Abstract
Purpose
Multiple studies have attempted to demonstrate the interest of the cell adhesion marker, E cadherin, as a diagnostic and prognosis marker in colorectal cancer (CRC). However, it was considered non specific.
Materials and methods
Studies were carried out with CRC cell lines and patients’ cohort operated for CRC. The expression of E cadherin was studied after 5 fluorouracil (5FU) treatment and correlated to CRC relapse, chemo-resistance and survival.
Results
In CRC cell lines derived from high tumor stages, extracellular domain of E cadherin expression decreased after 5FU treatment whereas it increased in supernatants. Interestingly, only specific cleaved forms at 55 kDa of E cadherin were detected in supernatants. In CRC surgical patients, more importantly concerning extracellular E cadherin domain, a decreased expression was observed in tissues in function of CRC stages whereas an increased expression was found in sera. Moreover, there is an increasing trend of survival with weak serum E cadherin secretion, reinforcing the implication of this protein in CRC evolution.
Discussion
The extracellular domain can be defined as a 5FU resistance marker and allow CRC monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal Cancer (CRC) is a leading cause of mortality in the Western world (Ferlay et al. 2013). It is surgically curable in early stages as 1 and 2, while metastatic stage is associated with a high mortality. It is a cancer with a good prognosis when diagnosed early: a 5-year relative survival is 91% for localized stages, 70% for stages with loco-regional invasion. However, the 5-year survival is approximately 11% in metastatic situations, which represent approximately 25% of diagnosed patients (INCA, 2010). The rate of CRC mortality has decreased over the last 20 years thanks to advances in the field of management (early diagnosis and improved treatment modalities).
For CRC, the reference treatment is surgery. In advanced stages, chemotherapy is administered. While numerous chemotherapies exist, like capecitabin or Xeloda® (oral), oxaliplatin or Eloxatine® (injection), irinotecan or Campto® (injection) and raltitrexed or Tomudex® (injection), 5 fluorouracil (5FU) (injection) is the historic reference molecule (Labianca et al. 2013). Along with chemotherapy, a new class of drugs has existed for several years: targeted therapies. These are molecules developed with the intention of blocking tumor angiogenesis and proliferation (Karapetis et al. 2008). These treatments lead to a decrease of mortality, but distant recurrences and resistances to treatment persist.
One of the most robust hypotheses to explain recurrences is the presence of tumor initiating cells (TICs): a small sub group of cells that initiate tumor growth and proliferation. They are characterized by self-renewal, multipotency, angiogenic potential, immune evasion features and chemoresistance (Vaiopoulos et al. 2012). In a previous study using sedimentation field flew fractionation (SdFFF) cell sorting and matrigel amplification (Mélin et al. 2012), we have obtained enriched fractions of activated TICs for HCT116 cell line and quiescent ones for WiDr cell line. After grafting these populations on the chorio allantoïc membrane (CAM) (Mélin et al. 2015), we demonstrated that they produced specific tumors. Activated TICs led to proliferative and invasive tumors with an overexpression of E cadherin, whereas quiescent TICs were less proliferative and invasive with a loss of expression of E cadherin.
Such an interesting link has been proved between E cadherin and TICs, and knowing that no sensitive and specific serum CRC marker of diagnosis, prognosis, and monitoring exists (Eche 2004), we have decided to focus on its expression.
E cadherin, a calcium-dependent transmembrane glycoprotein, is composed of a cytoplasmic domain, a single-pass transmembrane domain and an extracellular domain that consists of five tandemly repeated cadherin-motifs subdomains with putative calcium-binding sites. The cytoplasmic domain interacts with catenin molecules which mediate its binding to the actin cytoskeleton (Roya 2008).
E cadherin downregulation is correlated with malignancy parameters such as tumor progression, loss of differentiation, invasion and metastasis (Canel et al. 2013). Different mechanisms of E cadherin inactivation in malignant tumors have been described, including mutations, epigenetic silencing, increased endocytosis and proteolysis (Roya 2008).
As reviewed (Roya 2008), E cadherin is removed from the plasma membrane by endocytosis and recycled to sites of new cell–cell contacts. Abnormal activation of proto-oncogenes, such as Src and EGFR, results in increased phosphorylation of tyrosine residues in the cytoplasmic domain of E cadherin, which leads to the recruitment of the E3-ubiquitin ligase Hakai and subsequently enhances endocytosis and a ubiquitin-dependent degradation of E cadherin (Shen et al. 2008).
