Abstract
Purpose
To compare an inflammation score and collagen morphometry after incisional hernia repair with four different meshes at two time points.
Methods
Four types of mesh were used to repair an abdominal wall incisional defect in Wistar rats: high-density polypropylene (HW/PP); low-density polypropylene (LW/PP); polypropylene mesh encapsulated with polydioxanone coated with oxidized cellulose (PP/CE); and expanded polytetrafluoroethylene (ePTFE). An inflammation score based on histological analysis and collagen morphometry was performed after 7 and 28 days after operation (POD).
Results
Compared to LW/PP group at 7 POD, HW/PP group had lower (p = 0.014) and PP/CE group had higher inflammation scores (p = 0.001). At 28 POD, higher scores were seen in all the other groups compared to the LW/PP group (HW/PP, p = 0.046; PP/CE, p < 0.001; ePTFE, p = 0.027). Comparing groups individually at 7 and 28 PODs, all demonstrated lower inflammation score values at 28 POD (HW/PP, p < 0.001; LW/PP, p < 0.001; PP/CE, p = 0.002; ePTFE, p = 0.001). At 7 POD, higher amounts of collagen were detected in ePTFE compared to HW/PP (p < 0.001) and LW/PP (p = 0.004) and in PPCE group compared to HW/PP (p = 0.022). At 28 POD, no statistically significant difference was found. Comparing groups individually at 7 and 28 PODs, HW/PP and LW/PP showed larger amounts of collagen at the 28th POD, without any statistically significant differences for the PP/CE and ePTFE groups.
Conclusions
Inflammation scores decreased in all groups at 28 POD. Collagen deposition was higher for non-composite meshes at 28 POD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abdominal wall hernias are the most prevalent surgical diseases in the world, affecting about 10 % of the global population; in the United States, over one million surgical repairs are carried out per year [1]. Incisional hernias are the most common cause of reoperation after performing a laparotomy [2].
Despite the high prevalence, their etiology is not well defined. Wound infection, surgical technique, immunodeficiency, obesity and chronic obstructive disease, among others may be involved [3]. The importance of collagen and its subtypes in maintaining the tissue integrity and the hernia prevention is a topic of debate [4].
The incisional hernias’ recurrence rate, with primary corrections, was close to 50 % during the 1960s. With the introduction of prostheses, the number dropped to about 10 %. Their use still generates controversy, particularly in relation to their long-term effects, due to the biocompatibility of different materials that have been recently introduced [5]. Burger et al. [6] observed 63 % of recurrence after 10 years of surgeries with primary sutures, but only 32 % with mesh utilization.
Overall, the most common material used for the correction of inguinal and ventral hernias is polypropylene. This material can cause a chronic inflammatory response that results in a rapid and dense incorporation of surrounding tissue [7], which, together with abdominal wall elasticity restriction, can lead to chronic pain, retraction of the mesh, and decreased abdominal compliance [8]. Nowadays, more than 166 materials are available, making it difficult for the surgeon to choose the best type of prosthesis [9]. The ideal mesh would be the one that shows less inflammatory response and better healing to avoid recurrence. However, the collagen synthesis and the healing process are inflammation dependent [8]. It is still not clear how the early inflammatory response or late inflammatory response is associated with collagen deposition and, to a smaller level, of hernia recurrence.
Taking into account the available data, we hypothesized that high-density polypropylene (HW/PP) meshes and other meshes associated with absorbable materials could cause a more intense late inflammatory response, which, in turn, could lead to delayed or decreased collagen synthesis.
The objective of this study is to compare the early and late inflammatory responses and collagen deposition among the use of HW/PP, low-density polypropylene (LW/PP), polypropylene encapsulated with polydioxanone and coated with oxidized cellulose, or the expanded polytetrafluoroethylene (ePTFE) meshes when placed on the aponeurosis.
Methods
This experimental study of an acute wound model, not a hernia model, was submitted to the Ethics Committee of UNIFESP and approved in July 2012 (CEP 0226/12).
Animals
Eighty male Wistar rats (Rattus norvegicus albinos), 3 months of age, weighing about 250 g were used. The animals were kept in constant and similar conditions of temperature, light, and food were accompanied by the veterinary team of the institution. The research was conducted in accordance with the Brazilian College of Animal Experimentation Standards (COBEA).
Experimental design
The animals were divided into four groups of 20 animals each, according to the type of implanted mesh:
-
HW/PP group: high-density polypropylene mesh;
-
LW/PP group: low-density polypropylene mesh;
-
PP/CE group: polypropylene mesh encapsulated with polydioxanone and coated with oxidized cellulose;
-
ePTFE group: expanded polytetrafluoroethylene mesh.
