (2014) Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 259:700–707
Herbert RD (2000) How to estimate treatment effects from reports of clinical trials. II: Dichotomous outcomes. Aust J Physiother 46:309–313
Katoh H, Yamashita K, Wang G, Sato T, Nakamura T, Watanabe M (2011) Anastomotic leakage contributes to the risk for systemic recurrence in stage II colorectal cancer. J Gastrointest Surg 15:120–129
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jun Watanabe, Atsushi Ishibe, Yusuke Suwa, Hirokazu Suwa, Mitsuyoshi Ota, Chikara Kunisaki, and Itaru Endo have no conflicts of interest or financial ties to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Watanabe, J., Ishibe, A., Suwa, Y. et al. Indocyanine green fluorescence imaging to reduce the risk of anastomotic leakage in laparoscopic low anterior resection for rectal cancer: a propensity score-matched cohort study. Surg Endosc 34, 202–208 (2020). https://doi.org/10.1007/s00464-019-06751-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06751-9