Moreover, matrix metalloproteinases (MMP) cleave the E-cadherin extracellular domain near the plasma membrane region (Maretzky et al. 2005) releasing soluble E-cadherin (Noe et al. 2001), which stimulates tumor cells invasiveness (Okugawa et al. 2012). This is not connected to the E cadherin gene mutation (Efstathiou et al. 1999) but is probably in relation with post translational modifications. Likewise, Kokura et al. described the loss of E cadherin in anoxia-reoxygenation conditions in human CRC cell lines. No reduction of the amount of total E cadherin was found, suggesting that there was not a true decreased expression, but rather a redistribution, or an internalization of surface E cadherin and, as a consequence, a failure to bind the cytoskeleton (Kokura et al. 2004). Given that E cadherin is cleaved (Grieve and Rabouille 2014), all these data suggest a change in its distribution areas between intra and extracellular compartments.
It is well known now, that the cleavage of E cadherin generates different fragments with oncogenic and invasive properties (David and Rajasekaran 2012). Different fragments: extra cellular: 80 kDa, and intra cellular: 38, 33, 29 kDa have been highlighted. The 80 kDa form have been detected in urine and serum of different types of cancers (Chan et al. 2001) like CRC (Wilmanns et al. 2004).
In serum of CRC patients, E cadherin is expressed in soluble form. Pre-operative soluble E cadherin levels increase with disease extension (Wilmanns et al. 2004). Moreover, it is considered as a good prognosis marker of CRC (Okugawa et al. 2012).
However, no in vitro studies with CRC cell lines have been achieved. In particular, to search which specific form(s) of E cadherin is responsible for invasion and to correlate it to tumoral tissues and sera.
The objective of this study was to demonstrate, first of all, which specific form is implicated in the mechanism of invasiveness for CRC, and then its reliability to be a prognosis and monitoring biomarker.
For this, cells resistant to 5FU were obtained in vitro and E cadherin expression was studied both in cells and in supernatants. As a second step, in a preliminary study, we analyzed ex vivo the expression of E cadherin in tissues and sera from various stages of CRC surgical patients.
Materials and methods
Cell lines and culture
Human CRC cell lines corresponding to different tumor stages (Ahmed et al. 2013) WiDr, Colo205 (human colon carcinomas differentiated, Stage Dukes C for WiDr and Dukes D for Colo 205), SW480 and SW620 (human colon carcinomas dedifferentiated, Stage Dukes B for SW 480 and Dukes C for SW 620), were obtained from American Type Culture Collection (ATCC, VI, USA) and cultured according to their recommendations. Under basal conditions, WiDr cells were maintained in minimum essential media (MEM) (Thermofisher Scientific, MA, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Thermofisher Scientific, MA, USA), 1 mM sodium pyruvate (Thermofisher Scientific, MA, USA), 1% non-essential amino acids (Thermofisher Scientific, MA, USA), 100 IU/ml penicillin and 100 mg/ml streptomycin (Thermofisher Scientific, MA, USA). SW480, SW620 and Colo205 lines were cultured in RPMI medium (Thermofisher Scientific, MA, USA) supplemented with 10% FCS, 100 IU/ml penicillin and 100 mg/ml streptomycin, at 37 °C under humidified atmosphere and 5% CO2. Cells were used when they were at subconfluence, after three washes in PBS and trypsinization step (5 min in 0.5% Trypsin, Invitrogen, Thermofisher Scientific, MA, USA), 1500 rpm centrifugation (5 min) and counting using Trypan Blue (T8154; Sigma Aldrich, France) method on a Malassez lame.
Patients
A total of 50 consentant patients who underwent CRC resection at our institution (Limoges University Hospital, Department of Visceral Surgery, France) between March 2011 and April 2014 were enrolled in this retrospective study.
Prognostic values of the following clinic-pathologic parameters were evaluated (Table 1): tumor node metastasis classification (TNM). Locations of tumors and distant metastases were determined by colonoscopy, computed tomographic (CT).
All the patients had a uniform postoperative follow-up by the same team: they were examined within 1 month of resection, then every 3–4 months for the first year, every 6 months for the next 3 years and at gradually increasing intervals. CT was done every 6–12 months. A full colonoscopy was performed 1 year after surgery, then once every 3–5 years. Positron emission tomographic (pEt) scans were selectively performed when abnormalities or axial imaging raised the possibility of recurrence. Local recurrence was defined as the first clinical, radiological and/or pathological evidence of a tumor of the same histological type within the colon. Distant recurrence was defined as clinical, radiological and/or pathological evidence of systemic disease at sites including, but not limited to, the liver, lungs, peritoneum and para-aortic region.
