Abstract
Background
Ventral hernia is a commonly occurring surgical problem. Our earlier studies have shown that a 30 mg/kg dose of doxycycline can significantly impact the strength of polypropylene (PP) mesh in a rat hernia repair model at 6 and 12 weeks. The objective of the present study was to investigate the dose dependence of doxycycline treatment on hernia repair strengths in rats.
Study design
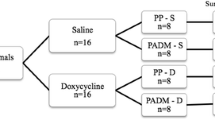
Fifty-six Sprague-Dawley rats underwent hernia repair with either PP mesh (n = 28) or sutures only (primary; n = 28); both groups were further divided into four doxycycline groups of seven animals each: control (0 mg/kg), low (3 mg/kg), medium (10 mg/kg), and high (30 mg/kg). One day before hernia repair surgery, animals received doxycycline doses by gavage and continued receiving daily until euthanasia. After 8 weeks, rats were euthanized and tissue samples from hernia repaired area were collected and analyzed for tensile strength using a tensiometer (Instron, Canton, MA, USA), while MMPs 2, 3, and 9, and collagen type 1 and 3 were analyzed by western blotting.
Results
In mesh-repaired animals, medium and high doxycycline dose repaired mesh fascia interface (MFI) showed significant increase in tensile strength when compared to control. In the primary repaired animals, there was no significant difference in MFI tensile strength in any dose group. In medium-dose MFI, there was a significant reduction in MMPs 2, 3, and 9. In this animal group, MFI showed significant increase in collagen 1 and significant reduction in collagen type 3 when compared to control.
Conclusion
It is possible to improve the strength of mesh-repaired tissue by administering a significantly lower dose of the drug, which has implications for translation of the findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ventral hernias are one of the leading surgical problems in the USA. Approximately 100,000 ventral hernia repairs are performed every year in the USA [1]. Incision hernias affect 11 % of all abdominal wall surgeries and have up to 50 % recurrence rates [1, 2]. This high rate of recurrence is a persisting problem causing a high rate of morbidity and mortality [3–7].
Many studies have found a link that connects the incisional hernias to impaired collagen metabolism [8, 9]. Collagen, primarily types 1 and 3, is one of the main structural components of extracellular matrix (ECM) in the abdominal wall. For a stronger abdominal wall, the ECM needs to contain a higher ratio of the more mature collagen type 1 to collagen type 3 [9]. An important factor affecting the levels of collagen subtypes in the ECM is the presence of endopeptidases called matrix metalloproteinases (MMPs) [10]. Levels of MMPs that can affect the collagen type 1–3 equilibrium can be an important factor in determining the collagen 1–3 ratio in the ECM and thereby the strength of the abdominal wall. Therefore, a lower level of MMPs could be more beneficial for a stronger abdominal wall.
Tetracyclines and their derivatives have been shown to inhibit MMPs, an activity that is independent of their antibiotic properties [10, 11]. In particular, the chemically modified derivative doxycycline has wide spectrum antibiotic and MMP inhibitory properties. Our previous studies have shown that doxycycline administration inhibits MMP levels in the repaired fascial interface of doxycycline-treated rats, leading to a stronger repaired fascia after 4, 6, and 12 weeks of treatment [12, 13]. Our previous studies, however, used a doxycycline dose that was almost 10 times more than an average adult human dose (3.5 mg/kg). Therefore, the objective of the present 8-week study was to investigate the dose dependence of doxycycline treatment on hernia repair strengths in rats.
Methods
Animal methodology
The experimental protocol used in this animal study was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Kentucky. Fifty-six male Sprague-Dawley rats weighing ~400 g were obtained from Harlan Laboratories (Indianapolis, IN), acclimatized for 5 days, and randomly assigned to one of two groups. For group 1 (n = 28), hernias were repaired with a monofilament polypropylene mesh (Davol Inc., Warwic, RI), while for group 2 (n = 28), hernia repair used sutures only (primary repair). Each group was further divided into four doxycycline groups (n = 7 each): control (0 mg/kg), low (3 mg/kg), medium (10 mg/kg), and high (30 mg/kg). One day before the hernia mesh implantation or primary repair surgery, animals received doxycycline doses as above by gavage, and they continued receiving the drug daily throughout the study. Oral administration of doxycycline was chosen as this would be the most easy to replicate in clinical trials. Also, topical administration would be limited in its duration, and IV administration would be challenging for prolonged periods of time. Before the implant or primary repair surgery, all animals were given a general anesthesia with inhaled 4 % isoflurane, and they were prepped for surgery by clipping and applying a 70 % chlorhexidine to the surgical area. Post-surgery analgesia was obtained by a subcutaneous buprenorphine injection (0.03 mg/kg) every 12 h. After 8 weeks, rats were euthanized, and tissue samples from hernia repaired area were collected and analyzed for tensile strength using a tensiometer (Instron, Canton, MA, USA), while MMPs and collagens were analyzed by western blotting.
