Abstract
Background
Endoscopic submucosal dissection (ESD) is accepted as a standard treatment in patients with early gastric cancer (EGC) who have a negligible risk of lymph node metastasis. The aim of this study was to compare the short-term and long-term outcomes between ESD and surgery in patients with EGC that fulfilled the expanded indication of ESD on their final pathologic report.
Methods
We reviewed the clinical data of patients who underwent gastric ESD and surgery between January 2007 and December 2012. Patients with pathologically confirmed EGC that fulfilled the expanded indication of ESD on their final pathologic report were analyzed.
Results
Among 2023 patients, 817 (40.4%) underwent ESD and 1206 (59.6%) underwent surgery. The proportion of cases meeting the absolute indication was significantly higher in the ESD group than in the surgery group (66.0 vs. 26.2%). Lesions on the middle third, >3 cm in size, flat or depressed, and of undifferentiated histology were significantly more common in the surgery group than in the ESD group. The ESD group showed lower acute complication rates [8.1% (66 of 817) vs. 18.1% (218 of 1206), P ≤ 0.001] and procedure-related mortality [0 vs. 0.3% (4 of 1206), P = 0.153] than the surgical group. The annual incidence of recurrent gastric cancer was 2.18% in the ESD group and 0.19% in the surgery group. The 5-year overall and disease-specific survival rates were not significantly different between the ESD group and the surgery group (overall survival: 96.4 vs. 97.2%, P = 0.423; disease-specific survival: 99.6 vs. 99.2%, P = 0.203).
Conclusions
Although EGC lesions had poorer features in the surgery group than in the ESD group, ESD was comparable to surgery for EGCs that fulfilled the expanded indication of ESD, with lower rates of acute complication and comparable overall survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Early gastric cancer (EGC) is defined as a gastric cancer with tumor invasion that is confined to the mucosa or submucosa, irrespective of the presence of lymph node metastasis [1]. For EGC, gastrectomy with lymph node dissection has long been considered as a standard treatment because of the presence of lymph node metastasis [2, 3]. The rate of lymph node metastasis is up to approximately 3% in mucosal gastric cancer and up to approximately 20% in submucosal gastric cancer [2, 4]. However, endoscopic submucosal dissection (ESD) is accepted as a standard treatment for EGC that meets the absolute indication of Japanese gastric cancer treatment guidelines because of the negligible risk of lymph node metastasis. In addition, on the basis of a large series of pathologic review cases, the expansion of the criteria for local endoscopic treatment was proposed for selected EGC cases [4]. ESD is currently widely performed for treating EGC that meets the absolute and expanded indications of ESD in Korea and Japan [5,6,7,8].
Because ESD is minimally invasive and preserves most of the stomach, it provides better quality of life to the patients than does surgical treatment [9]. Although there is a risk of metachronous cancer in the remnant stomach, recent studies reported that the short-term and long-term outcomes of ESD for EGC were excellent [10,11,12,13]. With the rapid development in the technique and devices in ESD, the rate of curative resection has increased [12, 13] and the reported rate of adverse events was relatively low [14, 15]. However, there are still concerns about the validity of ESD for EGC that meets the expanded indication, especially for the undifferentiated type of cancer [16,17,18]. There are also limited studies comparing the long-term outcomes of ESD with those of surgical treatment [19,20,21,22,23,24].
Therefore, in this study, we aimed to compare the short-term and long-term outcomes between ESD and surgery in patients with EGC that fulfilled the expanded indication of ESD including undifferentiated-type cancer.
Methods
Patients
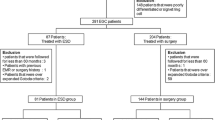
This is a single-center, retrospective study. We reviewed a prospective database of patients who underwent gastric ESD or surgery for newly diagnosed EGC at Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. The inclusion criteria were as follows: (i) patients aged ≥20 years, (ii) patients with newly diagnosed EGCs without previous treatment, (iii) patients with pathologically confirmed EGCs that met the expanded indication of ESD without lymphovascular infiltration on their final pathologic reports, and (iv) patients who did not have other organ malignancies at the time of treatment. From January 2007 to December 2012, there were a total of 4073 patients with newly diagnosed EGC based on their pathologic results. 1007 underwent ESD and 3066 underwent surgery (Fig. 1). Among them, 817 patients with 827 EGCs in the ESD group and 1206 patients with 1212 EGCs in the surgery group were analyzed in this study. The institutional review board of our hospital approved this study.
