Abstract
Background
Doxycycline, a nonspecific metalloproteinase (MMP) inhibitor, has been demonstrated to impact the strength of the polypropylene (PP) mesh-repaired hernia with an increase in the deposition of collagen type 1. The impact of doxycycline with porcine acellular dermal matrices (PADM) is unknown; therefore, we evaluated the impact of doxycycline administration upon hernia repair with PP and PADM mesh.
Methods
Sprague–Dawley rats weighing ~400 g underwent laparotomy with creation of a midline ventral hernia. After a 27-day recovery, animals were randomly assigned to four groups of eight and underwent intraperitoneal underlay hernia repair with either PP or PADM. Groups were assigned to daily normal saline (S) or daily doxycycline in normal saline 10 mg/kg (D) via oral gavage for 8 weeks beginning 24 h preoperatively. Animals were euthanized at 8 weeks and underwent tensiometric testing of the abdominal wall and western blot analyses for collagen subtypes and MMPs.
Results
Thirty-two animals underwent successful hernia creation and repair with either PADM or PP. At 8 weeks, 15 of 16 PP-implanted animals survived with only 12 of 16 PADM-implanted animals surviving. There were no differences in the mesh to fascial interface tensiometric strength between groups. Densitometric counts in the PADM-D group demonstrated increased collagen type 1 compared to PP-S (PADM-D [1286.5], PADM-S [906.9], PP-S [700.4], p = 0.037) and decreased collagen type 3 compared to PP-S (PADM-D [7446.9], PADM-S [8507.6], PP-S [11,297.1], p = 0.01). MMP-9 levels were increased in PADM-D (PP-S vs. PADM-D, p = 0.04), while MMP-2 levels were similar between PADM-D and PADM-S, respectively.
Conclusions
Collagen type 1 deposition at the mesh to fascial interface is enhanced following administration of doxycycline in ventral hernia repairs with porcine acellular dermal matrices. Doxycycline administration may have implications for enhancing hernia repair outcomes using biologic mesh.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of an incisional hernia is a predominant complication associated with laparotomies [1–3]. Of approximately 2 million laparotomies performed in the USA annually, 2 to 20 % will develop an incisional ventral hernia [4–9]. As the hernia progresses, patients may experience pain or obstruction leading to lost productivity and decreased quality of life [10]. Medical comorbidities, including diabetes and smoking, can lead to wound dehiscence, infection, and complications following laparotomy [11–13]. Of the patients who choose to undergo incisional hernia repair, the recurrence rates range widely from 10 to 50 % with significant morbidity and mortality [5, 14–17]. Multiple studies have reported a connection between the occurrence of incisional hernias and abnormal collagen metabolism and extracellular matrix diseases [18, 19].
The main structural component of the extracellular matrix (ECM) is made of mainly two types of collagen, types 1 and 3. For a stronger ECM, a higher ratio of mature collagen, type 1, to less mature collagen, type 3, is needed [20]. The ratios of collagen subtypes are influenced by the presence of a type of endopeptidase enzyme called matrix metalloproteinase (MMP) [21]. A lower level of MMPs has been shown to be beneficial in increasing the collagen type 1:3 ratios at the wound-healing site and thereby promoting the structural integrity of the ECM at the healing site. Collagenases MMP-2 and MMP-9 have the ability to cleave collagen once activated; MMP-2 specifically degrades type 1 collagen in the early stages of wound healing [22].
Doxycycline is a semisynthetic, chemically modified tetracycline widely used for its antibacterial properties; however, by scavenging for reactive oxygen species (ROS), doxycycline also inhibits the conversion of pro-MMPs to active MMPs [23, 24]. Previous studies from our laboratory have reported that doxycycline increased the strength of repaired fascia in a rat incisional hernia repair model using a synthetic polypropylene (PP) mesh [25–27]. In these studies, repaired fascia in animals treated with oral doxycycline had increased levels of type 1 collagen (thereby raising type 1:3 ratios), greater tensile strength, and reduced levels of MMP-2, MMP-9, or MMP-3. Levels of MMP-2 were reduced as early as 4 weeks posttreatment, therefore reducing the degradation of type 1 collagen. Additional dose-related trials were performed and published in 2015, revealing a medium dosage of doxycycline (10 mg/kg) is as effective as high dose (30 mg/kg) in producing a greater than 47 % increase in mesh–fascial interface (MFI) strength compared to saline controls [27].
