Abstract
Background
Indocyanine green (ICG) fluorescence imaging has been proven to be an effective tool to assess anastomotic perfusion. The aim of this systematic review and meta-analysis was to evaluate its efficacy in reducing the anastomotic leakage (AL) rate after colorectal surgery.
Methods
PubMed, Scopus, WOS, Google Scholar and Cochrane Library were searched up to January 2017 for studies comparing fluorescence imaging with standard care. ClinicalTrials.gov register was searched for ongoing trials. The primary outcome measure was AL rate with at least 1 month of follow-up. ROBINS-I tool was used for quality assessment. A meta-analysis with random-effects model was performed to calculate odds ratios (ORs) from the original data.
Results
One thousand three hundred and two patients from 5 non-randomized studies were included. Fluorescence imaging significantly reduced the AL rate in patients undergoing surgery for colorectal cancer (OR 0.34; CI 0.16–0.74; p = 0.006). Low AL rates were shown in rectal cancer surgery (ICG 1.1% vs non-ICG 6.1%; p = 0.02). There was no significant decrease in the AL rate when colorectal procedures for benign and malignant disease were combined. To date, there are no published randomized control trials (RCTs) on this subject, though 3 ongoing RCTs were identified.
Conclusions
ICG fluorescence imaging seems to reduce AL rates following colorectal surgery for cancer. However, the inherent bias of the non-randomized studies included, and their differences in AL definition and diagnosis could have influenced results. Large well-designed RCTs are needed to provide evidence for its routine use in colorectal surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anastomotic leakage (AL) is one of the most feared complications following colorectal surgery. It has been associated with increased postoperative morbidity and mortality rates [1, 2]. Due to the lack of a standardized definition for AL, there is still variability in studies reporting this condition [3]. The AL rate in colorectal surgery varies from 1 to 19% depending on the anatomic location of the anastomosis: ileocolic (1–8%); colocolic (2–3%); ileorectal (3–7%); colorectal or coloanal (5–19%) [3,4,5]. In the Rectal Cancer Project of the Spanish Society of Surgeons, the rate of AL for rectal cancer surgery was 10% [6]. The reduction in AL rates by improving its prevention, diagnosis and management continues to be a challenge nowadays. Finding new techniques to reduce AL has been highlighted as a research priority by the Association of Coloproctology of Great Britain and Ireland (ACPGBI) [7].
Multiple conditions have been associated with a greater risk of AL: male sex, age, comorbidities, high American Society of Anaesthesiologists (ASA) score, malnutrition, obesity, smoking, immunosuppression, alcohol abuse, preoperative chemotherapy and radiotherapy, advanced tumor stage, diverticulitis, low anastomoses, prolonged operative time, inadequate anastomotic blood supply, blood loss or perioperative blood transfusion and intraoperative septic conditions [3, 8,9,10]. Adequate perfusion of the anastomosis is essential for optimal healing and AL prevention [11,12,13]. Consequently, detection of bowel ischemia intraoperatively may reduce the risk of AL.
Different intraoperative techniques have been proposed to assess anastomotic integrity and bowel viability in colorectal surgery [14, 15]. Traditionally, usual anastomotic assessment includes direct visualization of the anastomosis, integrity of the doughnuts and the air leak test. Subjective signs indicating optimal anastomotic perfusion are evaluated, including serosal-mucosal color and/or bleeding at the cut edge of the bowel and/or palpable pulsations of the mesenteric arteries [10, 16]. However, a study by Karliczek et al. showed that the risk of AL is underestimated and the accuracy of surgeons’ prediction of AL risk low [17]. The authors indicated a need for a reliable predictive test that could be used intraoperatively.
Fluorescence imaging with indocyanine green (ICG) has been increasingly considered a potential intraoperative tool that could be used in routine practice to ensure adequate perfusion at the time of anastomosis formation. It allows surgeons to visualize bowel microperfusion in real time, being fast and easy to perform. Recent literature shows the potential benefit of fluorescence imaging with ICG in lowering AL rates by changing the surgical plan [18,19,20,21,22,23,24]. Moreover, it has already been proven to be safe and feasible in colorectal surgery [25,26,27,28,29]. However, further research is needed to validate its efficacy in reducing the AL rate [1].