Definition
EGCs that met the expanded indications of ESD and curative resection were defined based on the resected specimen according to the Japanese Gastric Cancer Treatment Guidelines 2010 (ver. 3) [3]. We classified the patients who underwent ESD or surgery into three groups according to the indication of ESD. (i) Absolute criteria group: intramucosal tumor, differentiated-type histology, without ulcerative findings (UL (−)), tumor size ≤2 cm. (ii) Differentiated EGCs in the expanded indication group: (a) intramucosal tumor, differentiated-type histology, UL (−), tumor size >2 cm; (b) intramucosal tumor, differentiated-type histology, UL (+), tumor size ≤3 cm; (c) submucosal invasion (SM1, <500 µm from the muscularis mucosa), differentiated-type histology, UL (−), tumor size ≤3 cm. (iii) Undifferentiated EGCs in the expanded indication group: intramucosal tumor, undifferentiated-type histology, UL (−), tumor size ≤2 cm. Complete resection of ESD was defined when resected specimens were cancer free in all lateral margins and in the vertical margin. The resection was considered as curative when all of the following conditions were fulfilled: en bloc resection, negative lateral and vertical margins, no lymphovascular infiltration, and EGCs that met the expanded indication of ESD. The T stage and N stage after surgery were determined according to the 7th edition of the American Joint Committee on Cancer staging system [25].
Any adverse events within 30 days and beyond 30 days after ESD or surgery were defined as acute complications and late complications. Bleeding was considered as a complication when there were signs of hemorrhage such as hematemesis, melena, or hematochezia, and adverse events requiring transfusion or intervention such as endoscopic hemostasis, embolization, or surgery after treatment. Intra-abdominal fluid collection or abscess, bowel obstruction, and leakage were considered as complications when confirmed on images of abdominal sonography, computed tomography (CT) scan, or fluoroscopy. Cardiac or pyloric stricture after ESD and anastomosis-site stricture after surgery was defined as the inability to pass a standard 1-cm endoscope.
Recurrent gastric cancer was classified into five types according to the time and site of recurrence. Residual disease was defined as recurrences within a year after treatment at the previous ESD site. Local recurrence was defined as recurrences after more than a year at the previous ESD site in the ESD group and at the anastomosis site in the surgery group. Synchronous and metachronous gastric cancers were defined as recurrence at a location other than the previous ESD site or the remnant stomach after surgery. Distant metastasis included peritoneal carcinomatosis and metastasis to other solid organs or distant lymph nodes. Newly diagnosed dysplasia in the stomach was not included in the definition of recurrent gastric cancer.
ESD and surgical procedures
Before ESD or surgical treatment, all patients underwent esophagogastroduodenography (EGD) and abdominal CT for the staging of EGC. According to the preoperative evaluation, treatment modality was selected after consideration of the characteristics of the lesions and patients’ comorbidities. All ESDs were performed by experienced endoscopists. The patients underwent ESD under conscious sedation with intravenous midazolam and propofol. A standard single-channel endoscope (GIF-Q260 J or GIF-H260Z; Olympus, Tokyo, Japan) was used. The ESD procedure sequence consisted of identifying the target lesion, placing circumferential marking dots, lifting the submucosal layer with saline injection, mucosal incision, and direct submucosal dissection with hemostasis. Direct submucosal dissection was performed by using an insulated-tip knife (KD-610L, Olympus) and endoscopic hemostasis with specialized hemostatic forceps (FD-410LR, Olympus) was performed to remnant vessels on the postresection surface, as needed.
Open or laparoscopic radical gastrectomy and lymph node dissection were performed in patients in the surgery group. Distal, proximal, or total gastrectomy was determined by experienced surgeons according to the location of tumor. The extent of lymph node dissection was performed based on the Japanese Gastric Cancer Association guidelines [3].
Follow-up schedules
After ESD for EGC, all patients underwent EGD at 3, 6, 12, 18, and 24 months after ESD for detecting residual or recurrent tumors. After 24 months, EGD was performed annually. In addition, abdominal CT was performed every 6 months for the first year and annually thereafter to detect lymph node metastasis or distant metastasis.
After surgery, EGD and abdominal CT were performed every 6 months for the first year and annually thereafter.