Ethics statements
The research was carried out according to The Code of Ethics of the World Medical Association (Declaration of Helsinki), and the author’s Institutional Review Board had previously approved the study, which was also approved by the Local Ethics Committee and signed consent forms were obtained from all the patients.
Serum from patients
Peripheral venous blood samples were obtained from patients in many cases within 4 days after surgery. Serum samples were centrifuged 10 min at 3000 rpm and serum was stored at −80 °C until use. Before collection, informed consent was obtained from patients to use the samples in future experiments.
Tissue from patients
A quantity of 30 mg of tumoral (T) and peri tumoral (PT) tissues were weighed and put into a tube containing lysis buffer with ceramic beads (MK 14 Precellys, Bertin Technology, France). Grinding of these tissues was ensured by stirring series of 5500 revolutions/s (2 times for 40 s, interrupted by a pause of 20 s) in Precellys mixer. Lysates obtained were then subjected to sonication (a 40 Hz pulse every 2 s for 1 min) to complete cell lysis. Finally, a 10 min centrifugation at 14,000g was achieved. Supernatants containing secreted protein were then recovered and stored at −80 °C.
Chemicals
5 Fluorouracil (5FU; F6627) was obtained from Sigma Aldrich and dissolved previously in 100% DMSO (dimethylsulfoxide; D2650; Sigma Aldrich, France). The final concentration of DMSO was under 0.05% when 5FU was used. To select rapidly 5FU resistant cells, 72 h of treatment were chosen. Colo205, SW480, SW620 and WiDr cells were submitted to different concentrations of 5FU during 72 h, and impact on cell behavior was studied.
Cellular metabolism activity analysis
In 96-well plates, 2500 cells were seeded. The next day, cells were treated and culture was sustained during different times. After treatment, cells were centrifuged (5 min at 1500 rpm) and media was replaced by media containing MTT (Cell Titer96® AQueous One Solution Cell Proliferation Assay, Promega, France) following manufacturer’s instructions. After 3 h of incubation at 37 °C under humidified atmosphere and 5% CO2, optical density was read at 490 nm. Results were expressed as percentage of viable cells with an active metabolism, using non treated cells as internal control (100%).
Cellular viability
Cells were seeded at 10 × 106 in 75 cm2 flask. The day after, 5FU treatment was made (treated cells) or not (untreated cells), and maintained during 72 h. Supernatants were collected and cells were trypsinized without washes to keep all cells. Living and dead cells were determined using Trypan blue method described just before in supernatants and in cell suspensions. Results were expressed as percentage of living and dead cells using total cells as 100%.
Cell cycle analysis
After treatment, total cells were collected (from supernatant and from cellular part), washed and counted. 106 cells were fixed in 70% cold ethanol at −20 °C during at least one night. The day of the analysis, cells were washed three times in PBS, and treated with RNase DNase free (1 mg/ml) for 20 min. Reaction was stopped in ice. Propidium iodure (0.5 mg/ml) was added and fluorescence assisted cell sorting (FACS) (FACS Vantage, BD Biosciences, USA) analysis was made immediately.
Western blot
Total proteins, from treated and untreated cells obtained as previously described for “cellular viability”, were extracted using Lysis Buffer 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 1 mM DTT, 0.1% Tween-20, 10% glycerol, 2 mM Na3VO4, 20 mM NaF and protease inhibitors (mixture set III 2 mg/ml; Calbiochem). The rest of the experiment is identical to the previous publication (Mélin et al. 2015). Specific primary antibodies, purified mouse anti human E cadherin (1.25 µg/ml, 610182 from BD, clone: 36/E-Cadherin, France; 1 µg/ml; MAB1838 from R&D system, clone # 180215, MN, USA) were used.
Proteome array analysis
Total proteins were extracted and quantified as described before. Following manufacturer’s instructions from “Proteome Profiler Human Pluripotent Stem Cell array” (ARY010 from RD system, Bio-techne, MN, USA), analysis was assessed on 300 μg proteins, with samples supplemented with Aprotinin (A6279, Sigma Aldrich, France), Leupeptin (1167, Tocris, UK) and Pepstatin (1190, Tocris, UK). Membranes were scanned with Syngene bio-imaging system. Reference spots were used as internal controls to compare samples. Results were explained as a ratio between target protein and internal control.
