Abstract
Background
Endoscopic resection is widely accepted as the primary treatment for early gastric cancer (EGC) without lymph node metastasis. A new and refined technique, endoscopic submucosal dissection (ESD), may prove to be more effective; however, incomplete resection and local recurrence present ongoing concerns. We sought to determine the clinicopathological features associated with local recurrence in patients with EGC following endoscopic resection.
Methods
We enrolled in this study 239 EGC patients treated by endoscopic resection between January 2002 and January 2008.
Results
Fifty EGC lesions were treated by conventional endoscopic mucosal resection (EMR group) and 189 EGC lesions were treated by ESD (ESD group). During the follow-up period (mean = 30.3 months), the rates for en bloc resection and complete resection (defined as en bloc resection with negative resection margin) were 64% (32/50) and 60% (30/50), respectively, in the EMR group, and 86.8% (164/189) and 79.9% (151/189), respectively, in the ESD group. We observed seven local recurrences in the ESD group, though only one with complete resection by ESD had a local recurrence. The EMR group showed a significantly higher recurrence rate than did the ESD group (18% vs. 3.7%, respectively, p < 0.001). Incomplete resection significantly increased local recurrence risk, and larger tumor size and use of EMR increased the risk for incomplete resection. Most lesions (3/4) treated with additional argon plasma coagulation for an initial recurrence had recurred again.
Conclusions
Despite the potential advantages in treating EGC with ESD, a risk for local recurrence remains. All patients treated with EMR, even with curative resection, and those with incomplete resection after ESD require conscientious surveillance for local recurrence. Furthermore, a large prospective study will be required to determine the best treatment modality for local recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer presents a common malignancy and cause of cancer-related death in many countries. Advances in diagnostic technology and the increasing prevalence of screening programs have increased the rate of early gastric cancer (EGC) detection. EGC is defined as a neoplasm confined to the mucosa or submucosa, regardless of regional lymph node metastasis [1]. Currently, EGC without evidence of nodal metastasis is first treated by endoscopic resection, either endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). This method is widely accepted because it is less invasive and less costly and requires a shorter hospital stay than surgical resection. Patients who undergo endoscopic resection have an excellent prognosis, with a 5-year survival rate of over 90% [2–4]. The conventional technique, EMR, however, may fail to remove the whole lesion, especially one that is large, which must then be removed in pieces. In this fragmented specimen, critical histological features, including depth of invasion, resection margin, lymphovascular involvement, and extent of resection, may be obscured. Piecemeal resection also results in a higher local recurrence rate (range = 2–35%) [5–9] than that of complete resection. In fact, of the lesions smaller than 20 mm, EMR may achieve en bloc resection of only 76%. In this respect, ESD shows distinct advantages. ESD may achieve the complete resection, not only of larger lesions but also of ulcerated lesions, regardless of the location. Thus, ESD allows for a precise histological assessment of resected specimens and may reduce the risk for residual disease and local recurrence [10–12].
Nevertheless, as with EMR, local recurrence remains a prominent concern with ESD. Few studies to date have fully evaluated long-term clinical outcomes regarding local recurrences after endoscopic resection or the risk factors for local recurrence following use of either ESD procedure. This information will be useful for treatment planning as well as to aid in the detection of high-risk groups. We therefore conducted this study to evaluate long-term outcome of endoscopic resection and clinicopathological features of patients with local recurrence of EGC after endoscopic resection.
Patients and methods
From January 2002 to January 2008, 422 patients with 427 early gastric cancers were treated by endoscopic resection at Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. A diagnosis of EGC was based on findings from endoscopy, endoscopic ultrasonography, computed tomography, and histopathological examination of biopsy specimens.
Standard indications for EMR included (1) differentiated adenocarcinoma, (2) intramucosal cancer, (3) lesion smaller than 20 mm, and (4) no endoscopic evidence of ulceration [13]. On the other hand, ESD was performed for EGC patients who met the following extended criteria [14]: (1) differentiated adenocarcinoma (well- to moderately differentiated tubular adenocarcinoma and papillary adenocarcinoma) confirmed histologically by biopsy, (2) depth of invasion limited to the mucosa or sm1 (≤500 μm penetration into the submucosa) as determined by endoscopic ultrasound (EUS), (3) lesions without ulceration, regardless of size, or 30 mm or less in size with ulceration, or sm1 invasion.
