Abstract
Background
This study aimed to evaluate the predictive value of the preoperative Controlling Nutritional Status (CONUT) score, which comprehensively reflects protein and lipid metabolism as well as the immunocompetence among patients with stage 2 or 3 gastric cancer.
Methods
From a retrospective database of 3484 patients who underwent gastrectomy for gastric cancer at nine Japanese institutions between 2010 and 2014, data for 626 patients with stage 2 or 3 cancer were retrieved. The study evaluated the significance of the associations between the optimal CONUT score cutoff values with the prognosis and the incidence of postoperative complications.
Results
The study determined that 2 was the optimal CONUT score cutoff value for predicting mortality 2 years after surgery. The patients with a CONUT score of 2 or higher (CONUT-high group) were significantly older and had a worse Eastern Cooperative Oncology Group performance status, lower body mass index, and more advanced tumor-node-metastasis stage than the patients with a CONUT score lower than 2 (CONUT-low group). Overall, the survival time was significantly shorter in the CONUT-high group than in the CONUT-low group [hazard ratio (HR) 1.97; P < 0.0001]. A multivariable analysis showed that the CONUT score was an independent prognostic factor of overall survival. The CONUT score more significantly reflected the overall survival for patients who underwent postoperative adjuvant chemotherapy than for those who underwent surgery alone. Additionally, a high preoperative CONUT score was significantly associated with an increased incidence of postoperative pneumonia and prolonged hospitalization.
Conclusions
The study results suggest that the preoperative CONUT score may be a useful predictor of postoperative short- and long-term outcomes for patients with stage 2 or 3 gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite improvements in diagnostic and treatment methods, gastric cancer remains a major cause of cancer-related mortality worldwide.1,2 Immune-nutritional status is known to be a factor that influences cancer treatment outcomes such as surgical morbidity and tolerance to chemotherapy.3,4,5 Patients with advanced gastric cancer typically are malnourished because of persistent bleeding and inflammation at the site of the tumor and because of digestive tract obstruction.1,6 Objective evaluation of impaired nutritional status, ideally at the latent stage, may therefore facilitate several decisions regarding the optimal management of patients with gastric cancer.
Ideal indicators that reflect immune-nutritional status should be simple to use, inexpensive, quickly measured, objective, and available before surgery. The Controlling Nutritional Status (CONUT) score comprehensively evaluates protein metabolism, immunocompetence, and lipid metabolism7,8 and is widely used to select patients for intervention by nutritional support teams and to predict patients’ responses to nutritional therapy.9,10,11
The CONUT score also has attracted attention as a prognostic indicator for cancer patients, and Kuroda et al.12 found in their analysis of 416 patients with resectable gastric cancer that patients with a high preoperative CONUT score had significantly shorter overall survival time than those with a low CONUT score. However, their study was limited because it was a single-institution study with a small sample and because it included stage 1 patients who likely had a small number of recurrences. Additionally, the study had a long period of data acquisition accompanied by large changes in disease backgrounds and standard treatments.
This study aimed to analyze a multicenter data set acquired within a 5-year period to evaluate the value of the preoperative CONUT score as a predictor of short- and long-term outcomes for patients with stage 2 or 3 gastric cancer.
Materials and Methods
Patients
We conducted a retrospective review of the clinical data for 3484 patients who underwent gastrectomy for gastric cancer at nine institutions between January 2010 and December 2014. The patients provided written informed consent for surgery and for use of their clinical data as required by the institutional review board at each participating institution.
We selected 626 patients based on the following inclusion criteria: no preoperative treatment, R0 resection with systematic lymphadenectomy performed in accordance with the Japanese Gastric Cancer Treatment Guidelines,13 pathologic stage 2 or 3 gastric cancer based on the tumor-node-metastasis (TNM) classification system of the International Union Against Cancer (UICC) Classification of Malignant Tumors, 8th edition,14 and availability of sufficient clinical data for analysis (Fig. 1a). Patients with gastric stump cancer and those who underwent extended surgery (e.g., pancreaticoduodenectomy and Appleby’s procedure) or limited surgery without systemic lymphadenectomy were excluded from the study.
