Abstract
Background
In clinical practice, it is not unusual to treat oncologic patients whose tumor markers are within normal range, even with advanced cancer. The Controlling Nutritional Status (CONUT) score could provide a useful nutritional and immunological prognostic biomarker for cancer patients. In this study, we assessed the prognostic value of the CONUT score for patients with gastric cancer, including a subgroup analysis with stratification based on serum carcinoembryonic antigen (CEA) level.
Methods
We retrospectively reviewed the medical records of 368 consecutive patients who underwent curative laparoscopy-assisted gastrectomy. The prognostic value of the CONUT score was compared between patients with a low (≤ 2) and high (≥ 3) score, with propensity score matching (PSM) used to control for biasing covariates (Depth of tumor, Lymph node metastasis, pathological TNM (pTNM) stage).
Results
Overall survival (OS) among all patients was independently predicted by the tumor stage (hazard ratio (HR): 2.231, p = 0.001), the CONUT score (HR: 2.254, p = 0.001), and serum CEA level (HR: 1.821, p = 0.025). Among patients with a normal preoperative serum CEA level, tumor stage (HR: 2.350, p = 0.007), and the CONUT score (HR: 1.990, p = 0.028) were independent prognostic factors of OS. In the high serum CEA level group, tumor size (HR: 2.930, p = 0.015) and the CONUT score (HR: 3.707, p = 0.004) were independent prognostic factors of OS.
Conclusions
It is advantageous to use both CEA level and the CONUT score to assess the prognosis of patients with gastric cancer, which reflect both tumor-related factors and host-related factors, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastrectomy is the mainstay curative treatment for gastric cancer, although surgical outcomes remain poor due to the aggressive biological behavior of these tumors [1]. To precisely individualize treatment, and thus, improve the survival of patients with gastric cancer, it would be useful to have a comprehensive set of perioperative biomarkers that are predictive of postoperative survival [2, 3]. Biological parameters for evaluating immunonutritional status or screening tools for predicting the prognosis of cancer patients, such as the Glasgow prognostic score, the nutritional index, and the geriatric nutritional risk index, are limited in their clinical application, with no consensus having been reached regarding their usefulness for patients with gastric cancer [4, 5].
The Controlling Nutritional Status (CONUT) score is calculated using three parameters, the serum albumin concentration, total cholesterol concentration, and total peripheral lymphocyte count, which reflect host protein metabolism, lipid metabolism, and immune function, respectively [6]. As the CONUT score reflects both nutritional and immune status, we hypothesized that this score could be a useful nutritional and immunological biomarker to predict prognosis among cancer patients, allowing for effective customization of treatment, based on an individual’s host-related factors, to improve long-term survival. The clinical significance of establishing an independent, and yet complementary prognostic biomarker other than conventional tumor markers, is emphasized by the findings that carcinoembryonic antigen (CEA), which is a useful tumor marker for several cancers, including gastric cancer [7,8,9], is not necessarily elevated in all patients with cancer, even in those with advanced cancer [10], making treatment challenging. Accordingly, the purpose of our study was to evaluate the prognostic and predictive value of the CONUT score, after curative gastrectomy, to predict survival among patients with gastric cancer.
Materials and methods
Patients
We retrospectively reviewed the medical records of 368 consecutive patients who underwent curative laparoscopy-assisted gastrectomy for histologically confirmed gastric adenocarcinoma, with R0 resection, at the Department of Digestive and General surgery, Shimane University Faculty of Medicine, between January 2010 and December 2016. The median follow-up period for survival analysis was 35.3 months (4.0–97.0 months). The pathological Tumor-Node-Metastasis (TNM) classification was determined based on the 7th edition of the American Joint Committee on Cancer TNM classification system [11]. Postoperative complications were evaluated using the Clavien–Dindo classification, and clinically relevant complications were defined as those with a grade ≥ 2 [12]. To reduce the biasing effect of different distributions of covariates between groups of patients with a low and high CONUT score, a propensity score-matched (PSM) analysis was performed for depth of tumor, lymph node metastasis, and pathological TNM (pTNM) stage.