E cadherin immunoassay
Cell supernatants and patients sera were used to quantify secreted E cadherin using Human E-cadherin Immunoassay (DCADE0, R&D systems, Biotechne, MN, USA) kit according to the manufacturer’s instructions. Results were calculated from a standard curve (recombinant human E cadherin; range, 0.050–2.604 ng/ml). Measurements were performed in triplicate, and mean values were used for data presentation.
Immunohistochemistry
E cadherin was immunohistochemically detected in paraffin-embedded tissues using VENTANA technology from Limoges University Hospital, Department of Anatomo-pathology, France. The processing of the bar-code-labeled slides was fully automated.
Survival analysis
Global recurrence-free survival and disease-specific survival were determined. Survival analyze was made using BiostatTGV free software.
Statistical analyses
Statistical analysis of differences was carried out by analysis of variance (ANOVA) using StatView version 5.0 software (Abacus Concepts, SAS Institute Inc). p values less than 0.05 (Fisher’s PLSD test) were considered significant.
Results
Study of E cadherin expression in colorectal cancer cell lines
Impact of 5FU treatment on cell behavior
To select rapidly the more 5FU resistant cells, 72 h of treatment were chosen. Colo205, SW480, SW620 and WiDr cells were submitted to different concentrations of 5FU during 72 h, and impact on cell behavior was studied. The metabolic activity was particularly affected because the inhibitory concentration of 50% (IC50) was obtained for 16 µM of 5FU (Fig. 1a1). For that reason and to keep more than 50% of metabolic activity and enough active cells to make studies, 8 µM of 5FU during 72 h was chosen for further experiments. This condition permitted to have 42.17% for Colo205, 64.97% for SW480, 74.75% for SW620 and 53.28% for WiDr of activated cells (Fig. 1a2). Concerning cellular viability, even if more pronounced mortality was observed after 5FU treatment, the four cell lines were able to keep more than 60% living or resistant cells (Fig. 1b). Finally, to check 5FU effectiveness, cell cycle analysis was made and showed a significant S cell step blockage for all cell lines (Fig. 1c).
Impact of 5FU treatment on cell behavior. 2500 cells were seeded in 96 wells plate. The day after, 5FU was used to treat cells with different concentrations and treatment was maintained during 72 h. Revelation was obtained using MTT test following manufacturer’s instructions. IC50 = inhibitory concentration for 50% metabolic activity. Results were representative of at least three independent experiments (a1). After 5FU treatment (8 µM for 72 h), metabolic activity (a2), viability (b), and cell cycle (c) were analyzed. Significance was obtained by comparing with untreated condition (NT). *p < 0.05; **p < 0.005; ***p < 0.0005
E cadherin expression variation is induced by 5FU treatment
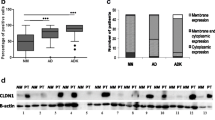
Cell E cadherin expression was studied based on proteome array analysis. It recognized specifically its extracellular domain. Concerning non-treated cells, the level of E cadherin expression was similar between Colo205 and WiDr, and between SW480 and SW620, as observed in Fig. 2a. After 5FU treatment, a trend of decrease in its expression was observed in all cell lines, which was only significant for SW620 (p = 0.0178).
Expression of E cadherin in cell lines before and after treatment with 5FU. Cells were treated with 5FU (8 µM, 72 h) and total proteins were extracted. Proteome array (a 300 µg of proteins) and Western blotting (b, c 50 µg of proteins) analyzes were made. a Quantification of specific extracellular domain of E cadherin signal’s intensity was represented with ratios of optical density (OD) values (E cadherin spot/ reference spot). b Western blot results with antibody against E cadherin recognizing intra-cellular part (BD antibody). c Western blot results with antibody against E cadherin recognizing extra-cellular part (RD antibody). For b, c, bêta actin was used as protein control. For a, the bar graphs show the mean ± standard error of the mean of optical density values ratios. Each graph represents the mean of three independent experiments. Statistical significance of the differences was calculated by ANOVA test. *p < 0.05. For b, c, Western blots and bar graphs are representative of three experiments. The bar graphs show ratios of optical density (OD) values between treated and untreated conditions for each cell line (E cadherin signal/reference actin signal). The dash line represents the expression of E cadherin in each cell line without 5FU treatment. This is a basic expression considered to be equal to 1
To complete this study, western blotting analyses were performed using antibodies recognizing different parts of E cadherin. The first (BD antibody) was able to fix the intracellular (cytoplasmic) domain of E cadherin, from amino acid 735 to 883. Whatever cellular line or treatment—or the absence thereof—, the expression of the total form of E cadherin (120 kDa) and specific cleaved forms at 55 and 35 kDa, was observed with this antibody (Fig. 2b). For the 55 and 35 kDa forms, an increased expression was obtained except for Colo 205 and WiDr. Another form under 25 kDa was also detected in WiDr and Colo205 (not shown). Using a second antibody (RD Antibody) able to recognize the extracellular domain of E cadherin starting from amino acid 709 to 155, only total form at 120 kDa was revealed for each cell line. Quantifications showed stable or increased (SW480 and SW620, early CRC stages) and decreased (WiDr and Colo 205, advanced CRC stages) expression after treatment. In conclusion, total and cleaved forms of E cadherin were detected in cell lines from CRC. A decrease of extracellular E cadherin expression (obtained by proteome analysis and western blotting with RD antibody) proved to be, after 5FU treatment, more significant for cell lines corresponding to advanced Dukes stage (Colo 205, WiDr).