Statistical analysis
The statistical tests performed to compare the results included Student’s t test or Mann–Whitney U-test for continuous variables, and the χ2 test or Fisher’s exact test for categorical variables. Comparisons between the two groups in long-term outcome, including overall survival, disease-specific survival, and cumulative incidence of recurrent gastric cancer, were done by using the Kaplan–Meier method and log-rank test. Cox regression analysis was used to adjust for possible confounding variables. A P- value of <0.05 was considered statistically significant. All statistical analyses were performed by using SPSS version 21.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of patients and lesions
From January 2007 to December 2012, a total 4073 patients underwent ESD or surgery for newly diagnosed EGC. The flowchart of patient enrollment is shown in Fig. 1. Of the enrolled patients, 2023 patients with 2039 lesions were analyzed in this study according to the predetermined inclusion and exclusion criteria. Among them, 817 patients with 827 lesions (40.4%) underwent ESD and 1206 patients with 1212 lesions (59.6%) underwent surgery [subtotal or distal gastrectomy, 1062 (88.1%) patients; total gastrectomy, 144 (11.9%) patients]. Among the patients in the ESD group, a large proportion had EGCs that met the absolute criteria (546 patients, 66.0%). On the contrary, a large proportion of patients among the surgery group had undifferentiated EGCs that met the expanded indication (564 patients, 46.5%).
The baseline characteristics of the enrolled patients and of the lesions are shown in Table 1. The patients in the ESD group were older, predominantly male, had more comorbidities (hypertension, cardiovascular disease), and more frequently treated with anticoagulants or antiplatelet drug than patients in the surgery group. More than half the patients (498 patients, 61.0%) in the ESD group first visited a division of gastroenterology, whereas more than half of the patients (814 patients, 67.5%) in the surgery group first visited a division of general surgery at our hospital.
The lesions were smaller, more frequently the flat or depressed type, and more frequently located in the lower third in the ESD group than those in the surgery group. The distribution of histology and the criteria of ESD indication were significantly different between the two groups. The rates of undifferentiated histology and lesions that met the expanded indication were significantly higher in the surgery group than in the ESD group. The characteristics of lesions according to each indication of ESD were analyzed (Table 2). In lesions that met the absolute indication, the tumors were larger and more frequently showed the flat or depressed type in the surgery group than in the ESD group. Likewise, in differentiated and undifferentiated EGCs in the expanded indication groups, the tumors were significantly larger and more frequently showed the flat or depressed type in the surgery group than in the ESD group. Especially, tumors >3 cm in size were more common in the surgery group than in the ESD group (26.4% (87 of 330) vs. 15.4% (29 of 188), P = 0.003]. The rates of ulceration and submucosal invasion were not significantly different between the two groups.
Comparisons of short-term outcomes according to the treatment modality
The morbidity and mortality after treatment are summarized in Table 3. The rate of acute complications was significantly higher in the surgery group than in the ESD group [18.1% (218 of 1206) vs. 8.1% (66 of 817], P ≤ 0.001]. Bleeding and perforation after treatment were the major complications after ESD. On the other hand, intra-abdominal fluid collection or abscess, wound infection, bowel obstruction, and leakage were major complications after surgery. Four patients died of procedure-related causes in the surgery group.
In the ESD group, 87 (10.65%) patients showed noncurative resection. Among them, 18 patients underwent additional endoscopic treatment (ESD or argon plasma coagulation), 14 patients underwent gastrectomy and lymph node dissection, and 55 patients were placed under close observation without additional treatment. All 1206 patients in the surgery group underwent R0 resection. Thirteen patients (1.1%) showed lymph node metastasis. Nine patients had N1 stage, two patients had N2 stage, and two patients had N3 stage disease.
The characteristics of patients and lesions with lymph node metastasis in the surgery group are shown in Table 4. Among 13 patients, 3 had EGCs that met the absolute indication and 10 had EGCs that met the expanded indication of ESD. Eight patients had undifferentiated-type histology. Most tumors [91.2% (11 of 12)] showed depressed-type morphology. In the preoperative evaluation, four patients had ulcer on endoscopy and showed CT findings that were suspicious of lymph node metastasis.
Comparisons of long-term outcomes according to the treatment modality
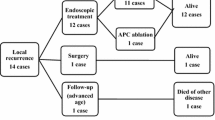
For the comparisons of long-term outcomes according to the treatment modality, 786 patients in the ESD group and 1202 patients in the surgery group were analyzed. Fourteen patients who underwent additional surgery directly after noncurative ESD and 21 patients who were lost to follow-up at our hospital within 1 month after curative ESD or surgery were excluded. Table 5 shows the long-term outcomes of treatments. The median follow-up period was 37.53 months [interquartile range (IQR) 26.26–59.36 months] in the ESD group, and 57.34 months (IQR 37.63–60.47 months) in the surgery group. In both groups, stricture was the most prevalent complication; however, the incidence was very low (0.3% in ESD, 0.7% in surgery). The annual incidence of recurrent gastric cancer was higher in the ESD group than in the surgery group (2.18 vs. 0.19%, P ≤ 0.001). The pattern of recurrence was different depending on the treatment (ESD or surgery). Among 60 cases of recurrence after ESD, recurrences at the previous ESD site were the most frequent [28 (46.7%)], followed by recurrences at locations other than the ESD site [27 (45.0%)]. Among nine cases of recurrence after surgery, there were five recurrences in the remnant stomach after more than a year (55.6%), followed by three cases with distant metastasis (33.3%). The depth of invasion and the treatment modality for recurrent cancer lesions were not significantly different between the two groups.