Laparoscopy-assisted gastrectomy was performed in all patients, including lymph node dissection performed as per the Japanese Gastric Cancer Treatment Guidelines (Version 3) [13]. Adjuvant chemotherapy, using 5-fluorouracil-based regimens, was recommended to patients with a histologically advanced gastric cancer.
The requirement for informed consent was waived because of the retrospective nature of this cohort study. All procedures were approved by the Institutional Review Board (IRB) and were in accordance with the Helsinki Declaration of 1965 and later versions (UMIN: 000030472).
Perioperative management
We introduced a multidisciplinary management team, including surgeon, anesthesiologist, dental hygienist doctor, pharmacist, nutritionist, and rehabilitation technician. This team mainly managed dental cleaning, medication assistance, physical exercise and rehabilitation, and nutritional support. We provided preoperative enteral nutrition to optimize preoperative condition as possible, which aimed to decrease the incidence rate of postoperative complications.
Postoperative outcome evaluation
Clinical findings and surgical outcomes were extracted from the medical records. The observation period was from the date of surgery until the date of death or loss to follow-up. Overall survival (OS) was calculated from the date of primary gastrectomy to the date of death from any cause or the date of the last follow-up.
Evaluation of preoperative nutritional indices
All laboratory data used for calculating the preoperative nutritional status were obtained within 1 week before surgery. The CONUT score was calculated according to three parameters (serum albumin concentration, total cholesterol concentration, and total lymphocyte count in peripheral blood), and was classified into four categories (normal, mild, moderate, and severe risk of malnutrition), as described in Table 1. A receiver operating characteristic curve of the preoperative CONUT score was generated from the outcomes of the multiple logistic regression analysis of OS. The area under the curve (AUC) estimation method was used to determine the predictive value of the CONUT for OS. Patients were classified in the high CONUT score group (the malnourished group), and in the low CONUT score group (the well-nourished group).
Statistical analyses
The PSM analysis was performed using R statistical package (version 3.1.3; http://www.r-project.org). All statistical analyses were conducted using the JMP software for Windows (version 12; SAS Institute, Cary, NC, USA). Continuous variables were expressed as the mean ± standard deviation when normally distributed, or as median and interquartile range for non-parametric data. The differences between groups were analyzed using Student’s t test for continuous variables with parametric distribution and Kruskal–Wallis test for non-parametric distributed variables. Frequencies were compared using the chi-squared test. OS was calculated using the Kaplan–Meier method, and between-group differences were evaluated using the log-rank test.
Cox proportional hazard regression models were constructed to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Univariate analyses were performed to identify the variables associated with OS. Variables with a p value < 0.05 on univariate analyses were entered into a multivariate logistic regression analysis, with a p value < 0.05 was considered statistically significant.
Results
Receiver operating curve of CONUT score for OS analysis and CONUT value according to the serum level of CEA
The optimal cutoff score of the CONUT was set at 2, based on the 5-year postoperative OS (sensitivity, 65.0%; specificity, 57.9%; and AUC, 0.625) (Fig. 1A).
A Receiver operating curve for postoperative survival was plotted to verify the optimum cutoff value of CONUT score for OS. B CONUT values in propensity score-matched 210 gastric cancer patients according to the serum level of CEA. Kruskal–Wallis test: p = 0.546. Each box represents interquartile ranges with median as horizontal line. Vertical bars indicate the maximum to minimum range
No association between the CONUT score and the serum CEA level was detected using a one-way Kruskal–Wallis analysis (p = 0.546). The mean CONUT score for patients with a normal serum CEA level (n = 157) was 2.6 ± 2.2, compared to 2.8 ± 2.3 for patients (n = 53) with a high serum CEA level (Fig. 1B).
Relationships between CONUT score and clinicopathological features
Based on the CONUT cutoff score, ≤ 2263 patients (71.5%) were classified into the well-nourished group, with 105 (28.5%) patients, having a CONUT score ≥ 3, classified into the malnourished group. As shown in Table 2, the CONUT score was significantly associated with age (p < 0.001), white blood cell count (WBC; p < 0.001), tumor size (p < 0.001), operative procedure (p = 0.041), depth of tumor (p < 0.001), lymph node metastasis (p = 0.005), pTNM stage (p < 0.001), CRP level (p < 0.001), and postoperative complications (p = 0.026). PSM adequately balanced the distribution of confounding variables (depth of tumor, lymph node metastasis, and pTNM stage) between the low and high CONUT score groups, with 105 matched pair identified, with a standardized or individualized follow-up, and used in subsequent analyses (Table 2).