Ten animals of each group were sacrificed on the seventh postoperative day (POD) (early operation subgroup). The remaining animals were kept in cages with the same feeding conditions and temperature for 21 additional days (28 days in total after mesh implantation) when they were also sacrificed (late operation subgroup). Immediately after euthanasia, the anterior abdominal wall was removed en bloc, fixed in formaldehyde solution and sent to the Pathology sector.
Surgical technique
An incision of approximately 4 cm was performed, and skin flaps were separated from anterior wall. A 1 × 2 cm fragment of the anterior wall of the abdominal musculature was removed with no violation of the posterior wall. For each experimental group, a 2 × 3 cm mesh was positioned above the created defect, without the fascia approximation. The respective mesh for each animal was positioned only with an overlap and fixated with six stitches 5–0 polypropylene sutures in the fascia border of each side of the intact wall. The skin was sutured with separated stitches using a monylon 4–0.
The surgical procedure was performed as previously described by our group [10, 11]. The animals were assessed daily to observe complications.
The samples were harvested after 7 or 28 days after the implant of the meshes. The samples were harvested from the center of the mesh and analyzed from the mesh to the transition tissue, which were found in the highest level of the analyzed elements.
Histological analysis
At the 7th and 28th PODs, the animals were euthanized with a lethal dose of ketamine. A sample of the abdominal wall block with the accompanying mesh was obtained for the histological analysis.
After fixation, the collected samples were submitted to routine xylene processing and paraffin embedding, cut by microtome, and stained by the hematoxylin–eosin method (HE) and Masson trichrome. For histological evaluation, we used a numerical scale with scores assigned to each item analyzed [12]. Briefly, this scale attributes one-to-four points (four being the most severe) to each item of interest, which were:
-
1.
number of cell layers at the periphery of granulomas;
-
2.
inflammatory reaction in the host tissue (type and number of cells);
-
3.
inflammatory response at the prosthesis surface (type and number of cells);
-
4.
interstitial tissue maturation.
Two additional items were also analyzed: the presence of giant cells and the inflammatory invasion of smooth muscle adjacent to the mesh, according to Pereira-Lucena et al. [13], using the same scoring system.
Both the author and a pathologist with recognized experience carried out the analyses, which were scored only by the pathologist. All 20 animals for each group were analyzed, and each animal had just one sample to be analyzed.
The analyzed microscopic field was the junction between the mesh and the host tissue, where the elements of interest to the study (inflammatory reaction and collagen deposition) could be found.
Morphometric analysis of collagen
For this analysis, we used the Picrosirius color with polarized light. The sections were dewaxed, hydrated, and stained with the Sirius Red solution of 0.1 % for a period of 1 h (Sirius Red F3B200, Mobay Chemical Co., Union, USA). Then, the slides were plated with saturated aqueous picric acid. Subsequently, the slides were washed in running water and counterstained with Harris hematoxylin for 5 min.
For the analyses, the slides were subjected to polarized light and their images captured by a system consisting of an Olympus BX51 microscope and a Pentium computer with 1-GB memory. The field area to be analyzed (400× augmentation) was defined as the one with the highest concentration of collagen, identified by the orange–red color.
The counting of the pixels corresponding to collagen was made on an Acer computer with 2 GB of memory and the Image Tool software (version 3.0). The following steps were performed to count the pixels corresponding to collagen, as shown in Fig. 1: (a) the color image (orange) selected was converted to grayscale; (b) the automatic threshold command was executed; and (c) the counting of white and black pixels and their percentage were performed by the command count black/white pixels.
Statistical analysis
The Shapiro–Wilk test was used to test the normality of the quantitative variables. Variables related to the inflammation scores were not normally distributed and were evaluated with non-parametric tests; the count of pixels by the Picrosirius method was normally distributed and was analyzed with parametric tests.
The Kruskal–Wallis test, followed by Mann–Whitney tests 2 × 2 or ANOVA with post hoc Bonferroni test, was used to determine the effect of the different types of meshes on the quantitative variables, separately for the early and late conditions. The Mann–Whitney test was used to assess the effect of early versus later conditions (7th vs. 28th POD), separately for each group. Analyses were performed using the Statistical Package for Social Sciences (SPSS) software (v18.0).
The level of significance was set at p < 0.05.
Results
Inflammation in the early subgroup
The median values related to early inflammatory response in the seventh POD for the four groups are shown in Table 1 and Fig. 2a. Post hoc analyses showed significant differences only for the group HW/PP compared to groups LW/PP (p = 0.014) and PP/CE (p = 0.001).
