Abstract
Background
Despite the proven benefits of laparoscopic abdominal hernia repair (LAHR), only 25 % of elective ventral hernia repairs are currently performed using this method. This surprising trend may be due to the current limitations of LAHR including lack of defect closure, high seroma rates, and longer OR times. To address these challenges, a new method was developed that uses an innovative “finned” mesh configuration to combine defect closure via open dissection and laparoscopic underlay mesh placement.
Methods
A new “finned” mesh is sutured within the defect edges using a traditional open method and then approached laparoscopically for final fixation onto the peritoneal surface of the abdominal wall. The “fin” provides a perpendicular plane for suturing to avoid unintentional contact with any underlying viscera, centers the mesh symmetrically around the closed defect, and prevents mesh migration without stay sutures.
Results
A retrospective review was performed on 108 consecutive patients that had a ventral, incisional, or umbilical hernia repaired using the “finned” mesh between 2007 and 2013. The mean follow-up was 40.83 months. Average operating time was 64.84 min (range 25–144 min) with an average length of stay of 0.80 days (range 0–10 days). There were two intraoperative complications (1.85 %): one small bowel injury and one unexplained incidence of tachycardia. Major post-operative complications included two recurrences (1.85 %) and one small bowel obstruction (0.96 %). Fourteen minor post-operative complications were observed (12.96 %), with the most common being post-operative ileus (n = 4) and urinary retention (n = 3). There were zero incidents of seroma, wound infection, or mesh infection in this study.
Conclusion
This innovative laparoscopic method incentivizes surgeons to embrace the technique and its universally accepted advantages by mitigating the most challenging aspects of LAHR. Promising results indicate a potential new standard of care for ventral hernia repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Over 400,000 ventral hernia repairs are performed each year in the USA with the ultimate purpose to restore the integrity of the abdominal wall and prevent complications such as pain, incarceration, or strangulation [1]. Recurrence remains the most serious and expensive long-term complication because it fails to accomplish the intended purpose of surgical intervention and leads to reoperation. Mesh reinforcement significantly reduces the risk of recurrence and is widely recommended over suture repair for this reason [2, 3].
In 1993, Dr. Karl LeBlanc introduced laparoscopic abdominal hernia repair (LAHR) and revolutionized underlay mesh placement as an intraabdominal bridge over the hernia defect for a “tension-free” repair. The surgical community was initially enthusiastic about this new approach because of its smaller incisions, direct access to the abdominal cavity, and increased visibility. These new advantages significantly improved patient outcomes with shorter lengths of stay, lower rates of infection, less post-operative complications, and a quicker recovery compared to open abdominal hernia repair (OAHR) [4–8].
Despite these benefits, only 25 % of elective ventral hernia repairs are currently performed laparoscopically due to several limitations [9, 10]. The “tension-free” aspect of LAHR bridges the defect without restoring the integrity of the abdominal wall, which is a main intention of the repair. This has led to unacceptably high seroma rates, decreased abdominal function, and increased risk of recurrence [11–14]. LAHR also requires a technically advanced skillset that leads to long operating times and a dangerous learning curve that surgeons may prefer to avoid [15, 16].
To address these disadvantages, the author (GCC) has developed a simplified method for LAHR that combines defect closure via open dissection with laparoscopic underlay mesh placement. The crux of this new method is an innovative three-dimensional mesh with a “fin” that projects 4–5 mm above the longitudinal centerline of the mesh (Fig. 1). The “fin” of the mesh is sutured within the hernia defect edges, which suspends the body of the mesh within the abdominal cavity and centers it around the closed defect. A pneumoperitoneum is then established to complete the repair laparoscopically by spreading the suspended mesh onto the peritoneal surface of the abdominal wall and anchoring it in place.
The purpose of the “finned” mesh method is to improve patient outcomes and provide surgeons with a simplified laparoscopic approach that mitigates its technical difficulty. This study retrospectively reviews the outcomes for 108 consecutive patients who had undergone a ventral, incisional, or umbilical hernia repair using this simplified “finned” mesh method by the authoring surgeon (GCC) between 2007 and 2013.
Materials and method
Materials
-
Broad-spectrum antibiotic: administered intravenously within 1 h prior to incision. For this study, one gram of Ancef was given unless the patient was allergic to cephalosporin or penicillin, in which case 500 mg of Levaquin or 900 mg of clindamycin was administered.
-
“Finned” mesh: a “fin” was fashioned along the longitudinal axis of a commercially available coated polypropylene mesh with a continuous 3-0 Vicryl suture and used for this study.
-
Gentamycin solution: 80 mg of gentamycin in 100 cc of normal saline used for soaking the prosthetic mesh before insertion into the abdomen.