Preoperative American Society of Anesthesiologists (ASA) physical status classification and preoperative comorbidities, such as hypertension, dyslipidemia, diabetes mellitus, respiratory functional disorder, history of the other organ cancers, cardiovascular disease, cerebrovascular disease, diabetes mellitus, or liver dysfunction were not significantly associated with CONUT (data unshown).
Cox regression analysis of propensity score-matched cohort
Univariate analyses identified advanced pTNM stage (p = 0.002), high CONUT score (p = 0.003), high serum CEA level (p = 0.027), and operative procedure (p = 0.007) as being significantly associated with a worse OS. On the multivariate analysis, pTNM stage (hazard ratio (HR): 1.970, 95.0% CI 1.199–3.235; p = 0.007), CONUT score (HR: 2.441, 95.0% CI 1.463–4.071; p < 0.001), serum CEA level (HR: 1.832, 95.0% CI 1.104–3.038; p = 0.019), and operative procedure (HR: 1.943, 95.0% CI 1.165–3.239; p = 0.011) were confirmed as independent prognostic factors for OS among the PSM subgroup (Table 3).
OS analysis stratified by the CONUT score and the serum CEA level in the propensity score-matched cohort
In the PSM subset, Kaplan–Meier analysis and the log-rank test revealed a worse prognosis, in terms of OS, among patients with a high, compared to a low, CONUT score (p < 0.001). The 5-year OS rates for patients with a low and high CONUT score was 73.8% and 49.8%, respectively (Fig. 2A).
Also, in the PSM subset, 53 patients with a high serum CEA level had a significantly lower OS, compared to the 157 patients with a normal CEA level (p = 0.020; Fig. 2B). The 5-year OS rates for patients with normal and high CEA levels were 67.5% and 45.4%, respectively.
Relationship between the CONUT score and the clinicopathological features of patients stratified by serum CEA level
Based on a CEA cutoff level of 5.0 ng/ml, 157 patients (74.8%) were included in the normal serum CEA group and 53 patients (25.2%) in the high CEA group. Among the 157 patients with a normal preoperative serum CEA level, 81 patients (51.6%) were classified in the low CONUT score group and the remaining 76 patients (48.4%) in the high CONUT score group, based on a CONUT cutoff score of 2. The CONUT score was significantly associated with age (p = 0.006), WBC (p = 0.006), tumor size (p = 0.003), and C-reactive protein (CRP) level (p < 0.001).
Among the 53 patients with a high preoperative serum CEA level, 24 (45.3%) were classified in the low CONUT score group (well nourished) and the remaining 29 (54.7%) in the high CONUT score (malnourished), based on a CONUT cutoff score of 2. The CONUT score was significantly associated with age (p = 0.015), WBC (p = 0.022), CRP level (p = 0.020), postoperative complications (p = 0.004), and adjuvant chemotherapy (p = 0.046) (Table 4).
Cox regression analysis of OS among groups stratified for serum CEA level
On univariate analyses for patients with a normal serum CEA level, an advanced pTNM stage (p = 0.008), high CONUT score (p = 0.032), and operative procedure (p = 0.038) were significantly associated with a worse OS. On multivariate analysis, pTNM stage (HR: 2.065, 95% CI 1.106–3.856; p = 0.023), CONUT score (HR: 2.240, 95% CI 1.181–4.249; p = 0.014), and operative procedure (HR: 1.825, 95% CI 0.951–4.249; p = 0.071) were independent prognostic factors of OS (data unshown).
On univariate analyses for patients with a high serum CEA level, a large tumor size (p = 0.004) and a high CONUT score (p = 0.014) were significantly associated to a worse OS. On multivariate analysis, tumor size (HR: 2.930, 95% CI 1.228–7.655; p = 0.015) and the CONUT score (HR: 3.707, 95% CI 1.500–10.607; p = 0.004) were independent prognostic factors of OS (data unshown).