The aim of this study was to systematically review the available literature reporting data on AL rates using ICG fluorescence imaging in contrast to standard surgical care in colorectal surgery.
Materials and methods
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [30].
Eligibility criteria
Studies that compared intraoperative use of ICG fluorescence imaging with standard care for the assessment of anastomotic perfusion or viability were eligible for inclusion. Patients of any age undergoing colon or rectal resection with anastomosis were included, regardless of operative approach, urgency of surgery or surgical indications. The primary outcome measure was the AL rate with at least 30 days of follow-up. Randomized controlled trials (RCTs), cohort studies, case–control studies and quasi-randomized studies were searched. Case reports were excluded. Studies using ICG fluorescence for purposes different from perfusion assessment were excluded, as well as those studies based on animal models.
Search strategy
An electronic search was carried out using PubMed, Scopus, Web of Science, Google Scholar databases and the Cochrane Library. The reference list of identified systematic reviews and review articles was hand-searched for additional references. Furthermore, the register ClinicalTrials.gov was searched to identify ongoing trials.
A combination of medical subject heading (MeSH) terms and keywords was searched: “indocyanine green,” “ICG,” “coloring agents,” “fluorescence,” “fluorescein angiography,” “fluorescent dyes,” “anastomotic leak,” “anastomotic leakage,” “anastomotic perfusion,” “anastomosis, surgical,” “bowel perfusion,” “blood supply,” “perfusion assessment,” “colorectal surgery,” “colon surgery,” “rectal surgery,” “colorectal resection,” “bowel resection” using the Boolean operator “OR” for each concept. Each concept was combined with “AND.” The complete search strategy is shown in the appendix. No search limits were applied, and all languages were included. Databases were search from their inception to January 24, 2017.
Study selection and data extraction
Studies were screened by title and abstract; then, the full text was obtained for those studies identified as potentially eligible.
From each study, data were extracted on: study characteristics and year of publication, patient inclusion period, sample size, surgical indication, surgical management (operative approach, procedure and whether a change in surgical plan was made), fluorescence imaging system used and AL rate.
Authors were contacted to provide additional information that was not available in the original studies. Two authors could not be contacted or were not able to provide the requested data [31, 32].
Risk of bias assessment
The quality of the included studies was evaluated using the ROBINS-I risk of bias assessment tool for non-randomized studies of interventions [33]. Seven domains were covered including confounding and selection of participants for the study, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes and selection of the reported result.
Statistical analysis
The odds ratios (ORs) were calculated from the original data and were assessed as the summary statistic. Values were reported with 95% confidence intervals (CIs). As there was a substantial level of heterogeneity expected across the included studies, Mantel–Haenszel (M–H) method and random-effects models were employed for quantitative statistical analysis of dichotomous variables. Also, statistical heterogeneity was assessed using I 2 test and visual inspection of forest plots. Statistical analyses were carried out using Review Manager (RevMan) software version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Study selection
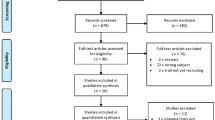
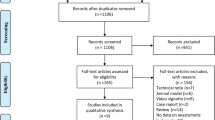
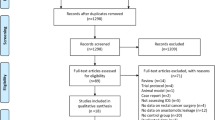
Results of literature search and selection process of eligible studies are presented in the PRISMA flow diagram (Fig. 1). From the 518 studies identified by the search, full text of 72 studies was evaluated. Finally, 5 non-randomized studies were included in the analysis [31, 32, 34,35,36]. To date, there are no published RCTs on this subject. On ClinicalTrial.gov search, 6 ongoing trials were identified, 3 of them were randomized studies with a control group [37,38,39].
Study characteristics
Characteristics of the analyzed studies are reported in Table 1 and differences in AL definitions in Table 2. The 5 studies included a total of 1302 adult patients. The sample size in the studies varied from 38 to 436 patients. Most studies included elective rectal surgery for rectal cancer. Follow-up ranged from 1 month to more than 6 months.
Four of the included studies were retrospective [31, 32, 34, 36], and all of them were single-center studies. Historical controls were used in Kudszus et al., Kin et al. and Boni et al. studies [31, 32, 36]. Most studies included elective rectal surgery. Jafari et al. [34] and Kim et al. [35] included patients undergoing robotic rectal resections. The commonest indication was cancer.