Animal surgical procedures
We used a single-step ventral defect creation/mesh implantation procedure for the mesh-repaired group. A 5-cm incision was made on the skin at the midline of the abdomen and extended the incision cranially and caudally. The skin was lifted up and raised 2 cm circumferentially from the body wall on either side of the linea alba. Then lifting up the linea alba, a vertical incision was made along the linea alba for 4 cm, and a 0.5-cm flap of the body wall was removed from both sides of the incision to make a defect. In the mesh-implanted group, a 3 cm × 4 cm piece of polypropylene mesh was cut, corners smoothed and placed under the body wall as an underlay with 1-cm overlap on to the native abdominal wall beyond the hernia defect. It was anchored to the abdominal wall using eight interrupted 4-0 vicryl stitches on the periphery of the mesh. In the primary repaired group, the created defect was closed by a running 4-0 vicryl suture. After the mesh or primary repair, the skin was closed in two layers: The subcutaneous layer was closed using a vicryl 4-0 interrupted suture, while the skin layer was closed with either wound clips or an interrupted monofilament 4-0 nylon suture. Triple antibiotic ointment was placed on the incision, and the animals were revived from anesthesia and observed until ambulant.
After the eighth week, animals were euthanized by CO2 asphyxiation and their abdominal wall tissues harvested. One half of the tissue was used for tensiometric analysis; one quarter was fixed in 4 % formalin, and one quarter was snap-frozen in liquid nitrogen for biochemical analysis.
Western blotting
Antibodies for MMPs (2, 3, and 9), collagens (types 1 and 3), and tissue inhibitors of metalloproteinases 1, 2, and 3 (TIMPs) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The blotting procedure was performed as previously described [12]. A lysis buffer containing 1 % Nonidet P-40, 0.1 % sodium dodecyl sulfate, 0.1 mg/mL phenylmethylsulfonyl fluoride, 2 mg/mL aprotinin, 2 mg/mL leupeptin, 2 mg/mL pepstatin A, and 1× phosphate-buffered saline (PBS) was added to the frozen abdominal wall samples (1 part tissue + 4 parts buffer) and homogenized. Homogenates were centrifuged at 10,000g for 20 min and supernatants centrifuged again at 100,000g for 20 min. The supernatants were again collected, aliquoted, and stored at −80 °C. One aliquot was used for assaying the protein content using the BCA Protein Assay (Thermo Fisher Scientific Inc., Rockford, IL, USA). The samples were denatured by boiling for 5 min with 2× gel loading buffer (17.3 % glycerol, 1.25 M β-mercaptoethanol, 5.2 % sodium dodecyl sulfate, 0.22 M Tris, pH 6.8, and 1–2 mg bromophenol blue). Thirty micrograms of protein from each sample was electrophoresed on a mini gel containing a 4 % stacking gel and 8.5 % separating gel at 175 V for 1 h and then electroblotted onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc, Hercules, CA, USA) at 100 V for 1 h. The membranes were then incubated for 1 h in a blocking buffer (5 % fat-free dry instant powdered milk, 1 mM Tris-base, 15 mM sodium chloride, and 0.05 % Tween-20) at room temperature with shaking, followed by primary and secondary antibodies diluted in blocking buffer for 1 h each at room temperature with shaking. After primary and secondary antibody incubations, the membranes were washed 3× with washing buffer (1 mM Tris-base, 15 mM sodium chloride, and 0.05 % Tween-20). A chemiluminescent detection kit (Pierce, Rockford, IL, USA) was used to detect the antibodies bound to the membrane, and the images were quantified using ImageJ software (NIH, Bethesda, MD, USA).