OS analysis stratified by the CONUT score among groups stratified for serum CEA level
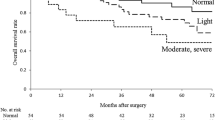
Among patients with a normal serum CEA level, the Kaplan–Meier analysis and log-rank test identified a significantly poorer OS among patients with a high CONUT score, compared to those with a low CONUT score (p = 0.041). The 5-year OS rates for patients with a low and high CONUT score were 76.2% and 58.6%, respectively.
Among patients with a high serum CEA level, a high CONUT score was associated with a significantly poorer OS than those with a low CONUT score (p = 0.004). The 5-year OS rates for patients with a low and high CONUT score were 67.9% and 28.3%, respectively (Fig. 3).
Discussion
There is good that cancer prognosis is not only related to tumor factors, but also patient status, including nutritional status and systemic inflammation [14, 15]. The clinical applicability of the CONUT score has been limited to the evaluation of nutritional status, although recently, it has received greater attention as a predictive biomarker of survival among patients with different types of cancers [16, 17]. The CONUT score is calculated from the serum albumin concentration, total cholesterol concentration, and total lymphocyte count in peripheral blood, and, thus, reflects protein reserves, caloric depletion, and impaired immune defenses, respectively.
Cholesterol is an essential immunocomponent of cellular membranes. It has numerous biological functions, including membrane fluidity and membrane protein activity, which potentially correlate with the initiation and progression of cancer and the immune response. Consequently, immunocompetent cells gain their capacity to exert an immunological response against the spread of tumor [18, 19]. Thus, hypocholesterolemia may contribute to a worse cancer prognosis. Lymphocytes play a fundamental role in the host’s anticancer immune status by inducing apoptosis and by inhibiting cancer cell proliferation, invasion, and migration [20]. Therefore, lymphocytopenia may lead to tumor progression. Hypoalbuminemia reflects not only malnutrition or hypercatabolism, but also systemic inflammation, which frequently induces a state of hypercytokinemia, resulting in an impaired immune response against cancer cells [21]. Therefore, the CONUT score reflects not only nutritional status but also the status of systemic inflammation and immune response [22]. In our retrospective study, we elucidated that patients with gastric cancer who had a high CONUT score (malnourished group) had a significantly poorer prognosis in terms of OS than those with a low CONUT score (well-nourished group). In addition, patients with a high CONUT score had a poorer tolerance to postoperative adjuvant chemotherapy than those with a low CONUT score (p = 0.011). Therefore, the poor tolerability of malnourished patients to anticancer treatment may explain the worse prognosis for OS in this group. These patients could possibly benefit from preoperative nutritional intervention and more intensive multimodal treatments.
Previous reports suggested that tumor-related factors, such as pTNM stage and CEA, were likely to be most reliable prognostic predictor for gastric cancer. Tumor markers play a significant role in the detection of tumors, treatment selection, the monitoring of the therapeutic response, and surveillance of various kinds of cancers. CEA is one of the most widely and frequently used tumor markers, especially for patients with gastrointestinal cancers [5, 6]. However, tumor markers, including CEA, are not always reliable in establishing a definitive diagnosis and determining appropriate management of cancers as they lack the high sensitivity and specificity needed [23], even for advanced cancers. Therefore, in this study, we evaluated the utility of the CONUT as a potent predictive factor of survival, with a high CONUT score (indicative of malnourishment) being significantly associated to a worse OS, regardless of serum CEA level. In other words, the CONUT score was a significantly reliable and independent predictive biomarker of survival among patients treated for gastric cancer using gastrectomy. These findings support the widely accepted hypothesis that the overall survival of cancer patients is not only determined by tumor characteristics alone, but also associated with host-related inflammation and malnutrition [14, 15]. Given these findings, patients with gastric cancer who have a high CONUT score could benefit from a more intensive follow-up, even after curative gastrectomy, with a preoperative nutritional intervention being of possible clinical benefit to improve the surgical outcomes in these patients.
On the other hand, the Nutritional Risk Index (NRI) was originally established by Bouillanne et al. for the screening index of the nutritional status of elderly hospitalized patients. NRI only requires measurements of height, weight, and serum albumin level [24]. Serum albumin may reflect chronic under-nutrition and deconditioning related to poor dietary habits. Malnutrition is one of the most common complicated disorders in gastrointestinal cancer patients. Therefore, biochemical markers, such as the albumin level, are the most commonly used markers of nutrition status. However, the relationship between the NRI and the morbidity of patients with gastrointestinal cancer has not yet been reported. Accordingly, the future study should evaluate the predictive value of short and long-term outcomes of gastrointestinal cancer patients after laparoscopic curative gastrectomy.