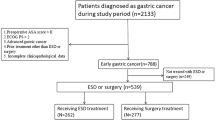
We excluded from the study those patients with follow-up periods of less than 1 year, patients lost to follow-up, and those who underwent gastrectomy after endoscopic treatment due to complications or incomplete resection (n = 111). We excluded an additional 77 patients treated with ESD because their characteristics exceeded those of the “extended criteria” (Fig. 1). Ultimately, 239 cases were included.
Endoscopic resection methods
All patients were hospitalized. The endoscopic resection procedure was performed with the patient under sedation with midazolam hydrochloride plus Demerol, administered intravenously just before the procedure.
After endoscopic evaluation of gastric lesions with indigocarmine spraying, the surrounding lesion was marked by electrocautery (ICC 200 or VIO 300D; ERBE, Tübingen, Germany) using a needle knife (Olympus, Tokyo, Japan).
For EMRL [15] and EMRC [16], the lesion was elevated by an injection of epinephrine-containing hypertonic saline solution. Thereafter, the lesion was aspirated, tightened using a ligating device (EMRL) or a cap-fitted panendoscope (EMRC), and resected. The EMRP [17] technique was circumferential precutting followed by snare resection.
For ESD, after confirming the lesion, the border between the lesion and normal mucosa was electrosurgically marked approximately 1-2 mm from the lesion. Marking facilitated the determination of resection completeness and the orientation of resected specimens [2, 18]. After fully marking the border, an epinephrine-containing hypertonic saline solution was injected into the submucosa to create a submucosal cushion. The lesion was incised using a needle knife along the outer border of the marked lesion. The lesion was then dissected with an insulation-tipped (IT) diathermic knife (Olympus) and electrosurgical units.
Histological evaluation and assessment of resection efficacy
The histopathological diagnoses were based on the Japanese Classification of Gastric Carcinoma [1]. Resected specimens were systematically sectioned at 2-mm intervals, centered on the part of the lesion closest to the margin and the site of deepest invasion. Lesions that remained within the mucosa were classified as mucosal cancers, and those invading the submucosa as submucosal cancers. The submucosal layer was categorized into three layers: sm1, upper third; sm2, middle third; and sm3, lower third.
Resection was judged to be complete if the tumor was removed en bloc and the horizontal and vertical margins were histologically free of malignant cells and lymph-vascular infiltration. Tumors resected in multiple fragments or tumors with histologically confirmed positive resection margins were defined as incomplete resections, even with an endoscopic confirmation of complete removal.
If gross examination after endoscopic resection suggested a high probability of residual tumor at the resection site, argon plasma coagulation was applied at the residual resection margin at the operator’s discretion.
Follow-up examinations and local recurrence
After resection, an upper GI endoscopic examination was conducted at 2–4-month intervals within the first year and annually thereafter. Biopsy specimens were taken during each procedure to detect the presence of a local recurrence. Patients with local recurrence underwent further treatments, which included repeat ESD, argon plasma coagulation (APC), and gastrectomy.
Statistical analyses
To analyze cumulative local recurrences we used the Kaplan–Meier method, with a log-rank test for the initial comparison. Multivariate analysis was performed using univariate predictors, entered stepwise into a multivariate Cox proportional hazard model (forward selection). Factors affecting incomplete resection and technical components in local recurrence in patients with incomplete resection were analyzed using logistic regression. Odds ratios (OR) and 95% CI (confidence intervals) were calculated to estimate the relative risks of incomplete resection.
Results
Patient characteristics and clinical outcomes of endoscopic resection
Of the 422 patients with 427 early gastric cancers treated by endoscopy, 239 were deemed eligible for this study (Fig. 1). The mean age of the patients was 63.05 ± 9.07 years, and the mean tumor size was 13.92 ± 8.73 mm (EMR: 11.5 ± 3.68 mm, ESD: 14.56 ± 9.54 mm) (Table 1). Fifty lesions were treated with EMR and 189 lesions with ESD. Rates of en bloc resection and complete resection for the EMR group were significantly lower than for the ESD group (64% vs. 86.8%, and 60% vs. 79.9%, respectively) (Table 2).
Local recurrence after endoscopic resection
During the follow-up period (mean = 30.3 months, range = 12–79.7 months), we observed nine (18%) and seven (3.7%) local recurrences in the EMR (n = 50) and ESD (n = 189) groups, respectively. In overall subjects, 181 early gastric cancers were completely resected and 7 of these experienced local recurrences. Among patients with complete resection using ESD, only one patient (0.66%) experienced local recurrence; however, among those who achieved complete resection in the EMR group, local recurrences affected six patients.