The Kaplan–Meier curve for overall survival, disease-specific survival, and cumulative incidence of recurrent gastric cancer are shown in Fig. 2. The 5-year overall survival rate was 96.4% in the ESD group and 97.2% in the surgery group. There was no significant difference between the two groups (Fig. 2A, P = 0.423). Furthermore, the 5-year disease-specific survival rate was not significantly different between the two groups (Fig. 2B, 99.6 vs. 99.2%, P = 0.203). The cumulative incidence of recurrent gastric cancer was significantly higher in the ESD group than in the surgery group (Fig. 2C, 5-year cumulative incidence rate: ESD group 10.9% vs. surgery group 0.95%, P ≤ 0.001).
A subgroup analysis of overall survival rates according to each indication of ESD was performed to evaluate the long-term outcomes of treatment among the same subgroups. For overall survival, there was no significant difference between the ESD group and the surgery group according to each indication of ESD (Fig. 3).
Comparisons of the overall survival rate between the endoscopic submucosal dissection (ESD) group and the surgery group according to the indication criteria of ESD: A Absolute indication; B differentiated early gastric cancers (EGCs) in the expanded indication; C undifferentiated EGCs in the expanded indication
Cox regression multivariate analysis for survival and cancer recurrence was done after adjustment for age and sex (Table 6). The adjusted hazard ratio for overall survival was 0.859 in the ESD group, and there was no significant difference compared with the surgery group [95% confidence interval (CI) 0.469–1.571, P = 0.621]. The adjusted hazard ratio for disease-specific survival was 0.323 in the ESD group, which was also not significantly different compared with the surgery group (95% CI 0.065–1.602, P = 0.167). On the other hand, the hazard ratio (HR) for gastric cancer recurrence was significantly higher in the ESD group than in the surgery group (HR 12.801, 95% CI 6.074–26.978, P ≤ 0.001). In the multivariate analysis for survival and cancer recurrence, there was no significant difference according to each indication of ESD.
Discussion
In this study, we compared a variety of aspects between ESD and surgery in patients with EGC that fulfilled the expanded indication of ESD on their posttreatment pathologic reports, in order to determine the advantages and disadvantages of the two treatment modalities. We found that the long-term overall survival and the disease-specific survival after ESD are comparable to those after surgery although we included undifferentiated EGC, for which it was controversial whether treatment with ESD or surgery should be performed. ESD had relatively low incidences of acute and chronic complications; however, frequent intragastric recurrence at the previous ESD site and at a location other than the ESD site was a problem for ESD.
As the number of patients with EGC who are undergoing ESD is increasing, the excellent long-term outcomes of ESD for EGCs meeting the absolute or expanded indications have been reported [10,11,12,13]. The expanded indication of ESD was proposed based on histologic examination of the surgically resected stomach and some retrospective research [4, 26]. However, a randomized controlled prospective study comparing oncologic outcomes between treatment modalities has not been performed yet. Therefore, a comparison of short-term and long-term outcomes between ESD and surgery is still needed to validate the current indication of ESD for EGC.
To date, there have been several retrospective studies that compared the long-term outcomes between ESD and surgery [19,20,21,22,23,24, 27]. These previous studies reported the noninferiority of ESD compared with surgery, showing that long-term overall survival and disease-specific survival rates were not significantly different between ESD and surgery [19,20,21,22,23,24, 27]. However, the rate of recurrent gastric cancer was significantly higher in the ESD group than in the surgery group [22,23,24, 27]. These findings were similar to our results. However, previous studies had a limitation that undifferentiated types of cancer were excluded in the analysis. In the present study, we included and analyzed EGC patients whose posttreatment pathology fulfilled the expanded indication of ESD including undifferentiated-type cancers, with a relatively long follow-up period.