Despite our important findings, there are a few limitations to the current study which need to be acknowledged. This study was a retrospective single-institution design using a relatively small sample size. Potential factors that can affect inflammation-based and nutritional markers, such as medications, cannot be excluded. In addition, other biomarkers such as rapid turnover proteins (transferrin, transthyretin, retinol-binding protein) were not adequately assessed. Therefore, future prospective randomized studies are warranted to investigate the significance of preoperative nutritional intervention for improving surgical outcome in gastric cancer patients.
Despite the above limitations, the CONUT is a convenient and useful biomarker to estimate nutritional status and for predicting OS among patients with gastric ulcer. Therefore, the CONUT score could be used as a complementary biomarker with the CEA level. In conclusion, the CONUT score is an objective, non-invasive, and readily available prognostic biomarker that has the potential to guide individualized treatment strategies to improve survival among patients with gastric cancer.
References
Torre LA, Siegel RL, Ward EM, Jemal A (2016) Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol Biomarkers Prev 25:16–27. https://doi.org/10.1158/1055-9965
Niccolai E, Taddei A, Prisco D, Amedei A (2015) Gastric cancer and the epoch of immunotherapy approaches. World J Gastroenterol 21(19):5778–5793. https://doi.org/10.3748/wjg.v21.i19.5778
Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, Taniura T, Kawabata Y (2018) Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: a propensity score matching analysis. BMC Cancer 13;18(1):285. https://doi.org/10.1186/s12885-018-4201-4
Kurahara H, Maemura K, Mataki Y, Sakoda M, Iino S, Hiwatashi K, Kawasaki Y, Arigami T, Ishigami S, Kijima Y, Shinchi H, Takao S, Natsugoe S (2015) Prognostication by inflammation-based score in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Pancreatology 15(6):688–693. https://doi.org/10.1016/j.pan.2015.09.015
Kanemasa Y, Shimoyama T, Sasaki Y, Hishima T, Omuro Y (2018) Geriatric nutritional risk index as a prognostic factor in patients with diffuse large B cell lymphoma. Ann Hematol 97(6):999–1007. https://doi.org/10.1007/s00277-018-3273-1
Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G (2005) CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 20(1):38–45
Kai D, Yang B, Wu HH, Zhu H, Tang C (2015) The prognostic significance of pretreatment serum cea levels in gastric cancer: a meta-analysis including 14651 patients. PLoS ONE 10(4):e0124151. https://doi.org/10.1371/journal.pone.012415. Published online 2015 Apr 16.
Shao Y, Sun X, He Y, Liu C, Hui Liu (2015) Elevated levels of serum tumor markers cea and ca15-3 are prognostic parameters for different molecular subtypes of breast cancer. PLoS ONE 10(7):e0133830. https://doi.org/10.1371/journal.pone.0133830 (Published online 2015 Jul 24).
Arrieta O, Saavedra-Perez D, Kuri R, Aviles-Salas A, Martinez L, Mendoza-Posada D, Castillo P, Astorga A, Guzman E, Garza J (2009) Brain metastasis development and poor survival associated with carcinoembryonic antigen (CEA) level in advanced non-small cell lung cancer: a prospective analysis. BMC Cancer 9:119. https://doi.org/10.1186/1471-2407-9-119 (Published online 2009 Apr 22).
Chen C, Chen Q, Zhao Q, Liu M, Guo J (2017) Value of combined detection of serum CEA, CA72-4, CA19-9, CA15-3 and CA12-5 in the diagnosis of Gastric Cancer. Ann Clin Lab Sci 47(3):260–263
Sobin L, Gospodarowicz M, Wittekind C (eds) (2010) International union against Cancer (UICC). TNM classification of malignant tumors, 7th edn. Wiley-Blackwell, New York
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Japanese Gastric Cancer Association (2011) Japanese gastriccancer treatment guidelines 2010 (ver.3). Gastric Cancer 14:113–123. https://doi.org/10.1007/s10120-011-0042-4
Ogino S, Galon J, Fuchs CS, Dranoff G (2011) Cancer immunology—analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol 9(12):711–719. https://doi.org/10.1038/nrclinonc.2011.122. 8) .