Median and interquartile range of inflammatory tissue reaction scores at the 7th POD (a) and the 28th POD (b) subgroups. HW/PP high-density polypropylene, LW/PP low-density polypropylene, PP/CE polypropylene encapsulated with polydioxanone and coated with oxidized cellulose, ePTFE expanded polytetrafluoroethylene mesh. N = 10 per group. *p < 0.05, **p = 0.001, ***p < 0.001
Inflammation scores in the late subgroup
The median values related to the late inflammatory response (28th DPO) of the four groups are shown in Table 2 and Fig. 2b. The LW/PP subgroup had lower values when compared to all other subgroups. The HW/PP subgroup also showed a lower value for the inflammation score compared to PP/CE subgroup (p = 0.02).
Inflammation scores in early versus late subgroups
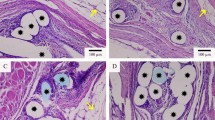
In Fig. 3, we can observe the decreasing number of inflammatory cells and rearrangement of connective tissue in animals from the LW/PP group (Fig. 3a, 7th POD; Fig. 3b, 28th POD) and macrophage phagocytosis of a prosthesis fragment in the late phase of PP/CE group (Fig. 3c).
Hematoxylin–eosin staining showing the inflammatory reaction in the host tissue and surround the mesh of group LW/PP (low-density polypropylene). a 7th POD; b 28th POD; c macrophage phagocytosis of a mesh fragment in the 28th POD of PP/CE (polypropylene encapsulated with polydioxanone and coated with oxidized cellulose) group (arrow)
Figure 4 shows the comparison of inflammation scores between the early and late values for the same type of mesh. All of them demonstrated significantly lower values for the 28th POD compared to the 7th POD (Mann–Whitney test with Bonferroni’s correction: HW/PP, p < 0.001; LW/PP, p < 0.001; PP/CE, p = 0.002; ePTFE, p = 0.001).
Comparison between the 7th and 28th POD inflammation scores. Black bars 7th POD; white bars 28th POD. HW/PP high-density polypropylene, LW/PP low-density polypropylene, PP/CE polypropylene encapsulated with polydioxanone and coated with oxidized cellulose, ePTFE expanded polytetrafluoroethylene mesh. N = 10 per group. *p < 0.001; # p = 0.002
Collagen analysis in the early subgroup
Morphometric analysis demonstrated that the ePTFE group had the greater collagen amount among the groups in the seventh POD (Table 3; Fig. 5a). The ePTFE group showed greater amounts of collagen compared to groups LW/PP (Fig. 6) and HW/PP; group PP/CE also showed a difference compared to group HW/PP (p = 0.022).
Collagen amount (percentage of pixels) for the 7th POD early (a) and 28th POD (b) subgroups. HW/PP high-density polypropylene, LW/PP low-density polypropylene, PP/CE polypropylene encapsulated with polydioxanone and coated with oxidized cellulose, ePTFE expanded polytetrafluoroethylene mesh. ***p < 0.001, **p = 0.004
Collagen analysis in the late subgroup
At 28 PODs, the initial evaluation among all groups revealed a statistically significant difference (p = 0.028), but after the 2 × 2 comparison and Bonferroni’s correction, no difference was found regarding collagen amount (Table 4; Fig. 5b).
Photomicrographs depicting collagen amount at the 28th POD in all groups are shown in Fig. 7.
Collagen in the early versus late subgroups
Figure 8 shows the comparison of the 7th and 28th PODs’ collagen analysis for each group. For both HW/PP and LW/PP groups, the analyses showed larger amounts of collagen at the 28th POD (p < 0.001 and p < 0.001, respectively). There was no statistically significant difference regarding PP/CE and ePTFE groups.
Discussion
This study shows that HW/PP meshes induce lower inflammatory responses at seven POD compared to low-density or encapsulated polypropylene meshes (Table 1). At 28 POD, the low-density mesh group had the lowest inflammatory scores compared to all other groups (Table 2). All the types of analyzed meshes produced lower inflammatory responses at 28 POD compared to 7 POD (Figs. 3, 4).
Regarding collagen deposition, both polypropylene meshes had lower values compared to the ePTFE mesh at seven POD (Table 3; Fig. 5a). No differences could be found among the evaluated meshes at 28 POD (Table 4; Fig. 7). When comparing collagen deposition at 7 and 28 PODs for each group, both LW/PP and HW/PP meshes showed larger amounts of collagen at the later period, while no difference could be found for the composite meshes (PP/CE and ePTFE), as shown in Fig. 8.