Factors without measurable effect on local recurrence included gender, treatment year, tumor size, endoscopic finding of EGC, and presence of ulcer (Table 3) . En bloc resection and complete resection (defined as en bloc resection with negative resection margin), however, significantly decreased local recurrence rate. In addition, treatment of EGC by ESD produced a significantly lower cumulative recurrence rate than did treatment by EMR (Fig. 2). On multivariate analysis, incomplete resection (p = 0.013, hazards ratio [HR] = 5.592) and EMR (p = 0.009, HR = 4.005) were proved to be significantly relevant to local recurrence (Table 3).
Factors affecting incomplete resection
Assuming the critical importance of complete resection as a determinant of local recurrence, we further analyzed factors that determine complete resection (Table 4). Larger tumor size (p = 0.048), and EMR method (p = 0.005) increased incomplete resection significantly, which was confirmed in multivariate analysis with an odds ratio (OR) = 2.65 (p = 0.004) and OR = 3.52 (p = 0.001), respectively. We also tested technical components of the procedure for their effects on local recurrence in the incomplete resection group, but found none (Table 5).
Clinical course after local recurrence
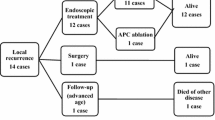
In the EMR and ESD groups combined, 16 patients experienced local recurrence, and all 16 received additional treatment. Eight patients underwent gastrectomy, four patients repeated ESD, and four patients were treated with APC. In surgical resection, one lesion was identified as submucosal (sm2) cancer without lymph node metastasis and the other seven as mucosal cancer without lymph node metastasis. One repeated endoscopic resection revealed a submucosal (sm1) cancer. There were no additional recurrences in the 12 patients who were treated with gastrectomy and repeated ESD during follow-up. However, all three of the four patients whose initial recurrence was treated with additional APC had another recurrence at the same location within 2 years after APC (9.3, 12.8, and 17.9 months, respectively). In addition, six patients developed metachronous cancers after initial endoscopic resection; four underwent gastrectomy and two were treated with ESD (Fig. 1). After treatment, there was no recurrence or newly developed lesion at the last follow-up.
Discussion
Planning for extended treatment of EGC and follow-up of patients at high risk for recurrence requires knowledge of the major determinants of treatment outcome. In this study we showed the long-term clinical outcome of EGC following endoscopic resection. The ESD technique with complete resection significantly reduced the risk of local recurrence. With EMR, however, a significant risk for recurrence remains, even with curative resection.
EMR was first introduced as a treatment for EGC in 1984, and variations of EMR, including EMR-ligation (EMRL), EMR-cap (EMRC), and EMR-strip biopsy, have since been developed [15, 16, 18, 19]. However, none of these conventional EMR techniques can adequately resect lesions greater than 20 mm in diameter [13]. EMR can resect larger lesions in multiple fragments, but this piecemeal removal may compromise the accuracy of histological analyses and the technical assessment of treatment and increase the risk for incomplete resection [2, 20]. In this retrospective study, various techniques of EMR were used. EMRP and EMRL were the most commonly used techniques for lesions 15 mm or smaller. The less commonly used technique was EMRC.
ESD was developed to facilitate en bloc resection, regardless of tumor size [2, 10, 12, 21]; however, ESD does not ensure complete resection or exclude the possibility of local recurrence.
Few studies to date have specifically addressed long-term clinical outcomes regarding local recurrences after endoscopic resection or clinical and pathological predictors of local recurrence following the use of either ESD procedure. Our study confirmed the superiority of ESD over EMR, as determined by en bloc resection rates (86.8% vs. 64%, respectively) and complete resection rates (79.9% vs. 60%, respectively). These parameters of performance for ESD continue to improve, with recent reports of en bloc and complete resection rates near 90% [22, 23], in contrast to previous reports [24, 25]. Our relatively low rates may stem from the involvement of six different endoscopists in the treatment of patients with EGC at our institution. Although all were highly trained specialists, their different preferences for devices and methods might have affected clinical outcome. Also, we enrolled all EGC patients from the beginning of EMR or ESD from 2002 to 2008. Most ESD techniques were performed after 2005.
Ryu et al. [8] reported recurrence rates of 9.6 and 3.5% in patients treated with conventional EMR and ESD, respectively, for gastric adenoma or EGC. Recent Japanese studies found local recurrence rates of 10-15% after incomplete resection by ESD, but 0-0.2% in patients with complete resection [22, 26]. We observed local recurrence in 18% of patients following EMR, 3.7% after ESD, and only one (0.66%) after complete resection by ESD. There were still local recurrences after complete resection. It is possible that remnant malignant cells might remain on the “negative” resection margin since the coagulated portion of the resection margin could not be assessed accurately.