Due to the lack of published RCTs, the 5 studies included for analysis were non-randomized studies of interventions. All the studies were at moderate risk of bias when they were evaluated according to the tool for assessing risk of bias in non-randomized studies of interventions (ROBINS-I) [33]. Items assessed for each study are found in Table 3.
Outcome assessment
The meta-analysis included 555 patients in the ICG group and 747 patients in the control group. Both groups included patients who had colon or rectal surgery for benign or malignant indications. The overall AL rate was 5.4%. There was no significant difference in AL rate with or without the use of ICG fluorescence (OR 0.51; 95% CI 0.23–1.13; p = 0.10) (Fig. 2). The I 2 value was 35%, which shows there was moderate heterogeneity.
Data from 956 cancer patients were obtained from 4 studies [31, 34,35,36]. AL risk was significantly reduced when using ICG fluorescence imaging in patients undergoing surgery for colon or rectal cancer (OR 0.34; CI 0.16–0.74; p = 0.006; I 2 = 0%) (Fig. 3).
Rectal cancer surgery was assessed in 554 patients in three studies [34,35,36]. ICG perfusion assessment in rectal surgery resulted in an 81% reduction in the odds of AL (OR 0.19; 95% CI 0.05–0.75; p = 0.02; I 2 = 0%) (Fig. 4), showing a lower AL rate in comparison with standard care (1.1 vs 6.1%, respectively).
A change in the planned anastomotic level was made in 41 of the 555 cases in the ICG group (7.4%), due to hypoperfusion seen with ICG. Moreover, Kim et al. [35] reported 13 cases out of 123 in the ICG group (10.6%), and Kudszus et al. [31] reported 5 cases out of 201 (2.5%) in which further exploration with ICG after anastomosis formation helped to identify adequate perfusion despite clinical impression of malperfusion. None of those patients underwent additional resection or reanastomosis.
Ongoing trials
Three ongoing RCTs were found on ClinicalTrial.gov register. Details of the identified studies are shown in Table 4.
AL rate is the primary outcome measure in the 3 studies, 2 of them with 30 days of follow-up [37, 38] and 1 with 2 months of follow-up [39]. One of the RCTs has included low anterior resections for rectal cancer [39], another is evaluating ICG use during rectal or left colectomies (benign and malignant disease) [38], and the third study includes robotic colorectal surgery for cancer, inflammatory bowel disease or diverticular disease [37].
Discussion
This systematic review and meta-analysis shows that intraoperative use of ICG fluorescence imaging is a potential tool to reduce the AL risk following colorectal surgery for cancer. However, the inherent bias of the non-randomized studies included should be taken into consideration when interpreting these findings.
Morbidity, mortality and costs generated by this postoperative complication may be reduced with a decrease in AL rate. The initial burden of a near-infrared (NIR) unit is 70.000€, and then, the cost for ICG dye is 13€ per patient [35]. In contrast, AL represents 1.6 to 5 million euros of the annual direct healthcare costs in the UK and over 22.000€ per patient in the USA [3]. AL also increases the mortality risk (from 1.9% without AL to 15.9% with AL) and the length of stay (from 7 days without AL to 23 days with AL) [9]. In colorectal cancer surgery, AL has been associated with reduced long-term cancer-specific survival and a greater risk of systemic and local recurrence [40, 41]. However, this association remains unclear when referring to rectal surgery [42].
Several studies have assessed the use of ICG fluorescence in colorectal surgery, but most of them are case series with a small sample size. Fluorescence imaging has been described in surgical procedures for benign and malignant indications and different operative approaches [20,21,22,23, 43] including robotic colorectal surgery [18, 24], transanal rectal surgery [25] and minimally invasive surgery [44].
ICG fluorescence seems to help in identifying the need for a change in the surgical plan, extending resection margins or requiring revision and reanastomosis. A change in the planned anastomotic level was decided in 7.4% (41 over 555 patients in the ICG group). Usually, a change is decided on if bowel hypoperfusion is detected by fluorescence, even if the bowel had seemed well-perfused on visual examination. In contrast, ICG fluorescence can also help in confirming adequate perfusion in those cases where there is a clinical impression of malperfusion, and therefore indicate that the resection margins do not need to be extended further.