The LW/PP mesh group showed a higher inflammatory response than the HW/PP mesh (Table 1; Fig. 2a). However, the analysis of the late period (28th POD) showed that the LW/PP mesh had lower inflammatory responses than the HW/PP mesh (p = 0.046) (Fig. 2b). Other authors concluded that the low-density, large pore meshes have a lower inflammatory response when compared to the conventional polypropylene [14].
Pascual et al. [8] did not observe this difference among different types of mesh on the 14th POD. Moreover, Zogbi et al. [15] studied 25 animals with HW/PP and LW/PP prostheses at three different times after surgery (7, 30, and 90 days), and found a greater inflammatory response in lower density polypropylene meshes in the 7th POD. This relationship is reversed at the 90th POD when the conventional HW/PP mesh begins to show a more intense inflammatory response.
We observed that although the groups have different inflammation values compared to each other, both in the early and the late periods, all performed similarly, with a decrease in inflammatory values at the 28th POD. We believe that the exacerbated inflammatory response at this point is harmful to tissue repair. These results corroborate the study of Pascual et al. [8], which suggests that the composite prostheses are responsible for the perpetuation of the inflammatory reaction and the delayed appearance of the repair tissue. In the process of healing and formation of a definitive repair tissue, collagen deposition begins with an immature collagen (type III), which becomes gradually replaced by type I collagen, of better quality, and the proportion of collagen I/III progressively increases [16]. In a study of laser skin of mice, de Noronha et al. [17] observed the formation of an immature collagen in the healing process already in the 7th POD, which was later replaced by a mature collagen that reaches its peak in the 28th POD. Vaz et al. [14] analyzing the collagen behavior after the placement of polypropylene prostheses on rats found that the total collagen shows a peak at 21° POD. These same authors also observed that the ratio of collagen type I/III progressively increases during the healing process. Peeters et al. [18] have performed studies in humans and found that the ratio of collagen type I/III of the rectus sheath and the skin was lower in patients with primary inguinal hernias and even lower in patients with recurrent hernias. Rosch et al. [16] concluded the same for patients with ventral hernias.
In our work, the total collagen production increased from the 7th to the 28th PODs, and the averages were higher in the group implanted with polypropylene meshes than those of composite materials (Fig. 5). According to Zogbi et al. [15], polypropylene meshes present an almost fivefold progressive increase in the ratio of collagen I/III, up to 90 days after implantation. Although we did not evaluate the type of collagen being deposited, a similar outcome may have occurred.
In a previous study of our group, Pereira-Lucena et al. [13] compared conventional polypropylene to polypropylene + low-density polyglactin and polypropylene + high-density polyglactin + low-density titanium. The animals were sacrificed on the 7th and 40th PODs. They concluded that the inflammatory process was maintained or raised in the composite groups and decreased in the polypropylene group between the 7th and the 40th PODs, suggesting that the absorbable materials in the composite meshes could prolong the inflammatory tissue reaction and damage collagen deposition.
Pascual et al. [8] reached a similar conclusion that the meshes using absorbable biological materials could increase the production of inflammatory mediators by decreasing the synthesis of growth factors in the new tissue. Rosch et al. [19] demonstrated that, in the long run, this difference did not exist in the composite fabrics coated with polyglactin when compared to pure polypropylene mesh. A similar result was found by Junge et al. [20] which compared with polypropylene mesh screens combined polypropylene and polyglecaprone. In our previous study, we concluded that an intense inflammatory reaction could reduce tissue maturation and collagen deposition [13].
Comparing our results with the above data, we can hypothesize that the meshes with large pores and, in this case, lower density, allow free flow of cells through the mesh, as have been shown by other authors [10, 11]. Thus, a more exacerbated reaction in the initial inflammatory phase could be observed, proceeding the proliferative period. This intense initial response would be beneficial, as it would generate a more suitable location for a greater deposition of collagen as well as the formation of better quality collagen. Consequently, the final product would be a stronger wound repair tissue.
The data from this study support the hypothesis that the prostheses that induce an intense early inflammatory response could lead to increased collagen deposition in a subsequent phase if this reaction is transitory and waning, which would allow a more organized and improved collagen deposition. On the contrary, the maintenance of an inflammatory condition, generated by the composite materials and verified in later stages, results in comparatively less deposition of collagen. Further work on the types of collagen that is deposited at different times should help clarify the issues around the choice of the types of meshes for hernia repair.