-
Three clamps: two Kelly clamps are used to clamp the rolled edges of the mesh for introduction into the abdomen through the open hernia defect. One straight clamp is applied at the center of the “fin” as a marker for positioning guidance while suturing the defect edges.
-
Nonabsorbable suture: used for closure of the hernia defect with the mesh fin incorporated within. In this series, either a zero or #1 polypropylene suture was used.
-
Three 5 mm ports: used to perform the laparoscopic portion of the hernia repair.
-
Laparoscopic shears and/or energy device: used for lysis of hard adhesions. A laparoscopic gauze dissector is used for lysis of soft adhesions as needed.
-
Laparoscopic gauze dissector and/or grasper: used for intraabdominal manipulation and positioning of the mesh prior to final anchoring.
-
Mesh fixation device: either absorbable or nonabsorbable tacks or anchors are used to secure the mesh onto the peritoneal surface of the abdominal wall.
Operative method
A broad-spectrum antibiotic is given intravenously within 1 h prior to incision. The patient is placed in the supine position, and general endotracheal anesthesia is administered. An incision is made over the hernia defect and extended through the skin, subcutaneous tissue, and peritoneum (sac) to the peritoneal cavity. Any adhesions between the sac and omentum or other viscera are lysed. A 5-mm port is inserted through the incision into the peritoneal cavity and secured in place with an airtight purse string. The 5-mm port is connected to the CO2 source, and a pneumoperitoneum is established at 15 mm Hg pressure. A 5-mm 0° scope is then introduced into the abdomen through the port for video exploration. Under video control, two 5 mm ports are inserted far from the defect and approximately 10 cm from each other. The scope is then inserted through the second or third port, and the first port is removed from the defect site. Under video control, the first port is reintroduced into the abdomen so that the lines between the ports form a triangle in the case of a midline hernia (this port placement would vary depending on the primary hernia site and the patient’s body habitus). Any additional adhesions are lysed laparoscopically, and any additional hernia defects are identified for inclusion in the repair. The purse string is then removed, and the pneumoperitoneum is evacuated. The hernia sac is dissected free all the way down to the fascial layer and excised. No additional dissection is carried out around the defect deep to the subcutaneous tissue.
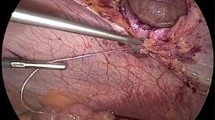
The “finned” mesh (Fig. 1) is then selected with appropriate dimensions so that it extends at least 5 cm beyond any point of the sutured hernia defect. The “finned” mesh is soaked in a solution of 80 mg of gentamycin in 100 cc of normal saline in preparation for implantation. The body of the mesh at either side of the “fin” is then rolled toward the “fin,” and the ends are engaged with two Kelly clamps. A straight clamp is placed at the center of the “fin” as a visual marker and to assist with the manipulation and control of the mesh. The mesh is then inserted into the abdomen through the hernia defect, and the two Kelly clamps at the end of the mesh are removed. The mesh is unrolled with either a clamp or finger and left unfolded inside the abdomen with the “fin” positioned between the edges of the hernia defect. The hernia defect is closed with a continuous zero or #1 polypropylene suture, incorporating the “fin” within the suture line between the approximated defect edges (Fig. 2). This leaves the mesh suspended from the sutured defect securely inside the peritoneal cavity and thus ready for laparoscopic anchoring (Fig. 3).
The pneumoperitoneum is then reestablished at a reduced pressure of 10 mm Hg. A 5-mm 30° scope is used to complete the laparoscopic portion of the procedure. The mesh is gently unfolded by manipulation with a laparoscopic gauze dissector or grasper and spread over the peritoneal surface of the abdominal wall symmetrically around the closed defect. The mesh is secured with two concentric rows of anchors or tacks to its final position of the abdominal wall extending at least 5 cm beyond any point of the suture line.
The pneumoperitoneum is then evacuated, and all three ports removed, concluding the laparoscopic portion of the repair. The skin and subcutaneous tissues are closed with absorbable sutures and subcuticular plastic closure. Proper dressing is applied, and the hernia repair is complete.
Data method
One hundred eight consecutive patients were identified between January 2007 thru October 2013 who had undergone a ventral, incisional, or umbilical hernia repair performed by the authoring surgeon using the “finned” mesh method.
Hospital and office patient records were reviewed to identify the following: age, sex and BMI of the patient, type and number of hernia defects, type and number of mesh used, length of stay, intraoperative complications, and post-operative complications. Mean operative time was calculated using operating room records for patients who underwent this particular hernia repair without any concomitant procedures (n = 97). Eleven patients were excluded from the mean operative time calculation (concurrent inguinal hernia repair, n = 10; concurrent hysterectomy, n = 1).