Prior studies were conducted using the synthetic monofilament polypropylene mesh (Davol Inc., Warwick, RI); however, due to the risk of infection or other adverse reactions with synthetic mesh, alternative biologic options have been developed. There are no prior publications investigating the use of a non-crosslinked biologic mesh material in the setting of doxycycline administration. We hypothesized that the biocompatibility of the porcine acellular dermal matrix (PADM, XenMatrix, Davol Inc., Warwick, RI) from which the biological mesh was derived would enhance abdominal wall fascia in terms of strength and durability.
Materials and methods
A randomized controlled prospective trial was designed to evaluate the effects of doxycycline. The animal experimental procedures used in this study were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Kentucky. Thirty-two Sprague–Dawley Rats weighing approximately 400 g each were obtained from Harlan Laboratories, (Indianapolis, IN) and acclimatized for 5 days before undergoing hernia creation surgery. 27 days after the hernia creation surgery, they were randomly divided into two groups of 16; one group received daily doxycycline, and the other received daily normal saline by gavage.
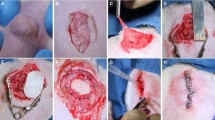
On the 28th day following hernia creation, rats were further allocated into repair arms using either a polypropylene (PP) mesh (n = 16, 8 doxycycline, 8 saline) or a PADM mesh (n = 16, 8 doxycycline, 8 saline) (Fig. 1). During both the hernia creation and subsequent mesh repair operations, the animals were maintained on a 4 % inhaled isoflurane, and their abdominal walls were clipped and prepped with 70 % chlorhexidine. Pain control after each operation was achieved by a subcutaneous buprenorphine injection (0.03 mg/100g).
Animal procedures
The hernia creation and repair protocols have been detailed in prior publications from our laboratory [25–27]. All rats underwent a hernia creation operation. Under anesthesia, after prepping the ventral midline area, a 1-cm transverse incision was made at the level of xyphoid on the skin, and a full thickness skin flap was made at the avascular prefascial plane above linea alba. Under direct view, a 4-cm incision was made along the linea alba to create a full thickness laparotomy. Then the transverse skin incision was closed in two layers, the inner layer using a 4-0 PGA suture and outer layer using either a monofilament nylon suture or wound clips. An antibiotic ointment was applied on the area, and the rats were monitored until ambulant and then for several days for possible complications. All 32 animals developed a pronounced bulging hernia immediately after the procedure. After 27 days from surgery, all animals were started on either a 10 mg/kg doxycycline or normal saline daily gavage depending on their treatment group until the end of the experiment.
On day 28, all animals underwent a second operation: a hernia repair surgery using one of the two meshes described above. The previous transverse surgical site was clipped and prepped, and a 5-cm incision on the skin was made on the mid-ventral line starting below the xyphoid and extending caudally. A subcutaneous flap was raised 2 cm around the incision. The linea alba was incised 4 cm in length including the defect area to expose peritoneum. From both sides of this midline incision, 0.5-cm fascia was excised to enlarge the defect. A 5 × 5 cm mesh was cut, and the corners smoothed out to an oval shape. The mesh was implanted on the defect as a bridging underlay with a 1-cm overlap and secured using eight interrupted 4-0 PGA sutures around the edges of the mesh. The subcutaneous flaps were brought together and approximated using buried deep dermal sutures with a 4-0 PGA suture. Wound clips were applied to approximate the epidermis. The animals were revived, monitored until ambulant, and maintained on analgesics for 48 h. They continued receiving doxycycline or saline daily for 8 weeks.
Following the 8th week, the animals were euthanized and the abdominal wall was harvested. A 1.5 × 8 cm strip of the anterior abdominal wall with the mesh en bloc was isolated to conduct a tesiometric analysis on an Instron tensiometer. Remaining tissue was processed for pathological and biochemical assays.