We identified curability and endoscopic resection technique as predictors for local recurrence, i.e., incomplete resection and use of EMR, even with complete resection, significantly increased the cumulative local recurrence rate. We may therefore assume that complete resection and use of ESD critically influence long-term, disease-free survival.
In the EMR group, there were more local recurrences in the complete resection group (6/30, 20%) than in the incomplete resection group (3/20, 15%) by simple numeric comparison, where time was not taken into consideration. However, since local recurrence was a time-dependent variable, the univariate Kaplan–Meier method and multivariate Cox proportional hazard model proved that complete resection had a lower local recurrence rate. Therefore, it is hard to conclude that the rate of local recurrence was really higher with complete resection than with incomplete resection in the EMR group.
In subsequent analysis, we identified tumor size and method of endoscopic resection as significant determinants of incomplete resection (Table 4). Takenaka et al. [26] reported tumor size and tumor location as independent risk factors for local recurrence. However, we found these factors to be more closely associated with complete resection than with local recurrence. This discrepancy between the two studies may be most likely due to direct correlation of tumor size and location with complete resectability. Therefore, we suggest that complete resection has more influence on local recurrences than do tumor size and location. Based upon the results of current study, we think that once the tumor is completely resected using ESD, the possibility of local recurrence might drop substantially and routine periodic follow-up might be sufficient. However, in selecting a candidate for endoscopic resection, the endoscopist should consider the feasibility of ESD in terms of complete resectability according to tumor size and endoscopic method in order to provide a longer disease-free survival for the patient. We also analyzed the clinical profiles of patients with incomplete resection (Table 5). The role of argon plasma coagulation (APC) in the extended treatment of incompletely resected EGC has not been fully evaluated [27–30], although it is reported to be effective. In the present study, immediate additional APC at the residual resection margin which suggested a high probability of residual tumor did not affect the local recurrence rate in patients with incomplete resection. However, most lesions treated with additional APC for an initial recurrence had recurred again at the same location. Therefore, physicians should be cautious about performing additional APC for recurring cancer lesions after endoscopic resection. A large prospective study will be required to determine whether and how APC should be used to treat EGC in the future.
Eight patients with local recurrence underwent surgical resection, while four were treated by further endoscopic resection. Among them, tumor invasion to the sm2 layer was found in one surgical specimen, and invasion to the submucosal layer was observed in one endoscopic resection specimen. These results indicate that care is required when deciding to perform surgery or repeated EMR/ESD after local recurrence, as lesions invading beyond the mucosal layer require additional treatments when resected by endoscopy. Furthermore, fibrotic mucosal change might impede the performance of a second endoscopic resection, since severe fibrosis and artificial ulcer scarring may develop around the recurrent lesion after the first resection. Chung et al. [31] reported a significantly lower en bloc resection rate for scar-positive lesions than for scar-negative lesions, probably because of the difficulty in lifting, by submucosal injection, the fibrotic lesion at the recurrence site and assessing histologically the depth of the resected specimen.
The retrospective design and confinement to a single institution limited the scope of this study. In addition, the six different endoscopists involved may possibly have developed preferences in their selection of knives and modes of electrocoagulation, thus affecting clinical outcomes. A prospective, multicenter study is necessary to confirm our results.
In conclusion, incomplete resection and use of EMR significantly increased local recurrence, while larger tumor size and use of EMR predicted incomplete resection. En bloc resection with a negative margin may be essential to achieve an outcome comparable to surgical resection. Therefore, patients treated with EMR, even those with curative resection, and patients with incomplete resection after ESD require vigilant follow-up. Furthermore, a large prospective study will be required to determine the best treatment modality for local recurrence.