Tensiometric analysis
The abdominal wall with the mesh-repaired area was tested for its tensile strength on an Instron E3000 tensiometer (Instron Corp, Canton, MA, USA) equipped with a 250-N load cell. The tissues were tested within 6 h of harvest after warming from 4 °C to room temperature. The tissue from the repaired area was cut into 4 cm long × 1.5 cm wide strips with the repaired area in the middle of the specimen and subsequently loaded onto the machine using pneumatic grips with ~20 lb pressure. The crosshead speed was set to 10 mm/min, and the tissues were loaded in tension until complete failure [14]. The displacement and load data were analyzed using Instron Bluehill software.
Statistical analysis
The data from the western blotting quantitation and tensiometric analysis were analyzed by ANOVA. The results were considered statistically significant when the P value was <0.05.
Results
For both the medium (10 mg/kg) and high (30 mg/kg) doxycycline doses, the mesh fascia interface (MFI) showed a significant increase in tensile strength (P < 0.05), 47–61 % greater, when compared to control animals (Figs. 1, 2). In the primary repaired animals, there was no significant difference in MFI tensile strength in all dosage groups (Fig. 1).
In our previous studies [12, 13], we used the high-dose (30 mg/kg) doxycycline treatment to study its effect on the mesh fascial interface. The current study analyzes the medium-dose group that showed a remarkable increase in the tensile strength of the MFI. In the medium-dose animal group, the MFI showed a marked increase in collagen 1 and a significant reduction in collagen type 3 when compared to control MFI (Figs. 3, 4). In the medium-dose MFI, there was a significant reduction in MMPs 2 and 9 but the reduction was not significant for MMP 3 (Figs. 5, 6, 7). In the medium-dose doxycycline group, the MFI tissue also showed an increase in TIMP-1 (Fig. 8).
Discussion
Tetracyclines have been shown to have diverse biological properties such as anticancer, antiparasitic, and antimalarial [15–17]. They are also found to be active in inhibiting biological enzymes including MMPs and scavenging of reactive oxygen species (ROS) [18, 19]. Wound sites are known to recruit polymorphonuclear neutrophils (PMNs) in the healing process, and they produce ROS that can induce even more MMP production [20]. The elevated MMP levels at the wound healing sites are known to destroy ECM [21–24]. Doxycycline has been shown to act as a ROS scavenger that can reduce the MMP levels by inhibiting the synthesis and activation of pro MMPs [25]. The mechanism of doxycycline-mediated prevention of ECM degradation has been shown to occur via three different pathways: (1) inhibiting active and pro MMPs, (2) down-regulation of MMP expression, and (3) protecting the host defense protein a-1 antitrypsin [24, 26].
In our previous studies, we demonstrated increased strength of the MFI in the doxycycline-treated animals [12, 13]. Treated animals also showed reduced MMP levels and an increased ratio of collagen type 1–3. This may be a direct result of the protective effects of doxycycline by blocking the MMP 2 activity by the three pathways mentioned above and thus preventing the degradation of collagen type 1. In the earlier studies, we used a doxycycline dose of 30 mg/kg that was considered to be a very high dose in terms of human dosing (~3.5 mg/kg). Thus, we sought to determine whether a lower dose could also be effective in increasing the fascial strength of the repaired hernia by reducing the MMP-mediated destruction of mature collagen 1 and thereby augmenting the repair. In the present study, we found that a lower dose of 3.5 mg/kg did not increase the repair strength, but at 10 mg/kg the doxycycline administration significantly increased the tensile strength of the MFI. Further, the MFI in these treated rats showed an increase in the collagen 1–3 ratio, similar to the higher-dose (30 mg/kg) doxycycline group. Also, we saw the same reduced levels of MMPs 2, 3, and 9 in the MFI of medium-dose (10 mg/kg) animals as seen previously in high-dose animals [12, 13]. In examining the role of TIMPs in affecting properties of the MFI, we observed a marked increase in TIMP-1 in the doxycycline-treated MFI. Although there was no change in TIMPs 2 or 3, TIMPs 1 and 2 are known to be involved in the regulation of MMPs 2 and 9, and they are also implicated in inflammation, suppression of apoptosis, and cell proliferation [27, 28]. The connection between up-regulation of TIMP-1 and the doxycycline administration is not known and needs to be investigated further.