Western blotting
All MMP-2, MMP-9, and collagen types 1 and 3 antibodies used in this study were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The blotting procedure was performed as previously described [19]. A lysis buffer containing detergents and protease inhibitors were prepared in phosphate-buffered saline and added to frozen tissue samples (1 part tissue + 4 parts buffer), then homogenized and centrifuged at 10,000g (10 min) followed by 100,000g (20 min). Supernatants were aliquoted and stored at −80 oC. One aliquot was used for protein assay using the BCA protocol (Thermo Fisher Scientific Inc., Rockford, IL USA). The samples were denatured at 95 oC in 2 × gel loading buffer, and 30 micrograms of protein from each sample was electrophoresed on a mini gel containing 4 % stacking and 10 % separating gel at 150 V for 1.5 h and then blotted onto PVDF membranes (Biorad Laboratories, Inc, Hercules, CA, USA) at 100 V for 1 h. The membranes were then washed and blocked in 5 % non-fat milk in wash buffer for 1 h. After an overnight 4 oC incubation with primary antibody and a 1-h incubation with secondary antibody, the membranes were washed three times in washing buffer and the bound antibodies were visualized using a chemiluminescent detection system (Pierce, Rockford, IL, USA). The images were quantified using Image J software (NIH, Bethesda, MD, USA) and expressed as arbitrary relative densitometry units.
Tensiometric measurements
Tensiometric measurements for abdominal wall strips were conducted on an Instron E3000 tensiometer (Instron Corp, Canton, MA, USA) that was equipped with a 250 Newton load cell. All tensiometric tests were completed within 6 h of harvesting the tissue. The harvested tissue representing the mesh–fascia interface (MFI) was shaped into 1.5 cm × 8 cm strips and stored in normal saline kept at 4 oC until testing. Before testing, the tissue strips were brought to room temperature and loaded into the pneumatic grips of the Instron. The grips were preloaded with 1 N tension; the tissue strips were deformed at 10 mm/min until complete failure of the strip occurred [28].
Masons trichrome
The tissue pieces from the abdominal wall with the implanted mesh were fixed in 10 % buffered formalin, processed into cut 4 µm sections then mounted on glass slides. After deparaffinizing, they were treated in Bouin’s solution in the microwave for 15 s and let stand for 2 more minutes. After washing, the slides were treated with Weigert’s hematoxylin for 10 min. Slides were then washed in water after confirming the nuclei are stained blue black. The slides were treated with Biebrich Scarlet solution for 3 min, then washed in distilled water and treated with 2 % phosphotungstic solution for 10 min. After an additional rinse in distilled water, slides were stained in 2 % light green solution for 3 min. They were then re-rinsed in distilled water, dipped in 1 % acetic acid, dehydrated through ascending grades of alcohol, cleared in xylene, and mounted in synthetic resin for microscopic analysis as immunoblots.
Statistical analysis
The western blotting quantitation and tensiometric analysis data from each group were analyzed by ANOVA. The outcomes were considered significant when the p value was <0.05.
Results
All 32 animals underwent successful hernia creation (Fig. 2A) and repair (Fig. 2B) with either PADM or PP. At 8 weeks, 15 of 16 PP-implanted animals survived with only 12 of 16 PADM-implanted animals surviving (Fig. 1). Two additional PADM-S animals showed a degraded mesh material at the time of abdominal wall harvest, which was encapsulated; therefore, they were excluded from tensiometric analysis.
Both PADM groups exhibited a slightly higher level of tensiometric strength compared to PP counterparts, but there were no statistically significant differences in the tensiometric strengths of the mesh to fascial interface (Fig. 3).