In this study, there were significant differences in the clinical and demographic characteristics of enrolled patients between the two groups. Patients in the ESD group were older, predominantly male, had a higher proportion of smoking history, and tended to have more underlying disease (hypertension and cardiovascular disease). Old age, smoking history, and comorbidity are risk factors for general anesthesia. Although patients in the ESD group were older and had more comorbidities, the rates of acute complications were lower in the ESD group than in the surgery group, and there was no procedure-related mortality. Some advantages of ESD over surgery are that ESD does not require general anesthesia and has lower acute complications. Therefore, ESD is a minimally invasive procedure and relatively safe for patients with more comorbidities and older age.
The characteristics of lesions were significantly different between the two groups. Tumors on the middle third, >3 cm in size, flat or depressed, and with an undifferentiated histology were significantly more common in the surgery group than in the ESD group. Although the lesions had fulfilled the expanded indication of ESD based on the resected specimen after treatment, there was a tendency that patients with tumors of a large size, flat, or depressed-type morphology, and undifferentiated histology underwent surgical treatments. This tendency was still observed when it was separately analyzed according to the absolute and expanded indications. The surgery group had tumors that were larger in size, and of the flat or depressed-type morphology regardless of the absolute and expanded indications. This finding implies that subtle differences that cannot be identified by the current criteria for ESD which includes tumor size, presence of ulcer, and tumor histology, exist in real clinics. On the basis of these subtle differences that was not identified yet, the surgeon and gastroenterologist may decide to perform surgery if they infer that EGC is more advanced, and they may decide to perform ESD if they infer that the EGC is in a less advanced stage. This finding means that further prospective studies about more precise criteria are needed to determine whether to recommend ESD or surgery for patients with EGC.
In present study, 87 (10.65%) patients in the ESD group underwent noncurative resection. Among them, 14 patients underwent additional gastrectomy and lymph node dissection, whereas four patients (0.3%) in the surgery group died of procedure-related causes even though they could underwent ESD instead of surgery. In addition, considering higher acute complication rates (ESD vs. Surgery; 8.1 vs. 18.1%) and unmeasurable aspect such as indigestion, vitamin deficiency from resection of the stomach, avoiding an unnecessary surgery is important. If these patients could have been treated by the other treatment, we could get better oncologic outcomes. However, considering the high risk of noncurative resection and metachronous recurrence after ESD than surgery, it is difficult to determine which treatment modality is better in terms of the oncologic outcomes and quality of patients’ life. Prospective studies with more long-term follow-up periods are needed to compare outcomes between two treatment modalities.
Previous studies reported that the rate of lymph node metastasis was up to approximately 3% in mucosal gastric cancer and up to approximately 20% in submucosal gastric cancer [2, 4]. In our study, 13 patients with EGC [1.1% (13 of 1206)] showed lymph node metastasis. There were even two patients with N3 stage EGCs that met the expanded indication of ESD. Because these two patients showed preoperative CT findings that were suspicious of lymph node metastasis, they underwent surgery although the lesions seemed to fulfill the expanded indication of ESD before treatment. Among 13 patients with lymph node metastasis, 4 patients underwent surgery because their preoperative CT findings showed the possibility of lymph node metastasis and 5 patients underwent surgery because of ulcer in the lesion on endoscopy. All of the 13 EGCs with lymph node metastasis detected after surgery were beyond the indication of ESD because of the CT findings, ulcerative lesion, and undifferentiated-type histology in the preoperative period. There were several studies about predicting the risk factors for lymph node metastasis in EGCs that met the indication of ESD [28,29,30]. Nevertheless, it is difficult to accurately predict lymph node metastasis in EGCs that met the expanded indication of ESD. Further prospective study is needed to investigate the risk factors of lymph node metastasis in EGCs that met the current indication of ESD.
Similar to previous studies [22,23,24, 27], the rate of recurrent gastric cancer was significantly higher in the ESD group than in the surgery group. Despite the many advantages of preserving the stomach, the development of recurrent gastric cancer is another main concern related to ESD. The incidence of metachronous cancer in the remnant stomach after partial gastrectomy was reported to be from 0.6 to 3.0% [31,32,33], whereas the incidence of local recurrence after ESD is 0.4–3.7% [34], that of metachronous recurrence after ESD is 2.7–20.9% [35, 36]. The potential risk of distant metastasis after ESD remains because lymph node dissection is not performed in patients undergoing ESD. The incidence of distant metastasis after curative ESD was reported to be extremely rare [35, 36]. However, as reported in 5- or 10-year long-term follow-up data, there were some extragastric recurrences after curative ESD [37]. In our study, three patients in the ESD group [0.25%, (2 of 786)] and three patients in the surgery group [0.25% (3 of 1206)] developed distant metastasis after the initial treatment. Two patients in the ESD group developed recurrent gastric cancer with distant lymph node metastasis at 3 and 4 years after the initial ESD, respectively. Among the three patients with distant metastasis in the surgery group, two patients developed other solid organ (liver, lung) metastasis and one patient developed peritoneal carcinomatosis within 2-3 years after surgery. Because of the possibility of distant metastasis after ESD and surgery, strict surveillance after treatment is needed for EGCs that meet the current indication of ESD.