Dosage effect of doxycycline administration on MMPs has been reported previously in studies related to MMP 2 inhibition in a 2K1C hypertensive rat model [29]. In that study, MMP 2 was shown to be dose dependently inhibited by doxycycline, resulting in downstream changes in the vascular function and structure. One of the main substrates of MMP 2 is collagen type 1, which is a more mature form of collagen in the ECM [30]. In the present study, we noticed that a lower dose pf 3.5 mg/kg did not produce any gain in fascial strength, but a 10 mg/kg dose produced a greater than 47 % increase in fascial strength in repaired fascia. A further increase in dosage (30 mg/kg) did not result in additional increase in MFI strength, but the gain in fascial strength remained significant when compared to control fascia. This finding about the effectiveness of lower-dose doxycycline (10 mg/kg) in increasing the fascial strength may have potential implications in applying this low-dose doxycycline effect in a long-term doxycycline application with reduced adverse side effects from the drug when compared to higher doses we used in our previous studies [12, 13].
Since we have already shown that doxycycline administration increased the tensile strength of the MFI in the mesh hernia repair, we also investigated whether this drug is effective in increasing the tensile strength of the primary hernia repair. In the current study, we did not find any difference between the primary repaired control group and the three doxycycline groups. This may be because of the differences in the repair process in the primary and the bridged mesh hernia repair. In the primary repair, there is less regenerated tissue in the repaired area when compared to the bridged mesh-repaired facial interface. Because of this, the ratio of collagen type 1 to 3 may not undergo significant changes after the repair process, resulting in less change in collagen type 1 to 3 ratio in the MFI.
Conclusion
The current study shows that doxycycline administration dose dependently influences the MFI tissue strength following hernia repair with mesh by altering MMP levels and, consequently, the ratio of collagen 1–3. Tissue from animals with primary repair was unaffected, however. Overall, these results demonstrate that it is possible to improve the strength of the mesh-repaired tissue by administering a significantly lower dose of the drug, which has implications for translation of the findings.
References
Rutkow IM, Robbins AW (1993) Demographic, classificatory, and socioeconomic aspects of hernia repair in the United States. Surg Clin N Am 73:413–426
Kingsnorth A (2006) The management of incisional hernia. Ann R Coll Surg Engl 88:252–260
Mudge M, Hughes LE (1985) Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg 72:70–71
George CD, Ellis H (1986) The results of incisional hernia repair: a twelve year review. Ann R Coll Surg Engl 68:185–187
Langer S, Christiansen J (1985) Long-term results after incisional hernia repair. Acta Chir Scand 151:217–219
Anthony T, Bergen PC, Kim LT (2000) Factors affecting recurrence following incisional herniorrhaphy. World J Surg 24:95–101
Leber GE, Garb JL, Alexander AJ, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133:378–382
Franz MG (2008) The biology of hernia formation. Surg Clin N Am 88:1–15
Salameh JR, Talbott LM, May W, Gosheh B, Vig PJ, McDaniel DO (2007) Role of biomarkers in incisional hernias. Am Surg 73:561–567; discussion 567–568
Antoniou SA, Antoniou GA, Granderath FA, Simopoulos C (2009) The role of matrixmetallo-proteinases in the pathogenesis of abdominal wall hernias. Eur J Clin Invest 39:953–959
Lee HM, Ciancio SG, Tüter G, Ryan ME, Komaroff E, Golub LM (2004) Subantimicrobial dose doxycycline efficacy as a matrix metalloproteinase inhibitor in chronic periodontitis patients is enhanced when combined with a non-steroidal antiinflammatory drug. J Periodontol 75:453–463