Milsom C, Yu J, May L, Meehan B, Magnus N, Al-Nedawi K, Luyendyk J, Weitz J, Klement P, Broze G, Mackman N, Rak J (2007) The role of tumor-and host-related tissue factor pools in oncogene-driven tumor progression. Thromb Res 120. Suppl 2:S82-91. Erratum in: Thromb Res. 2008;123(1):187-190/bib>
Hirahara N, Matsubara T, Hayashi H, Takai K, Nakada S, Tajima Y (2018) Prognostic importance of controlling nutritional status in patients undergoing curative thoracoscopic esophagectomy for esophageal cancer. Am J Ther 25(5):e524–e532. https://doi.org/10.1097/MJT.0000000000000414
Akamine T, Toyokawa G, Matsubara T, Kozuma Y, Haratake N, Takamori S, Katsura M, Takada K, Shoji F, Okamoto T, Maehara Y (2017) Significance of the preoperative CONUT score in predicting postoperative disease-free and overall survival in patients with lung adenocarcinoma with obstructive lung cancer. Anticancer Res 37(5):2735–2742
Verstraeten SL, Albert M, Paquot A, Muccioli GG, Tyteca D, Mingeot-Leclercq MP (2018) Membrane cholesterol delays cellular apoptosis induced by ginsenoside Rh2, a steroid saponin. Toxicol Appl Pharmacol 18:352:59–67. https://doi.org/10.1016/j.taap.2018.05.014
Zhang G, Zhang D, Wu J, Zhang F, Zhu Z, Chen K, Zhang N, Jin J, Feng J, Lin N, Zhang Y, Yu H, Su D, Ying L (2018) Low serum levels of pre-surgical total cholesterol are associated with unfavorable overall survival in patients with operable non-small cell lung cancer. Clin Lab 64(3):321–327. https://doi.org/10.7754/Clin.Lab.2017.170823
Lim JA, Oh CS, Yoon TG, Lee JY, Lee SH, Yoo YB, Yang JH, Kim SH (2018) The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: an in vitro analysis. BMC Cancer 7;18(1):159. https://doi.org/10.1186/s12885-018-4064-8
Lucijanic M, Veletic I, Rahelic D, Pejsa V, Cicic D, Skelin M, Livun A, Tupek KM, Stoos-Veic T, Lucijanic T, Maglicic A, Kusec R (2018) Assessing serum albumin concentration, lymphocyte count and prognostic nutritionalindex might improve prognostication in patients with myelofibrosis. Wien Klin Wochenschr 130(3–4):126–133. https://doi.org/10.1007/s00508-018-1318-z
Alcorta MD, Alvarez PC, Cabetas RN, Martín MA, Valero M, Candela CG (2018) The importance of serum albumin determination method to classify patients based on nutritional status. Clin Nutr ESPEN 25:110–113. https://doi.org/10.1016/j.clnesp.2018.03.124
Christou N, Perraud A, Blondy S, Jauberteau MO, Battu S, Mathonnet M (2017) The extracellular domain of E cadherin linked to invasiveness in colorectal cancer: a new resistance and relapses monitoring serum-bio marker? J Cancer Res Clin Oncol 143(7):1177–1190. https://doi.org/10.1007/s00432-017-2382-x
Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C (2005) Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 82:777–783
Funding
This study received no external sources of funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Noriyuki Hirahara, Yoshitsugu Tajima, Yusuke Fujii, Shunsuke Kaji, Tetsu Yamamoto, Ryoji Hyakudomi, Takahito Taniura, and Yasunari Kawabata have no conflicts of interest or financial ties to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hirahara, N., Tajima, Y., Fujii, Y. et al. Controlling Nutritional Status (CONUT) as a prognostic immunonutritional biomarker for gastric cancer after curative gastrectomy: a propensity score-matched analysis. Surg Endosc 33, 4143–4152 (2019). https://doi.org/10.1007/s00464-019-06723-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06723-z