In the present study, a relatively a large number of patients with undifferentiated-type EGCs in the expanded indication of ESD were analyzed compared with previous studies [20,21,22,23,24]. Although undifferentiated EGCs with intramucosal invasion, and those ≤2 cm in size were included in the expanded indication of ESD [3, 26], controversy remains because of the low curative resection rate together with the difficulty in accurate lesion demarcation and the high risk of lymph node metastasis compared with differentiated-type EGCs [18, 38]. In our study, more patients with undifferentiated EGCs that met the expanded indication of ESD underwent surgery than those who underwent ESD. The undifferentiated EGC lesions in the ESD group were smaller (9.00 ± 5.1 vs. 13.00 ± 5.3, P ≤ 0.001) and had a more elevated type of morphology [36.6% (34 of 93) vs. 0.9% (5 of 564), P ≤ 0.001]. To date, performing ESD for undifferentiated-type EGCs is discreet. However, on the basis of our study, the overall survival rate was not significantly different between the ESD group and the surgery group in undifferentiated EGCs in expanded indication group. Furthermore, it was revealed that the undifferentiated EGCs in the expanded indication were not predictors of overall survival, disease-specific survival, and gastric cancer recurrence on multivariate analysis, compared with the other groups of EGC. Our study shows that ESD is comparable to surgery for undifferentiated EGC that fulfills the expanded indication of ESD.
This study has several limitations. First, it has a single-center, retrospective design and selection bias might be present. However, the ESD and surgery data were prospectively collected at our institute to minimize the chance of bias. Although the lesions were from the same indication group of ESD, the lesions in the surgery group had poorer features and the patients in the ESD group had more comorbidities. These features might affect the choice between ESD and surgery at the time of diagnosis. Second, we excluded patients with EGCs that were beyond the indication of ESD. Patients with EGCs that had lymphovascular invasion, and with beyond the indication of ESD had to underwent additional surgery after ESD. Further studies are needed about the accurate diagnosis of lesions before ESD. Third, the Helicobacter pylori infection status of the enrolled patients was not accurately analyzed in the study because of its retrospective design. H. pylori infection might affect metachronous gastric cancer after ESD [39, 40].
In conclusion, this study showed that ESD is comparable to surgery for EGC that fulfilled the expanded indication of ESD, because of the lower rates of acute complication and comparable overall survival rates. However, a more precise method for deciding between surgery and ESD should be developed, and also a careful and strict surveillance program after ESD is needed, because of the possibility of distant metastasis and the development of metachronous gastric cancer after ESD.
References
Association JGC (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14(2):101–112. doi:10.1007/s10120-011-0041-5
Kitamura K, Yamaguchi T, Taniguchi H, Hagiwara A, Sawai K, Takahashi T (1997) Analysis of lymph node metastasis in early gastric cancer: rationale of limited surgery. J Surg Oncol 64(1):42–47
Association JGC (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14(2):113–123. doi:10.1007/s10120-011-0042-4
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y (2000) Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 3(4):219–225
Gotoda T (2007) Endoscopic resection of early gastric cancer. Gastric Cancer 10(1):1–11. doi:10.1007/s10120-006-0408-1
Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, Kim HJ, Kim JJ, Ji SR, Seol SY (2009) Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 69(7):1228–1235. doi:10.1016/j.gie.2008.09.027
Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S (2009) Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 58(3):331–336. doi:10.1136/gut.2008.165381
Gotoda T, Jung HY (2013) Endoscopic resection (endoscopic mucosal resection/endoscopic submucosal dissection) for early gastric cancer. Dig Endosc 25(Suppl 1):55–63. doi:10.1111/den.12003