Tharappel JC, Bower CE, Whittington-Harris J, Ramineni SK, Puleo DA, Roth JS (2014) Doxycycline administration improves fascial interface in hernia repair. J Surg Res 190:692–698
Tharappel JC, Ramineni SK, Reynolds D, Puleo DA, Roth JS (2013) Doxycycline impacts hernia repair outcomes. J Surg Res 184:699–704
Rice RD, Ayubi FS, Shaub ZJ, Parker DM, Armstrong PJ, Tsai JW (2010) Comparison of Surgisis, AlloDerm, and Vicryl woven mesh grafts for abdominal wall defect repair in an animal model. Aesth Plast Surg 34:290–296
White AC, Hayat CS, Kimball KT (1987) Effects of lipophilic tetracyclines against Giardia lamblia. J Infect Dis 28:781–787
Sezaki M, Inouye S, Kondo S (1992) A new tetracycline antibiotic with antitumor activity. I. Taxonomy and fermentation of the producing strain, isolation and characterization of SF-2575. J Antibiotics 45:320–324
Flanigan TP, Soave R (1991) Plasmodium falciparum growth and the effects of chlortetracycline. Clin Infect Dis 63:388–392
Castro MM, Tanus-Santos JE (2013) Inhibition of matrix metalloproteinases (MMPs) as a potential strategy to amelioratehypertension-induced cardiovascular alterations. Curr Drug Targets 14:335–343
Antonio RC, Ceron CS, Rizzi E, Coelho EB, Tanus-Santos JE, Gerlach RF (2014) Antioxidant effect of doxycycline decreases MMP activity and blood pressure in SHR. Mol Cell Biochem 386:99–105
Wysocki AB, Staiano-Coico L, Grinnell F (1993) Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol 101:64–68
Woessner JF (1994) The family of matrix metalloproteinases. Ann N Y Acad Sci 732:11–21
Mignatti P, Rifkin DB, Welgus HG, Parks WC (1996) Proteinases and tissue remodeling. In: Clark RAF (ed) The molecular and cellular biology of wound repair, 2nd edn. Plenum Press, New York, pp 427–474
Murphy G, Willenbrock F, Crabbe T, O’Shea M, Ward R, Atkinson S, O’Connell J, Docherty A (1994) Regulation of matrix metalloproteinase activity. Ann N Y Acad Sci 732:31–41
Smith APS (2003) The role of MMPs in chronic wound edema. Podiatry Today 16:22–26
Wilcox JR, Covington DS, Paez N (2012) Doxycycline as a modulator of inflammation in chronic wounds. Wounds 24:339–349
Ryan ME, Ashley RA (1998) How do tetracyclines work? Adv Dent Res 12:149–151
Lorenzl S, Albers DS, Narr S, Chirichigno J, Beal MF (2002) Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson’s disease. Exp Neurol 178:13–20
Guedez L, Courtemanch L, Stetler-Stevenson M (1998) Tissue inhibitor of metalloproteinase (TIMP)-1 induces differentiation and an antiapoptotic phenotype in germinal center B cells. Blood 92:1342–1349
Guimaraes DA, Rizzi E, Ceron CS, Oliveira AM, Oliveira DM, Castro MM, Tirapelli CR, Gerlach RF, Tanus-Santos JE (2011) Doxycycline dose dependently inhibits MMP-2-mediated vascular changes in 2K1C hypertension. Basic Clin Pharmacol Toxicol 108:318–325
Nagase N (2001) Substrate specificity of MMPs. In: Clendeninn NJ, Krzysztof A (eds) Cancer drug discovery and development: matrix metalloproteinase inhibitors in cancer therapy. Humana Press Inc., Totowa
Acknowledgments
This research was supported by a grant from Davol, Inc., Warwick, Rhode Island. Timely help in the preparation and submission of the manuscript by Ms. Linda Combs is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Tharappel, Harris, Zwischenberger, and Levy have no conflicts of interest or financial ties to disclose. Dr. Puleo receives Grant funding from the NIH (R01AR060964). Dr. Roth has received external grant funding from Davol, Inc. for the support of this study; he has also received external grant funding and consulting fees from CR Bard; he is on speaker bureaus for CR Bard and Ethicon; and he is a Board member of the Musculoskeletal Transplant Foundation.
Rights and permissions
About this article
Cite this article
Tharappel, J.C., Harris, J.W., Zwischenberger, B.A. et al. Doxycycline shows dose-dependent changes in hernia repair strength after mesh repair. Surg Endosc 30, 2016–2021 (2016). https://doi.org/10.1007/s00464-015-4434-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4434-0