Patients were contacted for voluntary follow-up either with a free office examination by the operating surgeon, a telephone interview, or a written questionnaire. These follow-ups were combined with existing office records to identify the following: post-operative complications, patient complaints of prolonged pain, seroma, recurrence, infection of the wound and/or mesh, and any other complaints.
Definitions
-
Recurrence: the presence of a hernia at the same general area of a previous repair
-
Prolonged pain: pain interfering with the patient’s quality of life, as described by the patient, that persists for more than 1 month post-operatively
-
Clinical seroma: the presence of a palpable or visible collection of fluid at the site of the hernia repair
-
Infection: purulence at the wound site with or without mesh involvement
-
Cellulitis: skin erythema at the incision site
-
Obese: BMI equal to or greater than 30 kg/m2
-
Morbidly obese: BMI equal to or greater than 40 kg/m2
-
Length of stay (LOS): number of nights spent in the hospital post-operatively
-
Mortality: patient death within 30 days of the operation
Results
Patient characteristics
There were 108 consecutive patients included in this study (47 females, 61 males) with an average age of 62.07 years (range 28–91). Twenty-eight patients (25.93 %) presented with incarcerated hernia, four of which were admitted as emergencies, with two patients having small bowel obstruction. The average BMI for all patients was 32.30 (range 20–52) with 64 obese patients (59.26 %) having a BMI equal to or over 30. The mean follow-up for all patients was 40.83 months ranging from 3 weeks to 83 months, with 99 patients having a follow-up of at least 1 year (Table 1).
Procedure information
There were a total of 110 “finned” meshes used for the repair of 139 defects, including 89 incisional, 20 ventral, and 30 umbilical herniae with four emergency admissions. Eighteen patients had more than one defect repaired during the same procedure, with two of those patients requiring two separate meshes for defects located in the epigastric and lower midline areas of the abdomen. The most common mesh size was 10 × 15 cm which was used to repair 64 defects, followed by the 10 × 10 cm mesh used in 17 defects. Average operating time was 64.8 min per procedure ranging between 25 and 144 min (Table 2).
Post-operative monitoring
Length of stay (LOS)
LOS ranged between zero and 10 days with an average of 0.80 days. Seventy-two patients (66.6 %) were discharged on the same day as the procedure and counted as a LOS of zero.
Mortality
There was no mortality in this series.
Complications
There were two intraoperative complications (1.85 %): one major (0.96 %) and one minor (0.96 %). The major complication was a small bowel injury with the harmonic scalpel during adhesiolysis which was immediately identified and repaired during surgery without further incident. The hernia repair proceeded as planned after the bowel injury was repaired. The minor complication presented as an unexplained tachycardia that was resolved intraoperatively with IV administration of labetalol without further incident.
There were two recurrences (1.85 %) in this study. One patient had an epigastric hernia recurrence identified 16 months after a periumbilical midline hernia repair. This 66-year-old male patient was too high a risk of reoperation due to severe cardiac and pulmonary comorbidities making him a prohibitive surgical risk. We therefore cannot identify the exact reason for this particular repair failure nor could we determine whether this is a true recurrence of the previous hernia or a new incisional hernia outside the boundaries of the repaired defect. The second patient to present with a recurrent hernia was a 45-year-old morbidly obese female with a BMI of 51. A CAT scan confirmed a recurrent epigastric hernia observed 17 months post-surgery, and reoperation had not yet been performed at the time of this review.
There was one other major post-operative complication (0.93 %). A patient was admitted through the emergency room 19 days after the hernia repair with small bowel obstruction secondary to adhesions. The small bowel obstruction was resolved with laparoscopic lysis of adhesions without any further complication.
A total of 14 minor post-operative complications were observed (12.96 %), with the most common being post-operative ileus (n = 4) and urinary retention (n = 3). There were zero incidents of wound or mesh infection, and zero incidence of seroma in this study (Table 3).
Post-operative pain
Six total patients complained of post-operative pain more than 1 month after surgery (5.61 %). One patient reported prolonged pain prior to surgery which continued for several months after the hernia repair; the pain was eventually resolved with treatment at a pain clinic. The remaining five patients reported a late onset “needle-like” pain. Upon physical examination of these patients, an excess polypropylene suture (suture stump) was palpable just under the skin at the incision site which was identified to be the cause of this specific pain sensation. Two of these patients had the excess suture removed under local anesthetic for complete resolution of the pain, while the remaining three patients did not consider the pain severe enough to warrant surgical intervention.