Patient Management
Postoperative follow-up evaluation included physical examinations, laboratory tests, and enhanced computed tomography (chest and abdominal cavity) once every 6 months for 5 years or until recurrence.15 Postoperative adjuvant chemotherapy with either S-1 (an oral fluoropyrimidine derivative) for 12 months or capecitabine plus oxaliplatin for 6 months was recommended to all patients as postoperative adjuvant treatment unless contraindicated by the patient’s condition or the patient’s refusal.16,17 Treatment after recurrence was determined on the basis of the evidence available at the time of treatment and the patient’s condition.
Quality of Surgery
To guarantee the quality of the gastrectomy and systemic lymphadenectomy, institutions at which more than 50 gastrectomies for gastric cancer were performed per year, in accordance with the Japanese Gastric Cancer Treatment Guidelines,13 were selected to participate in this multi-institutional study. Board-certified surgeons from the Japanese Society of Gastroenterological Surgery performed or supervised all surgery. A central review was not performed.
The CONUT Score
The CONUT score was calculated from the serum albumin value, total lymphocyte count (TLC), and serum cholesterol value (Table S1).8,9 Blood tests to calculate the CONUT score were performed within 3 days before surgery. To compare the predictive value of the CONUT score with that of other parameters, blood cell counts, total protein, urea nitrogen, creatinine, bilirubin, C-reactive protein (CRP), the estimated glomerular filtration rate (eGFR), the lymphocyte-to-monocyte ratio (LMR = TLC/monocyte count), the platelet-to-lymphocyte ratio (PLR = TLC/platelet count × 100), the neutrophil-to-lymphocyte ratio (NLR = neutrophil count/TLC), the platelet-to-neutrophil ratio (PNR = neutrophil count/platelet count × 100), Onodera’s prognostic nutritional index (PNI = 10 × albumin [g/dL] + 0.005 × TLC), and the albumin-to-bilirubin index (ALBI = [log 10 bilirubin {μmol/L}] × 0.66) + [albumin {g/L}] × − 0.085) were measured or calculated.6,18 The Glasgow Prognostic Scale (GPS) and modified GPS (mGPS) also were evaluated based on preoperative blood test results.19 Clinically relevant postoperative complications were classified into grades 2–5 based on the Clavien–Dindo classification.20
Statistical Analysis
The predictive value and optimal cutoff of the variables, including the CONUT score, were assessed using receiver operating characteristic (ROC) curve analysis of death within 2 years after surgery. Qualitative variables were compared between the two patient groups using the Chi square test, and quantitative variables were compared using the Mann–Whitney U test. Survival rates were estimated using the Kaplan–Meier method. The Cox proportional hazards model and multivariable analysis were used to determine the hazard ratio associated with each variable. Statistical analysis was performed using JMP 13 software (SAS Institute Inc., NC, USA). A P value lower than 0.05 indicated a significant difference.
Results
Patients’ Demographics and Clinical Characteristics
The mean age of the 626 patients was 67.9 ± 10.9 years, and the male-to-female ratio was 435–191. The median CONUT score was 1 (range 0–11), and total gastrectomy was performed for 229 patients (36.6%). Gastric cancer was pathologically diagnosed as stages 2A (n = 160), 2B (n = 121), 3A (n = 184), 3B (n = 117), and 3C (n = 44). Postoperative adjuvant chemotherapy was administered to 384 of the patients (61.3%). The median postoperative follow-up period was 49.2 months or until death.
Determination of the Optimal CONUT Score Cutoff Value for Predicting Survival
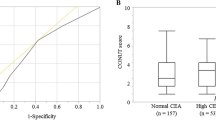
According to ROC curve analysis, the optimal CONUT score cutoff value that significantly correlated with mortality within 2 years after surgery was 2 (Fig. 1b). The CONUT score area under the curve was 0.656, which was higher than the AUC of its components as follows: TLC (0.595), serum albumin concentration (0.630), total cholesterol concentration (0.634), and other parameters, which are described earlier (Fig. 1c).