PADM animals treated with doxycycline demonstrated increased densitometric counts indicating collagen type 1 deposition (PADM-D 1286.5 vs. PP-S 700.4, p = 0.037) (Fig. 4). Collagen type 3 levels were decreased in both groups using PADM mesh; however, animals treated with doxycycline had a greater reduction in collagen type 3 levels (PADM-D 7446.9, PADM-S 8507.6 vs. PP-S 11,297.1, p = 0.01) (Fig. 5). Increased collagen type 1 in the setting of decreased collagen type 3 leads to a greater ratio and strength; however, as stated above, this increase did not translate to a statistically significant increase in overall tensiometric values. MMP-2 and MMP-9 levels were both increased in PADM animals treated with doxycycline; however, only MMP-9 was statistically significant (PADM-D 5600 vs. PP-S 3495, p = 0.04) (Figs. 6 and 7). The Trichrome staining analysis showed more compactly arranged fibrils in the PADM-D group, whereas the fibrils were loosely arranged in the control group (Fig. 8A, B).
Discussion
In our previous studies we have shown an increased tensile strength of fascia repaired with PP mesh in animals treated with doxycycline and a correlation to an increased collagen type 1:3 ratio. Previous studies indicated collagen type 1 was increased in doxycycline-treated animals, which might have contributed to the increased strength of the repaired fascia in those doxycycline-treated animals [24–26]. In the present study, our goal was to analyze the effect of same dose doxycycline on the PADM-implanted fascia in a rat model of hernia repair. Our biologic mesh group (PADM) showed an increased basal level of MMP-2 and MMP-9 when compared to the PP control group although it was not significant. After doxycycline administration, these MMP levels were found to be higher in all PADM animals. Higher MMP levels may have been a response to the immunogenicity of the xenograft itself as both PADM groups, independent of doxycycline, had elevated levels. Debris from de-cellularization process of the xenograft can react with the host immune system to induce pro-inflammatory cytokines, thus elevating MMP levels; this response can result in graft rejection [29]. Multiple studies have tested the remodeling effects of non-crosslinked acellular dermal matrices such as the type used in this study [30, 31]. A study by Sun et al. reported IgG levels elevated by 100-fold in primate studies for the entire 6-month experiment period, indicating a sustained humoral response [30]. We can attribute the encapsulated degraded mesh found in two of the saline control PADM animals as proof of a host inflammatory response to the mesh itself. Tensiometric analysis in these two animals could not be performed due to the missing mesh fascia interface, which resulted from mesh encapsulation. The inability to include these animals in analysis may have contributed to a type 2 error with dilution of possible statistical significance.
The basic function of a prosthetic mesh is to give structural support and a regenerative framework by supporting matrix remodeling through collagen deposition and also acts as a scaffold for tissue reconstruction on the affected area [32]. The architecture of XenMatrix differs from the native architecture of porcine dermal ECM due to changes secondary to manufacturer processing. Following processing, collagen fibrils and fibers within the PADM fuse and create the larger pores noted on the mesh [30]. There is an inherent existence of collagen fibers within the PADM used in this study compared to standard PP mesh; however, due to differences observed in both the PADM-D group and PADM-S group, we believe these changes reflect new collagen deposition rather than reflection of preexisting fibers.
A significant increase in collagen-1 and a slight decrease in collagen-3 were observed on the PADM-D-repaired fascia in our study compared to PADM saline controls. In the same manner, there was an increase in MMP-2 and MMP-9 levels over controls. These changes in collagen type 1:3 ratios may have progressed into a noticeably stronger fascia if the drug treatment was continued for a longer duration and/or with a higher dose doxycycline appropriate for the higher inflammatory response caused by the PADM. Collagen fibrils assessed with the trichrome stain showed a more compact arrangement in PADM animals treated with doxycycline (Fig. 7A), while it was more loosely arranged in the saline control animals (Fig. 7B). This may have been caused by more collagen deposition in the drug-treated animals as evidenced by increased collagen-1 in the treated animal fascia.