Secretion of E cadherin was increased in the cell supernatants after 5FU treatment
As decreased extracellular E cadherin expression was observed in cells after 5FU treatment, detection of this protein was performed in supernatants. Specific immunoassay of extracellular domain showed an increased expression of E cadherin after treatment for all cell lines (Fig. 3a) but was only significant for SW480 (p = 0.0001) and SW620 (p = 0.0425). Interestingly, the rate of secreted E cadherin was more important for Colo205. To confirm these results, expression of specific secreted E cadherin was sought with both antibodies recognizing the intracellular part of E cadherin (BD antibody) and the extracellular domain of E cadherin (RD antibody). To compare our results, supernatants were submitted previously to a Coomassie Blue analysis (data not shown). With the antibody recognizing the intracellular part of E cadherin, only specific cleaved forms were detected at 55 and 35 kDa, whatever the cell line analyzed (Fig. 3b). The most important intensities after treatment were observed for the 55 kDa form of Colo 205 and SW620, and the 35 kDa form of Colo205 and SW 480. Using the antibody recognizing extracellular part of E cadherin, only one form, cleaved at 55 kDa, was revealed (Fig. 3c). Intensities were more important after treatment for Colo205, SW480, and WiDr.
Expression of E cadherin in supernatants of cell lines before and after treatment with 5FU. Cells were treated with 5FU (8 µM, 72 h) and supernatants were collected. a ELISA (recognizing specifically extracellular domain of E cadherin) quantification was made for treated (5FU) and non treated conditions (NT). The bar graph shows the mean ± standard error of the mean of signal intensity. Each graph represents the mean of three independent experiments. Statistical significance of the differences was calculated by ANOVA test (*p < 0.05, ***p < 0.0005). b Western blot was made with antibody against E cadherin recognizing intra-cellular part (BD antibody). c Western blot was made with antibody against E cadherin recognozing extra-cellular part (RD antibody). For b, c, supernatants were submitted previously to a Coomassie blue analysis to compare results. Western blots and bar graphs are representative of three experiments. The bar graphs show ratios of optical density (OD) values between treated and untreated conditions for each cell line. The dash line represents the expression of E cadherin in each cell line without 5FU treatment. This is a basic expression considered to be equal to 1
These experiments showed that only cleaved forms of E cadherin were detected in supernatants of CRC cell lines. Higher E cadherin intensity was detected after 5FU treatment in the majority of CRC cell lines and mostly its extracellular domain for cell lines corresponding to advanced Dukes stages (Colo 205 and WiDr).
Preliminary study of E cadherin expression in patients undergoing surgery for colorectal cancer
Detection of E cadherin in CRC patients tissues
A study of E cadherin expression in CRC patients was made. The cohort comprised 50 patients, 31 males and 19 females, with 73.1% of patients older than 65. The number of patients in each tumor stage was similar. When associated with tubulovillous adenomas, stages 0 and 1 were made. Tumor location was, respectively, 32.7% in the right colon, 9.6% in the transverse colon, 19.2% in the left colon and 34.6% in the rectum (Table 1). Twelve patients had metastases: 15.4% were synchronous to the initial tumor, whereas 7.7% were metachronous.
Constitutional expression of E cadherin was first evaluated by immunohistochemistry and examined in tumoral tissues of each CRC stage, but also in the adjacent tumoral tissues of each patient, used as internal control (PT). This analysis showed the same detection in peri tumoral (PT) tissues, whatever the CRC stage, while a decreased expression was observed in tumoral tissues (Fig. 4) according to CRC stages.