Comparison of Patients’ Clinical Characteristics
Based on the optimal CONUT score cutoff determined using ROC curve analysis, 626 patients were subdivided into the CONUT-low group (score < 2; n = 337) and the CONUT-high group (score ≥ 2, n = 289). The patients in the CONUT-high group were significantly older and had a worse Eastern Cooperative Oncology Group performance status, a lower body mass index, larger tumors, and more advanced TNM stages (Table 1). However, no significant differences were observed regarding sex, type of gastrectomy, or whether the postoperative adjuvant chemotherapy was administered.
Prognostic Impact of the Preoperative CONUT Score
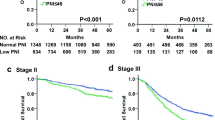
The patients in the CONUT-high group were more likely to experience a shorter overall survival after curative gastrectomy than those in the CONUT-low group (Fig. 2a). However, disease-free survival was only marginally shortened in the CONUT-high group, and the difference in the survival curves was not statistically significant (Fig. 2a).
We performed multivariable analysis to further evaluate the prognostic significance of the CONUT score. A preoperative CONUT-high score, age of 65 years or older, increased preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels, total gastrectomy, pathologic vascular invasion, invasive growth, and lymph node metastasis were identified as independent predictors of poor overall survival (Table 2). The CONUT-high group experienced a slightly higher incidence of recurrence at all sites (peritoneum, lymph nodes, liver, lung, and bone) (Fig. S1).
When the patients were categorized into the three groups based on their CONUT score (CONUT score of 0–1, 2–5, or 6–11), their overall survival became incrementally worse as the CONUT score increased (Fig. 2b).
Postoperative Treatment and the CONUT Score
To further explore the clinical implications of the preoperative CONUT score for patients with stage 2 or 3 gastric cancer, we performed a subgroup analysis after stratifying the patients based on whether they received postoperative adjuvant chemotherapy. The overall survival times were significantly shorter for the patients in the CONUT-high group than for those in the CONUT-low group, regardless of the presence or absence of postoperative adjuvant chemotherapy (Fig. S2).
CONUT Score and Postoperative Complications
We next evaluated the association between the preoperative CONUT score and the incidence of postoperative complications. The overall incidence of clinically relevant postoperative complications was 31.5% in the CONUT-high group and 26.4% in the CONUT-low group. Death within 90 days after surgery occurred for one patient in the CONUT-low group (0.3%) and four patients in theCONUT-high group (1.4%). The median postoperative stay was significantly longer in the CONUT-high group (14 days; range 7–306 days) than in the CONUT-low group (13 days; range 4–198 days) (P < 0.0001). The incidence of postoperative pneumonia was significantly higher in the CONUT-high group than in the CONUT-low group (Fig. 3a).
When the patients were subdivided based on age, preoperative body mass index, surgical procedure, and disease stage, those in the CONUT-high group experienced more postoperative complications among all the subpopulations, particularly those with a body mass index of 22 kg/m2 or higher and partial gastrectomy (Fig. 3b).
Discussion
We used a multicenter consolidated database with a large contemporary patient cohort amassed during a short period to determine whether the preoperative CONUT score predicted postoperative complications and long-term postoperative survival for patients who underwent radical gastrectomy. The findings showed that the preoperative CONUT score served as a significant predictor of the short- and long-term outcomes for patients with stage 2 or 3 gastric cancer.