In the present meta-analysis, the study by Kin et al. [32] was the only one that reported no reduction in the AL rate when using intraoperative fluorescence. However, this study has some limitations that could have influenced results. Only proximal bowel perfusion was assessed, and therefore, rectal stump perfusion was not confirmed. In contrast to the other studies, which only included patients undergoing surgery for cancer, this study also included patients with inflammatory bowel disease and diverticular disease.
The results of this study must be taken with caution as it has several limitations that could have influenced them. One of the limitations of this meta-analysis is the lack of randomization in the studies included. Moreover, when the quality of the studies was assessed with ROBINS-I tool [33], all of them showed moderate risk of bias. In addition, 4 studies were retrospective [31, 32, 34, 36] and results from ICG fluorescence group were compared with a control group from a different time period. Also, the risk of publication bias in the studies reporting the effect of fluorescence imaging on AL rates should be considered.
Other limitations including variability in the definition of AL as well as differences in the length of follow-up, use of neoadjuvant therapy, surgical technique and application of ICG should also be considered. In all the included studies, ICG fluorescence was used before anastomosis formation. However, differences in its use could have influenced the rates of change of surgical plan. In the studies of Kudszus, Kin and Boni et al., anastomotic perfusion with ICG was assessed after resection [31, 32, 36]. In the study of Jafari et al. [34], the optimal transection point was decided under white light; then after ICG injection, the transection point was revised. In contrast to the other studies, Kim et al. [35] checked the perfusion status of the left colon and rectum with ICG before the division of the distal rectum. Then, the transection point was decided on depending on the perfusion assessment. In some cases, ICG fluorescence imaging was also used after anastomosis formation [35, 36].
Furthermore, the quantitative definition of adequate or inadequate preanastomotic perfusion is not well defined, mainly because most of the actual imaging systems lack the ability to quantify tissue perfusion. However, some experimental studies assessing fluorescence quantification in animal models have been published [45]. Additionally, Sherwinter et al. [25] used a fluorescence score in their study based on the sequence of fluorescence uptake and time of maximal excitation.
Conclusions
Despite the limitations of the available studies, this systematic review and meta-analysis show that ICG fluorescence imaging is a promising tool that could be of help in clinical practice. It may reduce the AL rate in patients having colorectal resection for cancer. Moreover, ICG perfusion assessment in rectal anastomosis has shown a lower AL rate in comparison with standard care. However, its efficacy in reducing AL risk is uncertain as the presented data come from poor quality studies. To date, there is no published RCT on the subject, though 3 ongoing RCTs were identified on ClinicalTrials.gov register. There is a need of larger, well-designed RCTs to assess whether the AL rate can be reduced by incorporating ICG fluorescence imaging in routine colorectal surgery for benign or malignant disease.
References
Vallance A, Wexner S, Berho M et al (2017) A collaborative review of the current concepts and challenges of anastomotic leaks in colorectal surgery. Colorectal Dis 19(1):O1–O12. https://doi.org/10.1111/codi.13534
Alves A, Panis Y, Trancart D, Regimbeau JM, Pocard M, Valleur P (2002) Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World J Surg 26(4):499–502. https://doi.org/10.1007/s00268-001-0256-4
McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC (2015) Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 102(5):462–479. https://doi.org/10.1002/bjs.9697
Phitayakorn R, Delaney CP, Reynolds HL et al (2008) Standardized algorithms for management of anastomotic leaks and related abdominal and pelvic abscesses after colorectal surgery. World J Surg 32(6):1147–1156. https://doi.org/10.1007/s00268-008-9468-1