Using a proteome array specific to the extracellular domain of E cadherin, no significant difference was found in peri tumoral tissues (Fig. 5a). On the contrary, a decrease of expression was obtained between early and advanced stages with significant differences: Stage TV 0 1 versus Stage 2: p = 0.0121; Stage TV 0 1 versus Stage 4: p = 0.0017 (Fig. 5b). Moreover, using antibodies recognizing different parts of E cadherin, western blots of patients’ tissues were performed, using individual patients representing each stage. First, with the antibody recognizing the intracellular domain of E cadherin, the full length E cadherin at 120 kDa but also at 95 kDa was observed (Fig. 5c). Both expressions were more important for the stages 2 and 4. Later on, with the antibody recognizing the extracellular domain of E cadherin, the full length E cadherin at 95 kDa was identified with a decreased expression with different tumor stages (Fig. 5d).
Protein analysis of E cadherin expression in peri tumoral and tumoral tissues of CRC patients. Proteome array (a, b 300 µg of proteins) and Western blotting (c, d 50 µg of proteins) analyzes were made. a, b Quantification of extracellular domain of E cadherin signal’s intensity was represented with ratios of optical density (OD) values (E cadherin spot/reference spot). For each stage the number of patients (N) was: Stage TV 0 1, N = 6; Stage 2, N = 6; Stage 3, N = 8; Stage 4, N = 4. For A and B, the bar graphs show the mean ± standard error of the mean of optical density values ratios. Each graph represents the mean of three independent experiments. Statistical significance of the differences was calculated by ANOVA test. *p < 0.05; **p < 0.005. Western blots were made with antibody against E cadherin recognizing intra-cellular part (BD antibody) (c) and against E cadherin recognizing extra-cellular part (RD antibody) (d). For c, d, bêta actin was used as protein control. The bar graphs show ratios of optical density values. Each graph represents one experiment
E cadherin detection in CRC patient’s serum
Because a decreased expression of the E cadherin extracellular domain was found in the patients’ tissues, studying this expression in sera was made. Using ELISA analysis which is specific to the released extracellular domain of E cadherin, an increased expression in serum with tumor stages was observed (Fig. 6a).
E cadherin expression in patients sera. Sera were collected as described in "Materials and methods" section. For each stage the number of patients (N) was: Stage TV 0 1, N = 12; Stage 2, N = 12; Stage 3, N = 13; Stage 4, N = 11. Quantification of extracellular domain of E cadherin was made. The bar graph shows the mean ± standard error of the mean of signal intensity. Each graph represents the mean of three independent experiments. Statistical significance of the differences was calculated by ANOVA test. *p < 0.05. Stage TV 0 1 versus stage 3: p = 0.0229; Stage TV 0 1 versus stage 4: p = 0.0438
Survival in function of E cadherin expression
The mean expression of E cadherin in patient’s serum was 80.2 ng/ml. A better survival after 30 months for post-surgical patients having a weak E cadherin concentration in serum under or equal to 80.2 ng/ml, was observed but it was never significant (p over 0.81) (Fig. 7).
Survival analysis in function of E cadherin expression in patients sera. Survival curves were obtained using BiostatTGV free software. Mean of E cadherin was 80.2 ng/ml. Blue correspond to CRC patients having E cadherin under or equal to 80.2 ng/ml. The number of patients (N) in this group was: N = 30. Red corresponds to CRC patients having E cadherin over 80.2 ng/ml. N = 20. Global survival was defined as the period between the start of treatment and the last control performed or the patient’s death. It takes into account the death event and whatever the cause. p = 0.81. Free survival was defined as survival without recurrence. p = 0.84. Specific survival was amputated overall survival of non-cancerous disease deaths. p = 0.81
Discussion
Despite effective therapeutic approaches, CRC remains a public health problem due to resistance or relapse processes.
The complete complex E cadherin-catenin leads to intercellular connection, and its dissociation in CRC leads to invasion.
To demonstrate the ability of E cadherin to be a good diagnosis (Wilmanns et al. 2004) or prognosis marker (Okugawa et al. 2012), all clinical studies were based on the dosage of soluble E cadherin before treatments. The interest of our study was to analyse E cadherin in vitro before and after 5FU treatment and in a preliminary study, ex vivo after surgery, to follow its expression with more specificity.