Choi JH, Kim ES, Lee YJ, Cho KB, Park KS, Jang BK, Chung WJ, Hwang JS, Ryu SW (2015) Comparison of quality of life and worry of cancer recurrence between endoscopic and surgical treatment for early gastric cancer. Gastrointest Endosc. doi:10.1016/j.gie.2015.01.019
Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, do Kim H, Song HJ, Lee GH, Kim JH, Park YS (2011) Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 74(3):485–493. doi:10.1016/j.gie.2011.04.038
Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, Yoshinaga S, Saito Y (2016) High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 19(1):198–205. doi:10.1007/s10120-015-0469-0
Park CH, Shin S, Park JC, Shin SK, Lee SK, Lee YC, Lee H (2013) Long-term outcome of early gastric cancer after endoscopic submucosal dissection: expanded indication is comparable to absolute indication. Dig Liver Dis 45(8):651–656. doi:10.1016/j.dld.2013.01.014
Oda I, Oyama T, Abe S, Ohnita K, Kosaka T, Hirasawa K, Ishido K, Nakagawa M, Takahashi S (2014) Preliminary results of multicenter questionnaire study on long-term outcomes of curative endoscopic submucosal dissection for early gastric cancer. Dig Endosc 26(2):214–219. doi:10.1111/den.12141
Okada K, Yamamoto Y, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Chino A, Tsuchida T, Fujisaki J, Nakajima A, Hoshino E, Igarashi M (2011) Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc 25(1):98–107. doi:10.1007/s00464-010-1137-4
Iizuka H, Kakizaki S, Sohara N, Onozato Y, Ishihara H, Okamura S, Itoh H, Mori M (2010) Stricture after endoscopic submucosal dissection for early gastric cancers and adenomas. Dig Endosc 22(4):282–288. doi:10.1111/j.1443-1661.2010.01008.x
Oka S, Tanaka S, Higashiyama M, Numata N, Sanomura Y, Yoshida S, Arihiro K, Chayama K (2014) Clinical validity of the expanded criteria for endoscopic resection of undifferentiated-type early gastric cancer based on long-term outcomes. Surg Endosc 28(2):639–647. doi:10.1007/s00464-013-3222-y
Kim JH, Lee YC, Kim H, Song KH, Lee SK, Cheon JH, Kim H, Hyung WJ, Noh SH, Kim CB, Chung JB (2009) Endoscopic resection for undifferentiated early gastric cancer. Gastrointest Endosc 69(4):e1–e9. doi:10.1016/j.gie.2008.10.040
Kim YY, Jeon SW, Kim J, Park JC, Cho KB, Park KS, Kim E, Chung YJ, Kwon JG, Jung JT, Kim EY, Kim KO, Jang B, Lee SH, Yang CH (2013) Endoscopic submucosal dissection for early gastric cancer with undifferentiated histology: could we extend the criteria beyond? Surg Endosc 27(12):4656–4662. doi:10.1007/s00464-013-3099-9
Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, do Kim H, Lee JH, Kim MY, Kim BS, Oh ST, Yook JH, Jang SJ, Yun SC, Kim SO, Kim JH (2011) EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc 73(5):942–948. doi:10.1016/j.gie.2010.12.032
Chiu PW, Teoh AY, To KF, Wong SK, Liu SY, Lam CC, Yung MY, Chan FK, Lau JY, Ng EK (2012) Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc 26(12):3584–3591. doi:10.1007/s00464-012-2371-8
Kim DY, Hong SJ, Cho GS, Jeong GA, Kim HK, Han JP, Lee YN, Ko BM, Lee MS (2014) Long-term efficacy of endoscopic submucosal dissection compared with surgery for early gastric cancer: a retrospective cohort study. Gut Liver 8(5):519–525. doi:10.5009/gnl13061
Cho JH, Cha SW, Kim HG, Lee TH, Cho JY, Ko WJ, Jin SY, Park S (2015) Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc. doi:10.1007/s00464-015-4672-1
Choi IJ, Lee JH, Kim YI, Kim CG, Cho SJ, Lee JY, Ryu KW, Nam BH, Kook MC, Kim YW (2015) Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc 81(2):333–341. doi:10.1016/j.gie.2014.07.047
Kim YI, Kim YW, Choi IJ, Kim CG, Lee JY, Cho SJ, Eom BW, Yoon HM, Ryu KW, Kook MC (2015) Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy 47(4):293–301. doi:10.1055/s-0034-1391284
Washington K (2010) 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 17(12):3077–3079. doi:10.1245/s10434-010-1362-z
Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T (2009) Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 12(3):148–152. doi:10.1007/s10120-009-0515-x
Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, Sohn TS, Bae JM, Kim KM, Ahn JH, Carriere KC, Kim JJ, Kim S (2016) Long-term outcome of endoscopic resection vs. surgery for early gastric cancer: a non-inferiority-matched cohort study. Am J Gastroenterol 111(2):240–249. doi:10.1038/ajg.2015.427
Pyo JH, Shin CM, Lee H, Min BH, Lee JH, Kim SM, Choi MG, Lee JH, Sohn TS, Bae JM, Kim KM, Kim HS, Jung SH, Kim JJ, Kim S (2016) A risk-prediction model based on lymph-node metastasis for incorporation into a treatment algorithm for signet ring cell-type intramucosal gastric cancer. Ann Surg. doi:10.1097/sla.0000000000001602
Takizawa K, Ono H, Yamamoto Y, Katai H, Hori S, Yano T, Umegaki E, Sasaki S, Iizuka T, Kawagoe K, Shimoda T, Muto M, Sasako M (2015) Incidence of lymph node metastasis in intramucosal gastric cancer measuring 30 mm or less, with ulceration; mixed, predominantly differentiated-type histology; and no lymphovascular invasion: a multicenter retrospective study. Gastric Cancer. doi:10.1007/s10120-015-0569-x
Kang HJ, Kim DH, Jeon TY, Lee SH, Shin N, Chae SH, Kim GH, Song GA, Kim DH, Srivastava A, do Park Y, Lauwers GY (2010) Lymph node metastasis from intestinal-type early gastric cancer: experience in a single institution and reassessment of the extended criteria for endoscopic submucosal dissection. Gastrointest Endosc 72(3):508–515. doi:10.1016/j.gie.2010.03.1077
Hosokawa O, Kaizaki Y, Watanabe K, Hattori M, Douden K, Hayashi H, Maeda S (2002) Endoscopic surveillance for gastric remnant cancer after early cancer surgery. Endoscopy 34(6):469–473. doi:10.1055/s-2002-32007
Nozaki I, Hato S, Kobatake T, Ohta K, Kubo Y, Nishimura R, Kurita A (2014) Incidence of metachronous gastric cancer in the remnant stomach after synchronous multiple cancer surgery. Gastric Cancer 17(1):61–66. doi:10.1007/s10120-013-0261-y
Nozaki I, Nasu J, Kubo Y, Tanada M, Nishimura R, Kurita A (2010) Risk factors for metachronous gastric cancer in the remnant stomach after early cancer surgery. World J Surg 34(7):1548–1554. doi:10.1007/s00268-010-0518-0
Park JC, Lee SK, Seo JH, Kim YJ, Chung H, Shin SK, Lee YC (2010) Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience. Surg Endosc 24(11):2842–2849. doi:10.1007/s00464-010-1060-8
Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, Saito D (2006) Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer 9(2):93–98. doi:10.1007/s10120-006-0372-9
Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N, Nishihara A, Nakanishi F, Nakahara M, Ogiyama H, Kinoshita K, Yamada T, Iijima H, Tsujii M, Takehara T (2013) Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut 62(10):1425–1432. doi:10.1136/gutjnl-2011-301647
Min BH, Kim ER, Kim KM, Park CK, Lee JH, Rhee PL, Kim JJ (2015) Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy 47(9):784–793. doi:10.1055/s-0034-1392249
Horiuchi Y, Fujisaki J, Yamamoto N, Shimizu T, Miyamoto Y, Tomida H, Omae M, Ishiyama A, Yoshio T, Hirasawa T, Yamamoto Y, Tsuchida T, Igarashi M, Takahashi H (2015) Accuracy of diagnostic demarcation of undifferentiated-type early gastric cancers for magnifying endoscopy with narrow-band imaging: endoscopic submucosal dissection cases. Gastric Cancer. doi:10.1007/s10120-015-0488-x
da Jung H, Kim JH, Chung HS, Park JC, Shin SK, Lee SK, Lee YC (2015) Helicobacter pylori eradication on the prevention of metachronous lesions after endoscopic resection of gastric neoplasm: a meta-analysis. PLoS ONE 10(4):e0124725. doi:10.1371/journal.pone.0124725
Mitsunaga A, Tagata T, Hamano T, Teramoto H, Kan M, Mitsunaga Y, Tobari M, Shirato I, Shirato M, Yoshida S, Shimada M, Nishino T (2014) Metachronous early gastric cancer over a period of 13 years after eradication of Helicobacter pylori. Clin J Gastroenterol 7(6):490–495. doi:10.1007/s12328-014-0536-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors (Hahn KY, Park CH, Lee YK, Chung H, Park JC, Shin SK, Lee YC, Kim HI, Cheong JH, Hyung WJ, Noh SH, Lee SK) declare that they have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Hahn, K.Y., Park, C.H., Lee, Y.K. et al. Comparative study between endoscopic submucosal dissection and surgery in patients with early gastric cancer. Surg Endosc 32, 73–86 (2018). https://doi.org/10.1007/s00464-017-5640-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5640-8