The cost of ventral hernia repair remains a significant challenge with overall financial losses in many procedures, particularly those involving the use of biologic mesh [33]. As a result, the role of biologic mesh in uncomplicated hernia repairs has been criticized not only due to cost [34], but also due to the high rate of hernia recurrence relative to synthetic mesh repairs [35]. However, the role of biologic mesh in contaminated ventral hernia repair is less controversial due to the potential for device infection or need for removal when implanting permanent mesh in a contaminated environment [36]. The use of doxycycline as an adjunct to ventral hernia repair may impact the value associated with biologic mesh hernia repairs, if clinical outcomes are ultimately improved. Whether the use of doxycycline results in fewer complications or enhanced clinical outcomes remains unknown. Our prior work has demonstrated doxycycline to augment collagen type 1 and tensiometric strength in polypropylene mesh repairs [25]. This study suggests that the addition of doxycycline to a porcine dermal matrix ventral hernia repair may impact collagen type 1 deposition favorably. In light of this evidence, further studies evaluating the impact of perioperative doxycycline administration upon ventral hernia outcomes are needed. We feel that the use of pharmacotherapy as an adjunct to ventral hernia repair represents an interesting and innovative approach with the potential to improve outcomes through the alteration of collagen deposition.
In conclusion, doxycycline administration improved collagen type 1 deposition in porcine acellular dermal matrix hernia repairs. This may have implications for impacting mesh design, hernia repair techniques, postoperative drug therapy, etc. Further studies with a longer duration are needed to determine the degree of ingrowth and remodeling that occurs to the ECM.
References
Israelsson LA, Jonsson T (1996) Incisional hernia after midline laparotomy: a prospective study. Eur J Surg 162:125–129
Read RC, Yoder G (1989) Recent trends in the management of incisional herniation. Arch Surg 124:485–488
Ditzel M, Deerenberg EB, Grotenhuis N, Harlaar JJ, Monkhorst K, Bastiaansen-Jenniskens YM, Jeekel J, Lange JF (2013) Biologic meshes are not superior to synthetic meshes in ventral hernia repair: an experimental study with long-term follow-up evaluation. Surg Endosc 27:3654–3662
Santora TA, Rosalyn JJ (1993) Incisional hernia. Surg Clin North Am 73:557–570
Bucknall TE, Cox PJ, Ellis H (1982) Burst abdomen and incisional hernia: a prospective study of 1129 major laparotomies. BMJ 284:931–983
Regnard JF, Hay JM, Rea S, Fingerhut A, Flamant Y, Maillard JN (1988) Ventral incisional hernias: incidence, date of recurrence, localization and risk factors. Ital J Surg Sci 18:259–265
Mudge M, Hughes LE (1985) Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg 72:70–71
Urschel JD, Scott PG, Williams HT (1988) Etiology of late developing incisional hernias—the possible role of mechanical stress. Med Hypotheses 25:31–34
Cengiz U, Israelsson LA (1998) Incisional hernias in midline incisions: an eight-year follow up. Hernia 2:175–177
Millikan KW (2003) Incisional hernia repair. Surg Clin N Am 83:1223–1234
Sugerman HJ, Kellum JM Jr, Reines HD, DeMaria EJ, Newsome HH, Lowry JW (1996) Greater risk of incisional hernia with morbidly obese than steroid-dependent patients and low recurrence with prefascial polypropylene mesh. Am J Surg 171:80–84
Hesselink VJ, Luijendijk RW, DeWitt JH, Heide R, Jeekel J (1993) An evaluation of risk factors in incisional hernia recurrence. Surg Gynecol Obstet 3:228–235
Lamont PM, Ellis H (1988) Incisional hernia in re-opened abdominal incisions: an overlooked risk factor. Br J Surg 75:374–376
Rutkow IM (1998) Epidemiologic, economic and sociologic aspects of hernia surgery in the United States in the 1990s. Surg Clin North Am 78:941–951
George CD, Ellis H (1986) The results of incisional hernia repair: a twelve year review. Ann R Coll Surg Engl 68:185–187