Cells contained total and cleaved forms (Fig. 2b, c). No comparison could be made as there are no CRC cell lines studies in litterature. However, others cancers in vitro were studied. For example in human breast cancer cell line: MCF-7, the 80 kDa form of E cadherin have been found (Wever et al. 2007). In our study, the E cadherin forms found were different. Tissues contained only total forms (Fig. 5c, d). The cleaved forms were detected in cells containing only the intracellular domain, whereas tissues contained the total form with intra and extracellular domains of E cadherin, coming from different cellular types constituting tumor heterogeneity.
All of these results, therefore, attest the presence of a cleavage of this protein and probably a redistribution of its different domains. Indeed, E cadherin can be cleaved in different fragments. Several proteases such as MMP9, MMP7 (matrilysin) and MMP3 (stromelysin 1) (Noe et al. 2001), plasmin (Hayashido et al. 2005), kallikrein 7 (Johnson et al. 2007), MT1-MMP and ADAM10 (Maretzky et al. 2005) can cleave E-cadherin in vitro and shed the E cadherin ectodomain (soluble E-cadherin, sE-CAD). Other enzymes such as caspases (Steinhusen et al. 2001) and calpain (Rios-Doria et al. 2003) cleave E cadherin in its cytoplasmic part releasing an intracellular 37 kDa C-terminal fragment. This fragment disturbs β-catenin subcellular localization and stimulates β-catenin dependent cyclin D1 expression in the human keratinocyte HaCaT cell line.
Moreover, analysis of E cadherin expression in cells (Fig. 2c) and tissues (Fig. 5d) overlapped and showed decreased expression of extracellular domain with tumor stages (WiDr and Colo 205 for CRC lines and stages 3 and 4, for CRC patients). As a consequence, cell–cell adhesion could be inhibited whereas migration could be stimulated.
To confirm E cadherin cleavage and released, we decided to study E cadherin expression in cell supernatants and in patients’ sera. Supernatants had an increased expression of E cadherin with tumor stages (Fig. 3a) and contained only E cadherin cleaved forms originating from intra and extracellular domains (Fig. 3b, c). Total form was never found in supernatants, as maybe a consequence of the activity of secretase or MMP proteins. The major form found was a part of the extracellular domain at 55 kDa. (Fig. 3c) (ratios near 8 contrary to those quantified for intracellular domain, near 2).
Moreover, an increased E cadherin expression after 5FU treatment was identified (Fig. 3a). This result reinforced the constant link of E cadherin extracellular domain along 5FU chemo-resistance.
In CRC sera, an increased extracellular domain of E cadherin expression was revealed in function of tumor stages (Fig. 6). This result is in agreement with those of Katayama et al. (1994). They showed that E cadherin in the serum of cancer patients was a soluble form near 80 kDa. Moreover, its levels in the serum were significantly elevated, particularly in colon or rectum cancer patients.
To summarize, we have shown the increase of extracellular E cadherin with tumor stages both in supernatants and in CRC patients’ sera who have undergone surgery. In vitro study shows that it was a specific extra cellular form at 55 kDa. As a consequence, this domain seems to be a CRC invasiveness marker.
So, it could help us to follow relapse of CRC in both asymptomatic and symptomatic patients. Okugawa et al. (2012), had already demonstrated the interest of pre-operative serum E cadherin concentration as a prognostic marker in CRC patients. Our study tends to follow its use for monitoring patients with CRC. The ELISA test seemed to be a suitable assay to follow E cadherin level changes in patients’ sera because of its sensitivity and its specific recognition of the extracellular domain. So, it will be very interesting to test an important number of patients before and after chemotherapy whatever tumor stage.
Concerning survival, no significant differences were found in terms of overall or disease-free survival, and no specific survival based on the expression of E cadherin (Fig. 7). However, using a weak number of patients, we showed a trend of increase E cadherin level with a decrease of survival. This should be investigated further and on a larger cohort.
To our knowledge, it does not exist monitoring and resistance CRC marker. For the first time, we have shown interest of cleaved E cadherin protein, more specifically the extracellular part of it, as a marker of CRC invasiveness.