The CONUT score was originally proposed by Gonzalez et al.21 as an integrated nutritional index to predict the length of hospitalization. Although the CONUT score can be calculated from parameters that are easy to acquire such as the serum albumin concentration, total cholesterol concentration, and lymphocyte count (representing protein metabolism, lipid metabolism, and immunocompetence, respectively), it is known to correlate closely with results of the Subjective Global Assessment, which is frequently used to assess a patient’s nutritional condition.9,11,21,22,23 These easy-to-use indices could provide inexpensive, prompt, and unbiased preoperative evaluation. Moreover, several reports have suggested that the CONUT score predicts perioperative complications and the long-term prognosis of patients with malignancies such as colorectal cancer, lung cancer, and breast cancer.9,11,12,23,24
Consistent with these reports, our data showed that the CONUT score had a greater predictive value for patient prognosis than each of its components and other blood parameters, and that it was an independent predictor of overall survival for patients with gastric cancer. Moreover, the overall survival for patients increased incrementally as the CONUT score increased. The prognostic value of this index, originally intended as an integrated nutritional index, is not surprising because malnutrition and immunosuppression are known to influence a cancer patient’s prognosis adversely.25,26,27 For example, the concentration of circulating vascular endothelial cell growth factors increase in malnourished, immunocompromised patients with ovarian and gastrointestinal cancer.28,29 Additionally, the complex state of malnutrition and immunosuppression facilitates chronic inflammatory reactions that are mediated by cytokines, which are secreted from cancer cells.26,30,31 Chronic inflammation and malnutrition also are major causes of the decrease in helper T cell population levels, interleukin-2 and -3 levels, and T cell blastogenic responses, which lead to compromised tumor immunity and increased dissemination of tumor cells.30,31,32
To the best of our knowledge, this is the first study to determine the correlation between the CONUT score and complications after gastrectomy with systematic lymphadenectomy among patients with stage 2 or 3 gastric cancer. For example, the incidence of pneumonia was significantly higher in the CONUT-high group, which may have been directly associated with the patients’ compromised immune-nutritional status. Conversely, although malnutrition adversely affects wound healing,6,18 the incidence of anastomotic leakage, which is among the worst complications after gastric cancer surgery, was unexpectedly similar between the CONUT-low and -high groups. However, our data suggest that the CONUT score may be an objective perioperative risk-assessment tool. Efforts to decrease preoperative CONUT scores through implementation of aggressive nutritional support before surgery may contribute to reducing the incidence of complications and could improve patient prognosis. A prospective large-scale intervention trial using the CONUT score as the eligibility criterion or as a parameter for evaluating the effect of treatment is warranted to explore these possibilities and to improve general practice procedures.
The current study had certain limitations, primarily because of its retrospective nature. Moreover, we did not have detailed information on compliance and the relative dose intensities of postoperative chemotherapy. Additionally, the issue of the influence that neoadjuvant treatments have on immune-nutrition status and the postoperative course remains unresolved because pretreated patients were excluded from the analysis.
In conclusion, the study results indicate that the preoperative CONUT score is a significant predictor of short- and long-term outcomes for patients with stage 2 or 3 gastric cancer. Routine calculation of the CONUT score is therefore advisable before patients undergo gastrectomy.
References
Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654–64.
Kanda M, Shimizu D, Tanaka H, et al. Significance of SYT8 for the detection, prediction, and treatment of peritoneal metastasis from gastric cancer. Ann Surg. 2018;267:495–503.
Heys SD, Park KG, Garlick PJ, Eremin O. Nutrition and malignant disease: implications for surgical practice. Br J Surg. 1992;79:614–23.
Miyata H, Yano M, Yasuda T, et al. Randomized study of clinical effect of enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Clin Nutr. 2012;31:330–36.
Andreyev J, Ross P, Donnellan C, et al. Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol. 2014;15:e447–60.
Kanda M, Mizuno A, Tanaka C, et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Med Baltim. 2016;95:e3781.
Iseki Y, Shibutani M, Maeda K, et al. Impact of the preoperative Controlling Nutritional Status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS ONE. 2015;10:e0132488.
Nakagomi A, Kohashi K, Morisawa T, et al. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb. 2016;23:713–27.
Toyokawa T, Kubo N, Tamura T, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016;16:722.
Formiga F, Chivite D, Corbella X. Utility of the Controlling Nutritional Status (CONUT) score in patients admitted due to acute heart failure. Int J Cardiol. 2017;235:203.
Ishihara H, Kondo T, Yoshida K, et al. Preoperative Controlling Nutritional Status (CONUT) score as a novel predictive biomarker of survival in patients with localized urothelial carcinoma of the upper urinary tract treated with radical nephroureterectomy. Urol Oncol. 2017;35:539.