References
Williams KB, Belyansky I, Dacey KT, Yurko Y, Augenstein VA, Lincourt AE, Horton J, Kercher KW, Heniford BT (2014) Impact of the establishment of a specialty hernia referral center. Surg Innov 21(6):572–579
DuBay DA, Wang X, Adamson B, Kuzon WM, Dennis RG, Franz MG (2006) Mesh incisional herniorrhaphy increases abdominal wall elastic properties: a mechanism for decreased hernia recurrences in comparison with suture repair. Surgery 140(1):14–24
Akinci M, Yilmaz KB, Kulah B, Seker GE, Ugurlu C, Kulacoglu H (2013) Association of ventral incisional hernias with comorbid diseases. Chirurgia 108(6):807–811
Calvi ENC, Nahas FX, Barbosa MV, Calil JA, Ihara SSM, Juliano Y, Ferreira LM (2014) Collagen fibers in the rectus abdominis muscle of cadavers of different age. Hernia 18(4):527–533
Cassar K, Munro A (2002) Surgical treatment of incisional hernia. Br J Surg 89(5):534–545
Burger JWA, Luijendijk RW, Hop WCJ, Halm JA, Verdaasdonk EGG, Jeekel J (2004) Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 240(4):578–583 (discussion 583–585)
Goldenberg A, Matone J, Marcondes W, Herbella FAM, Farah JFM (2005) Comparative study of inflammatory response and adhesions formation after fixation of different meshes for inguinal hernia repair in rabbits. Acta Cir Bras 20(5):347–352
Pascual G, Rodríguez M, Sotomayor S, Pérez-Köhler B, Bellón JM (2012) Inflammatory reaction and neotissue maturation in the early host tissue incorporation of polypropylene prostheses. Hernia 16(6):697–707
Klinge U, Klosterhalfen B (2012) Modified classification of surgical meshes for hernia repair based on the analyses of 1,000 explanted meshes. Hernia 16(3):251–258
Klosterhalfen B, Junge K, Klinge U (2005) The lightweight and large porous mesh concept for hernia repair. Expert Rev Med Devices 2(1):103–117
Klinge U, Klosterhalfen B, Birkenhauer V, Junge K, Conze J, Schumpelick V (2002) Impact of polymer pore size on the interface scar formation in a rat model. J Surg Res 103(2):208–214
Harrell AG, Novitsky YW, Cristiano JA, Gersin KS, Norton HJ, Kercher KW, Heniford BT (2007) Prospective histologic evaluation of intra-abdominal prosthetics four months after implantation in a rabbit model. Surg Endosc 21(7):1170–1174
Pereira-lucena CG, Artigiani Neto R, de Rezende DT, Lopes-Filho GJ, Matos D, Linhares MM (2014) Early and late postoperative inflammatory and collagen deposition responses in three different meshes: an experimental study in rats. Hernia 18(4):563–570
Vaz M, Krebs RK, Trindade EN, Trindade MRM (2009) Fibroplasia after polypropylene mesh implantation for abdominal wall hernia repair in rats. Acta Cir Bras 24(1):19–25
Zogbi L, Trindade EN, Trindade MRM (2013) Comparative study of shrinkage, inflammatory response and fibroplasia in heavyweight and lightweight meshes. Hernia 17(6):765–772
Rosch R, Junge K, Knops M, Lynen P, Klinge U, Schumpelick V (2003) Analysis of collagen-interacting proteins in patients with incisional hernias. Langenbecks Arch Surg 387(11–12):427–432
de Noronha L, Chin EWK, Kimura LY, Graf R (2004) Estudo morfométrico e morfológico da cicatrização após uso do laser erbium: YAG em tecidos cutâneos de ratos. J Bras Patol Med Lab 40(1):41–48
Peeters E, De Hertogh G, Junge K, Klinge U, Miserez M (2014) Skin as marker for collagen type I/III ratio in abdominal wall fascia. Hernia 18(4):519–525
Rosch R, Junge K, Quester R, Klinge U, Klosterhalfen B, Schumpelick V (2003) Vypro II® mesh in hernia repair: impact of polyglactin on long-term incorporation in rats. Eur Surg Res 35(5):445–450
Junge K, Rosch R, Krones CJ, Klinge U, Mertens PR, Lynen P, Schumpelick V, Klosterhalfen B (2005) Influence of polyglecaprone 25 (Monocryl) supplementation on the biocompatibility of a polypropylene mesh for hernia repair. Hernia 9(3):212–217
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CTM declares no conflict of interest. RAN declares no conflict of interest. GJLF declares no conflict of interest. MML declares no conflict of interest.
Statement of human and animal rights
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Informed consent
No informed consent.
Rights and permissions
About this article
Cite this article
Maeda, C.T., Artigani Neto, R., Lopes-Filho, G.J. et al. Experimental study of inflammatory response and collagen morphometry with different types of meshes. Hernia 20, 859–867 (2016). https://doi.org/10.1007/s10029-016-1513-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-016-1513-7