Langer S, Christiansen J (1985) Long-term results after incisional hernia repair. Acta Chir Scand 151:217–219
Anthony T, Bergen PC, Kim LT, Henderson M, Fahey T, Rege RV, Turnage RH (2000) Factors affecting recurrence following incisional herniorrhaphy. World J Surg 24:95–101
Leber GE, Garb JL, Alexander AJ, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133:378–382
Franz MG (2008) The biology of hernia formation. Surg Clin N Am 88:1–15
Salameh JR, Talbott LM, May W, Gosheh B, Vig PJ, McDaniel DO (2007) Role of biomarkers in incisional hernias. Am Surg 73:561–568
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Mignatti P, Rifkin DB, Welgus HG, Parks WC (1996) Proteinases and tissue remodeling. In: Clark RAF (ed) The molecular and cellular biology of wound repair, 2nd edn. Plenum, New York, pp 427–474
Wilcox JR, Covington DS, Paez N (2012) Doxycycline as a modulator of inflammation in chronic wounds. Wounds 24:339–349
Antoniou SA, Antoniou GA, Granderath FA, Simopoulos C (2009) The role of matrix metalloproteinases in the pathogenesis of abdominal wall hernias. Eur J Clin Invest 39:953–959
Tharappel JC, Bower CE, Whittington-Harris J, Ramineni SK, Puleo DA, Roth JS (2014) Doxycycline administration improves fascial interface in hernia repair. J Surg Res 190:692–698
Tharappel JC, Ramineni SK, Reynolds D, Puleo DA, Roth JS (2013) Doxycycline impacts hernia repair outcomes. J Surg Res 184:699–704
Tharappel JC, Harris JW, Zwischenberger BA, Levy Salomon M, Puleo DA, Roth JS (2015) Doxycycline shows dose-dependent changes in hernia repair strength after mesh repair. Sur Endosc 30:2016–2021
Rice RD, Ayubi FS, Shaub ZJ, Parker DM, Armstrong PJ, Tsai JW (2010) Comparison of Surgisis, AlloDerm, and Vicryl woven mesh grafts for abdominal wall defect repair in an animal model. Aesth Plast Surg 34:290–296
Badylak SF (2014) Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann Biomed Eng 42:1517–1527
Sun WQ, Xu H, Sandor M, Lombardi J (2013) Process-induced extracellular matrix alterations affects the mechanisms of soft tissue repair and regeneration. J Tissue Eng 4:2041731413505305. doi:10.1177/2041731413505305
Pui CL, Tang ME, Annor AH, Ebersole GC, Frisella MM, Matthews BD, Deeken CR (2012) Effects of repetitive loading on the mechanical properties of biologic scaffold materials. J Am Coll Surg 215:216–228
Gaertner WB, Bonsack ME, Delaney JP (2007) Experimental evaluation of four biologic prostheses for ventral hernia repair. J Gastrointest Surg 11:1275–1285
Reynolds D, Davenport DL, Korosec RL, Roth JS (2013) Financial implications of ventral hernia repair: a hospital cost analysis. J Gastrointest Surg 17:159–166 (discussion 166-167)
Totten CF, Davenport DL, Ward ND, Roth JS (2016) Cost of ventral hernia repair using biologic or synthetic mesh. J Surg Res 203:459–465
Bondre IL, Holihan JL, Askenasy EP, Greenberg JA, Keith JN, Martindale RG, Roth JS, Liang MK, Ventral Hernia Outcomes Collaborative (2016) Suture, synthetic, or biologic in contaminated ventral hernia repair. J Surg Res 200:488–494
Carbonell AM, Criss CN, Cobb WS, Novitsky YW, Rosen MJ (2013) Outcomes of synthetic mesh in contaminated ventral hernia repairs. J Am Coll Surg 217:991–998
Acknowledgments
This research was supported by a grant from SAGES.
Authors’ contributions
JCT and JSR contributed to study design and collection of data; JCT, JWH, and BAZ performed surgical procedures; JCT, JSR, and CFT made critical revisions of the article. The authors acknowledge the support provided by Ms. Linda Combs in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. JSR is a speaker for CR Bard, MTF, and LifeCell; he receives grant support from Bard, LifeCell, MTF, and Gore; and he is a shareholder in Miromatrix.
Rights and permissions
About this article
Cite this article
Tharappel, J.C., Harris, J.W., Totten, C. et al. Doxycycline alters collagen composition following ventral hernia repair. Surg Endosc 31, 1659–1666 (2017). https://doi.org/10.1007/s00464-016-5155-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5155-8