Kuroda D, Sawayama H, Kurashige J, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21:204–12.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19.
Liu JY, Peng CW, Yang XJ, Huang CQ, Li Y. The prognosis role of AJCC/UICC 8th edition staging system in gastric cancer, a retrospective analysis. Am J Transl Res. 2018;10:292–303.
Kanda M, Kobayashi D, Tanaka C, et al. Adverse prognostic impact of perioperative allogeneic transfusion on patients with stage II/III gastric cancer. Gastric Cancer. 2016;19:255–63.
Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93.
Kanda M, Murotani K, Kobayashi D, et al. Postoperative adjuvant chemotherapy with S-1 alters recurrence patterns and prognostic factors among patients with stage II/III gastric cancer: a propensity-score-matching analysis. Surgery. 2015;158:1573–80.
Kanda M, Tanaka C, Kobayashi D, et al. Preoperative albumin-bilirubin grade predicts recurrences after radical gastrectomy in patients with pT2-4 gastric cancer. World J Surg. 2018;42:773–81.
Inaoka K, Kanda M, Uda H, et al. Clinical utility of the platelet-lymphocyte ratio as a predictor of postoperative complications after radical gastrectomy for clinical T2-4 gastric cancer. World J Gastroenterol. 2017;23:2519–26.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
de Ulibarri IJ, Gonzalez-Madrono A, de Villar NG, et al. CONUT: a tool for Controlling Nutritional Status. first validation in a hospital population. Nutr Hosp. 2005;20:38–45.
Miyata T, Yamashita YI, Higashi T, et al. The prognostic impact of Controlling Nutritional Status (CONUT) in intrahepatic cholangiocarcinoma following curative hepatectomy: a retrospective single institution study. World J Surg. 2018;42:1085–91.
Takagi K, Umeda Y, Yoshida R, et al. Preoperative Controlling Nutritional Status score predicts mortality after hepatectomy for hepatocellular carcinoma. Dig Surg. 2018. https://doi.org/10.1159/000488215.
O’Hara LM, Thom KA, Preas MA. Update to the Centers for Disease Control and Prevention and the Healthcare Infection Control Practices Advisory Committee Guideline for the Prevention of Surgical Site Infection (2017): a summary, review, and strategies for implementation. Am J Infect Control. 2018;46:602–9.
Giger U, Buchler M, Farhadi J, et al. Preoperative immunonutrition suppresses perioperative inflammatory response in patients with major abdominal surgery-a randomized controlled pilot study. Ann Surg Oncol. 2007;14:2798–806.
Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71.
Ryan AM, Reynolds JV, Healy L, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:355–63.
Nakamura I, Shibata M, Gonda K, et al. Serum levels of vascular endothelial growth factor are increased and correlate with malnutrition, immunosuppression involving MDSCs and systemic inflammation in patients with cancer of the digestive system. Oncol Lett. 2013;5:1682–6.
Watanabe T, Shibata M, Nishiyama H, et al. Elevated serum levels of vascular endothelial growth factor is effective as a marker for malnutrition and inflammation in patients with ovarian cancer. Biomed Rep. 2013;1:197–201.
Braumuller H, Wieder T, Brenner E, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–5.
Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15:362–74.
Kanda M, Tanaka C, Kobayashi D, et al. Proposal of the coagulation score as a predictor for short-term and long-term outcomes of patients with resectable gastric cancer. Ann Surg Oncol. 2017;24:502–9.
Acknowledgements
We thank Jodi Smith, PhD, from the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
DISCLOSURE
The author declares that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ryo, S., Kanda, M., Ito, S. et al. The Controlling Nutritional Status Score Serves as a Predictor of Short- and Long-Term Outcomes for Patients with Stage 2 or 3 Gastric Cancer: Analysis of a Multi-institutional Data Set. Ann Surg Oncol 26, 456–464 (2019). https://doi.org/10.1245/s10434-018-07121-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-07121-w