References
Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma, 2nd English edition. Gastric Cancer 1:10–24
Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S (2001) Endoscopic mucosal resection for treatment of early gastric cancer. Gut 48:225–229
Okamura T, Tsujitani S, Korenaga D, Haraguchi M, Baba H, Hiramoto Y, Sugimachi K (1988) Lymphadenectomy for cure in patients with early gastric cancer and lymph node metastasis. Am J Surg 155:476–480
Sue-Ling HM, Martin I, Griffith J, Ward DC, Quirke P, Dixon MF, Axon AT, McMahon MJ, Johnston D (1992) Early gastric cancer: 46 cases treated in one surgical department. Gut 33:1318–1322
Noda M, Kodama T, Atsumi M, Nakajima M, Sawai N, Kashima K, Pignatelli M (1997) Possibilities and limitations of endoscopic resection for early gastric cancer. Endoscopy 29:361–365
Watanabe K, Ogata S, Kawazoe S, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M, Tsunada S, Iwakiri R, Fujimoto K (2006) Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc 63:776–782
Kojima T, Parra-Blanco A, Takahashi H, Fujita R (1998) Outcome of endoscopic mucosal resection for early gastric cancer: review of the Japanese literature. Gastrointest Endosc 48:550–554 discussion 554
Ryu CB, Kim SG, Jung SW, Jung IS, Kwon KW JHS, Jin SY, Cho JY, Lee JS, Lee MS, Shim CS, Kim BS (2005) The usefulness and limitation of endoscopic mucosal resection using the incision and submucosa dissection methods (EISD) for early gastric cancer and gastric flat adenoma in Korea. Gastrointest Endosc 61:AB238
Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K (2006) Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 64:877–883
Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, Yokota T (1999) A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc 50:560–563
Yokoi C, Gotoda T, Hamanaka H, Oda I (2006) Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc 64:212–218
Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K, Sugano K (2003) Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy 35:690–694
Nakajima T (2002) Gastric cancer treatment guidelines in Japan. Gastric Cancer 5:1–5
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y (2000) Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 3:219–225
Suzuki Y, Hiraishi H, Kanke K, Watanabe H, Ueno N, Ishida M, Masuyama H, Terano A (1999) Treatment of gastric tumors by endoscopic mucosal resection with a ligating device. Gastrointest Endosc 49:192–199
Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M (1993) Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc 39:58–62
Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S (2001) New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy 33:221–226
Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N (1988) Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc 34:264–269
Inoue H, Noguchi O, Saito N, Takeshita K, Endo M (1994) Endoscopic mucosectomy for early cancer using a pre-looped plastic cap. Gastrointest Endosc 40:263–264
Miyamoto S, Muto M, Hamamoto Y, Boku N, Ohtsu A, Baba S, Yoshida M, Ohkuwa M, Hosokawa K, Tajiri H, Yoshida S (2002) A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc 55:576–581
Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H (2005) Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 17:54–56
Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S (2009) Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 58:331–336
Goto O, Fujishiro M, Kodashima S, Ono S, Omata M (2009) Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy 41:118–122
Jeong G, Lee JH, Yu MK, Moon W, Rhee PL, Paik SW, Rhee JC, Kim JJ (2006) Non-surgical management of microperforation induced by EMR of the stomach. Dig Liver Dis 38:605–608
Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y, Hamada T, Inoue H, Gotoda T, Yoshida S (2006) A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer 9:262–270
Takenaka R, Kawahara Y, Okada H, Hori K, Inoue M, Kawano S, Tanioka D, Tsuzuki T, Yagi S, Kato J, Uemura M, Ohara N, Yoshino T, Imagawa A, Fujiki S, Takata R, Yamamoto K (2008) Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest Endosc 68:887–894
Sagawa T, Takayama T, Oku T, Hayashi T, Ota H, Okamoto T, Muramatsu H, Katsuki S, Sato Y, Kato J, Niitsu Y (2003) Argon plasma coagulation for successful treatment of early gastric cancer with intramucosal invasion. Gut 52:334–339
Kitamura T, Tanabe S, Koizumi W, Mitomi H, Saigenji K (2006) Argon plasma coagulation for early gastric cancer: technique and outcome. Gastrointest Endosc 63:48–54
Ogata M, Maejima K, Chihara N, Mizutani S, Komine O, Bo H, Shioya T, Watanabe M, Tokunaga A, Tajiri T (2007) Successful use of endoscopic argon plasma coagulation for patients with early gastric cancer and diabetes mellitus. J Nippon Med Sch 74:246–250
Akhtar K, Byrne JP, Bancewicz J, Attwood SE (2000) Argon beam plasma coagulation in the management of cancers of the esophagus and stomach. Surg Endosc 14:1127–1130
Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, Kim HJ, Kim JJ, Ji SR, Seol SY (2009) Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 69:1228–1235
Acknowledgments
The authors thank the members of Biostatistics and Dr. Beom Kyung Kim for statistical assistance.
Disclosures
Jun Chul Park, Sang Kil Lee, Ju Hee Seo, Yu Jin Kim, Hyunsoo Chung, Sung Kwan Shin, and Yong Chan Lee have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J.C., Lee, S.K., Seo, J.H. et al. Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience. Surg Endosc 24, 2842–2849 (2010). https://doi.org/10.1007/s00464-010-1060-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-1060-8