European Society of Coloproctology collaborating group (2017) The relationship between method of anastomosis and anastomotic failure after right hemicolectomy and ileo-caecal resection: an international snapshot audit. Colorectal Dis 38(1):42–49. https://doi.org/10.1111/codi.13646
Ortiz H, Biondo S, Codina A et al (2016) 001. Cir Esp 94(4):213–220. https://doi.org/10.1016/j.ciresp.2015.11.008
Tiernan J, Cook A, Geh I et al (2014) Use of a modified Delphi approach to develop research priorities for the association of coloproctology of Great Britain and Ireland. Colorectal Dis 16(12):965–970. https://doi.org/10.1111/codi.12790
Kingham TP, Pachter HL (2009) Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 208(2):269–278. https://doi.org/10.1016/j.jamcollsurg.2008.10.015
Frasson M, Flor-Lorente B, Ramos Rodríguez JL et al (2015) Risk factors for anastomotic leak after colon resection for cancer: multivariate analysis and nomogram from a multicentric, prospective, national study with 3193 patients. Ann Surg 262(2):321–330. https://doi.org/10.1097/SLA.0000000000000973
Chadi SA, Fingerhut A, Berho M et al (2016) Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest Surg 20(12):2035–2051. https://doi.org/10.1007/s11605-016-3255-3
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V (2000) Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum 43(1):76–82
Rutegård M (2015) Anastomotic leakage in rectal cancer surgery: the role of blood perfusion. World J Gastrointest Surg 7(11):289–292. https://doi.org/10.4240/wjgs.v7.i11.289
Sparreboom CL, Wu ZQ, Ji JF, Lange JF (2016) Integrated approach to colorectal anastomotic leakage: communication, infection and healing disturbances. World J Gastroenterol 22(32):7226–7235. https://doi.org/10.3748/wjg.v22.i32.7226
Nachiappan S, Askari A, Currie A, Kennedy RH, Faiz O (2014) Intraoperative assessment of colorectal anastomotic integrity: a systematic review. Surg Endosc 28(9):2513–2530. https://doi.org/10.1007/s00464-014-3520-z
Karliczek A, Benaron DA, Baas PC et al (2008) Intraoperative assessment of microperfusion with visible light spectroscopy in esophageal and colorectal anastomoses. Eur Surg Res 41(3):303–311. https://doi.org/10.1159/000155880
Hirst NA, Tiernan JP, Millner PA, Jayne DG (2014) Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Colorectal Dis 16(2):95–109. https://doi.org/10.1111/codi.12411
Karliczek A, Harlaar N, Zeebregts C, Wiggers T, Baas P, van Dam G (2009) Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 24(5):569–576. https://doi.org/10.1007/s00384-009-0658-6
Bae SU, Min BS, Kim NK (2015) Robotic low ligation of the inferior mesenteric artery for rectal cancer using the firefly technique. Yonsei Med J 56(4):1028–1035. https://doi.org/10.3349/ymj.2015.56.4.1028
Boni L, David G, Dionigi G, Rausei S, Cassinotti E, Fingerhut A (2016) Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc 30(7):2736–2742. https://doi.org/10.1007/s00464-015-4540-z
Foppa C, Denoya PI, Tarta C, Bergamaschi R (2014) Indocyanine green fluorescent dye during bowel surgery: are the blood supply “guessing days” over? Tech Coloproctol 18(8):753–758. https://doi.org/10.1007/s10151-014-1130-3
Gröne J, Koch D, Kreis ME (2015) Impact of intraoperative microperfusion assessment with Pinpoint Perfusion Imaging on surgical management of laparoscopic low rectal and anorectal anastomoses. Colorectal Dis 17(3, SI):22–28. https://doi.org/10.1111/codi.13031
Nishigori N, Koyama F, Nakagawa T et al (2016) Visualization of lymph/blood flow in laparoscopic colorectal cancer surgery by ICG fluorescence imaging (Lap-IGFI). Ann Surg Oncol 23:266–274. https://doi.org/10.1245/s10434-015-4509-0
Protyniak B, Dinallo AM, Boyan WP, Dressner RM, Arvanitis ML (2015) Intraoperative indocyanine green fluorescence angiography—an objective evaluation of anastomotic perfusion in colorectal surgery. Am Surg 81(6):580–584
Hellan M, Spinoglio G, Pigazzi A, Lagares-Garcia JA (2014) The influence of fluorescence imaging on the location of bowel transection during robotic left-sided colorectal surgery. Surg Endosc 28(5):1695–1702. https://doi.org/10.1007/s00464-013-3377-6
Sherwinter DA, Gallagher J, Donkar T (2013) Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: a feasibility study. Colorectal Dis 15(1):91–96. https://doi.org/10.1111/j.1463-1318.2012.03101.x
Jafari MD, Wexner SD, Martz JE et al (2015) Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg 220(1):82–92. https://doi.org/10.1016/j.jamcollsurg.2014.09.015