References
Ahmed D, Eide PW, Eilertsen IA et al (2013) Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2(9):e71
Canel M, Serrels A, Frame MC et al (2013) E-cadherin–integrin crosstalk in cancer invasion and metastasis. J Cell Sci 126(2):393–401
Chan AOO, Lam SK, Chu KM et al (2001) Soluble E-cadherin is a valid prognostic marker in gastric carcinoma. Gut 48(6):808–811
David JM, Rajasekaran AK (2012) Dishonorable discharge: the oncogenic roles of cleaved E-cadherin fragments. Cancer Res 72(12):2917–2923
Eche N (2004) Marqueurs des cancers digestifs: côlon–rectum, pancréas, foie. Immuno-Anal Biol Spéc 19(5):279–285
Efstathiou JA, Liu D, Wheeler JMD et al (1999) Mutated epithelial cadherin is associated with increased tumorigenicity and loss of adhesion and of responsiveness to the motogenic trefoil factor 2 in colon carcinoma cells. Proc Natl Acad Sci 96(5):2316–2321
Ferlay J, Soerjomataram I, Ervik M et al (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase. International Agency for Research on Cancer, Lyon
Grieve AG, Rabouille C (2014) Extracellular cleavage of E-cadherin promotes epithelial cell extrusion. J Cell Sci 127(15):3331–3346
Hayashido Y, Hamana T, Yoshioka Y et al (2005) Plasminogen activator/plasmin system suppresses cell–cell adhesion of oral squamous cell carcinoma cells via proteolysis of E-cadherin. Int J Oncol 27(3):693–698
Johnson SK, Ramani VC, Hennings L et al (2007) Kallikrein 7 enhances pancreatic cancer cell invasion by shedding E-cadherin. Cancer 109(9):1811–1820
Karapetis C, Khambata-Ford S, Jonker D et al (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Eng J Med 359:1757–1765
Katayama M, Hirai S, Kamihagi K et al (1994) Soluble E-cadherin fragments increased in circulation of cancer patients. Br J Cancer 69(3):580–585
Kokura S, Yoshida N, Imamoto E et al (2004) Anoxia/reoxygenation down-regulates the expression of E-cadherin in human colon cancer cell lines. Cancer Lett 211(1):79–87
Labianca R, Nordlinger B, Beretta GD et al (2013) Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(suppl 6):vi64–vi72
Maretzky T, Reiss K, Ludwig A et al (2005) ADAM10 mediates E-cadherin shedding and regulates epithelial cell–cell adhesion, migration, and β-catenin translocation. Proc Natl Acad Sci USA 102(26):9182–9187
Mélin C, Perraud A, Akil H et al (2012) Cancer stem cell sorting from colorectal cancer cell lines by sedimentation field flow fractionation. Anal Chem 84:1549–1556
Mélin C, Perraud A, Christou N et al (2015) New ex-ovo colorectal-cancer models from different SdFFF-sorted tumor-initiating cells. Anal Bioanal Chem 407:8433–8443
Noe V, Fingleton B, Jacobs K et al (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114(1):111–1118
Okugawa Y, Toiyama Y, Inoue Y et al (2012) Clinical significance of serum soluble E-cadherin in colorectal carcinoma. J Surg Res 175(2):e67–e73
Rios-Doria J, Day KC, Kuefer R et al (2003) The role of calpain in the proteolytic cleavage of E-cadherin in prostate and mammary epithelial cells. J Biol Chem 278(2):1372–1379
Shen Y, Hirsch DS, Sasiela CA et al (2008) Cdc42 regulates E-cadherin ubiquitination and degradation through an epidermal growth factor receptor to Src-mediated pathway. J Biol Chem 283(8):5127–5137
Steinhusen U, Weiske J, Badock V et al (2001) Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem 276(7):4972–4980
Vaiopoulos AG, Kostakis ID, Koutsilieris M et al (2012) Colorectal cancer stem cells. Stem Cells 30(3):363–371
Van Roya F, Berxb G (2008) The cell–cell adhesion molecule E-cadherin. CMLS Cell Mol Life Sci 65:3756–3788
Wever OD, Derycke L, Hendrix A et al (2007) Soluble cadherins as cancer biomarkers. Clin Exp Metastasis 24(8):685–697
Wilmanns C, Grossmann J, Steinhauer S et al (2004) Soluble serum E-cadherin as a marker of tumour progression in colorectal cancer patients. Clin Exp Metastasis 21(1):75–78
Acknowledgements
We are thankful to the Anatomo-pathology department who made the immunohistochemical experiments. All this work was supported by a grant from CHU de Limoges [CORC Comité d’Orientation Recherche en Cancer Limousin, 2013], sponsored by Conseil Régional and ARS Limousin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Christou, N., Perraud, A., Blondy, S. et al. The extracellular domain of E cadherin linked to invasiveness in colorectal cancer: a new resistance and relapses monitoring serum-bio marker?. J Cancer Res Clin Oncol 143, 1177–1190 (2017). https://doi.org/10.1007/s00432-017-2382-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-017-2382-x