Ris F, Hompes R, Lindsey I, Cunningham C, Mortensen NJ, Cahill RA (2014) Near infra-red laparoscopic assessment of the adequacy of blood perfusion of intestinal anastomosis—a video vignette. Colorectal Dis 16(8):646–647. https://doi.org/10.1111/codi.12593
Guraieb-Trueba M, Frering T, Atallah S (2016) Combined endoscopic and laparoscopic real-time intra-operative evaluation of bowel perfusion using fluorescence angiography. Tech Coloproctol 20(12):883–884
Keller DS, Joshi HM, Rodriguez-Justo M, Walsh D, Coffey JC, Chand M (2017) Using fluorescence lymphangiography to define the ileocolic mesentery: proof of concept for the watershed area using real-time imaging. Tech Coloproctol 21(9):757–760. https://doi.org/10.1007/s10151-017-1677-x
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8(5):336–341. https://doi.org/10.1016/j.ijsu.2010.02.007
Kudszus S, Roesel C, Schachtrupp A, Höer JJ (2010) Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbeck’s Arch Surg 395(8):1025–1030. https://doi.org/10.1007/s00423-010-0699-x
Kin C, Vo H, Welton L, Welton M (2015) Equivocal effect of intraoperative fluorescence angiography on colorectal anastomotic leaks. Dis Colon Rectum 58(6):582–587. https://doi.org/10.1097/DCR.0000000000000320
Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J 355:i4919. https://doi.org/10.1136/bmj.i4919
Jafari MD, Lee KH, Halabi WJ et al (2013) The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 27(8):3003–3008. https://doi.org/10.1007/s00464-013-2832-8
Kim JC, Lee JL, Yoon YS, Alotaibi AM, Kim J (2016) Utility of indocyanine-green fluorescent imaging during robot-assisted sphincter-saving surgery on rectal cancer patients. Int J Med Robot Comput Assist Surg 12:710–717. https://doi.org/10.1002/rcs.1710
Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E (2017) Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc 31(4):1836–1840. https://doi.org/10.1007/s00464-016-5181-6
ClinicalTrials.gov (2015) The role of indocyanine green (ICG) fluorescence imaging on anastomotic leak in robotic colorectal surgery. https://clinicaltrials.gov/ct2/show/NCT02598414. Accessed 24 Jan 2017
ClinicalTrials.gov (2017) Evaluation of intestinal vascolarization with indocianine green angiography during rectal resection or left colectomy. https://clinicaltrials.gov/ct2/show/NCT02662946. Accessed 24 Jan 2017
ClinicalTrials.gov (2017) A study assessing perfusion outcomes with PINPOINT® near infrared fluorescence imaging in low anterior resection (PILLAR III). https://clinicaltrials.gov/ct2/show/NCT02205307. Accessed 24 Jan 2017
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P (2011) Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak systematic review and meta-analysis. Ann Surg 253(5):890–899. https://doi.org/10.1097/SLA.0b013e3182128929
Katoh H, Yamashita K, Wang G, Sato T, Nakamura T, Watanabe M (2011) Anastomotic leakage contributes to the risk for systemic recurrence in stage II colorectal cancer. J Gastrointest Surg 15(1):120–129. https://doi.org/10.1007/s11605-010-1379-4
Espín E, Ciga MA, Pera M, Ortiz H (2015) Oncological outcome following anastomotic leak in rectal surgery. Br J Surg 102(4):416–422. https://doi.org/10.1002/bjs.9748
Kawada K, Hasegawa S, Wada T et al (2017) Evaluation of intestinal perfusion by ICG fluorescence imaging in laparoscopic colorectal surgery with DST anastomosis. Surg Endosc 31(3):1061–1069. https://doi.org/10.1007/s00464-016-5064-x
Ris F, Hompes R, Cunningham C et al (2014) Near-infrared (NIR) perfusion angiography in minimally invasive colorectal surgery. Surg Endosc 28(7):2221–2226. https://doi.org/10.1007/s00464-014-3432-y
Diana M, Agnus V, Halvax P et al (2015) Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg 102(2):e169–e176. https://doi.org/10.1002/bjs.9725
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval is not needed as this study corresponds to a meta-analysis of studies already published.
Informed consent
Informed consent is not needed as this study corresponds to a meta-analysis of studies already published.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blanco-Colino, R., Espin-Basany, E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol 22, 15–23 (2018). https://doi.org/10.1007/s10151-017-1731-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-017-1731-8