Discussion
The standard bridge repair used in LAHR became a convenient solution to a lack of suitable options for defect closure in the minimally invasive environment. This tension-free repair has been found to significantly increase the risk of recurrence and overall complications compared to mesh reinforcement with fascial coaptation [17]. A comprehensive review by Nguyen et al. [18] indicates that LAHR combined with fascial closure results in reduced recurrence rates (0–5.7 % with closure vs. 4.8–16.7 % without closure), reduced seroma formation rates (5.6–11.4 % with closure vs. 4.3–27.8 % without closure), improved abdominal wall function, and higher patient satisfaction compared to the standard laparoscopic bridge repair. All eleven relevant studies reviewed by Nguyen et al. used either extracorporeal or intracorporeal sutures to approximate the defect. The “finned” mesh method expands on this emerging technique by adding limited open dissection and a new mesh configuration to enhance the laparoscopic portion of the repair.
In addition to improving abdominal wall function and overall patient outcomes, approximating the hernia defect also eliminates the dead space between the defect edges. Reducing the defect from a two-dimensional area to a one-dimensional suture line likewise reduces the mesh size required to cover the defect with sufficient overlap. Mesh extension is recommended to be at least 5 cm beyond the edges of the defect to prevent mesh migration, infection, and ultimately recurrence [19, 20]. Using a 5 cm × 5 cm hernia defect as an example, a tension-free repair would require a 15 × 15 cm (225 cm2) mesh for adequate coverage, whereas the same repair over an approximated defect would require a 10 × 15 cm mesh (150 cm2)—or 33 % less area—to accomplish the same ideal 5 cm overlap. This reduction in mesh size makes it easier to manipulate and also reduces the risk of post-operative complications [21, 22].
One advantage of using an open approach to approximate the hernia defect is the ability to directly access the hernia sac. Excision of the hernia sac allows the open defect gap to be used as the initial port site for laparoscopic exploration, which avoids additional dissection and provides a wide insertion point into the abdominal cavity for the mesh. The hernia sac is also a significant factor in the unacceptably high rate of clinical seroma in LAHR, which has been reported as high as 100 % [11, 23–25]. Seroma formation is a unique complication that is often considered a minor esthetic problem, but can lead to more serious complications such as infection, mesh explantation, and recurrence [11, 26, 27]. It was expected with the “finned” mesh method that defect approximation combined with hernia sac excision would decrease the risk of seroma, and there were no clinical seromas observed in this study.
The most important benefit of using an initial open incision is the direct access to suture the “fin” of the mesh between the edges of the defect. This unique mesh configuration greatly reduces the technical difficulty of the laparoscopic repair, and the “fin” provides a perpendicular plane for suturing to help avoid any unintentional contact with underlying viscera. The most difficult and time-consuming tasks of LAHR are the manipulation of the mesh and the application of stay sutures. Specifically, positioning the mesh so that it is centered over the defect with sufficient overlap is challenging to perform under video control with laparoscopic instruments. Since the mesh “fin” coincides with its centerline and there is at least 5 cm on either side, its incorporation within the defect automatically centers the mesh around the defect with sufficient overlap symmetrically in each direction. Furthermore, the “fin” fixation removes the need for stay sutures, and the surgeon is instead able to skip directly to spreading and anchoring a securely prepositioned mesh. Compounding these difficult tasks saves valuable operating time by delegating only the most basic elements—spreading and anchoring the mesh—to be completed in a minimally invasive environment.
Even though it is challenging to measure an intangible trait such as “technical difficulty” without a controlled analysis, the difficulty of any procedure has a direct effect on operating time. The median operating time in LAHR has been shown to be significantly longer when compared to traditional open repair (149 vs. 89 min, respectively) [28]. A comprehensive analysis of 6266 published laparoscopic ventral hernia repairs by Carlson et al. [29] shows LAHR to have an average operating time of 110 min (range 43–252 min), while the mean operating time with the “finned” mesh method was almost half that at 64.8 min (range 25–144 min). This significant decrease in operating time lowers the risk of patient readmission and mesh infection and decreases overall healthcare costs [30, 31].
Limiting the extent of open dissection is a large factor in keeping the superior outcomes associated with LAHR intact with this new method. Even though the “finned” mesh technique begins with an “open” incision, dissection is not extended beyond the edges of the defect since adhesiolysis and mesh anchoring are performed laparoscopically. These specific tasks require extensive dissection to perform in an exclusively open environment, but by delegating these to the laparoscopic approach, the “finned” mesh method reduces the risk of infection.
Surgical site infections (SSI) are an expensive complication that leads to longer hospital stays with an increased risk of reoperation and mesh explantation [32, 33]. The odds of acquiring a surgical site infection (SSI) in OAHR are up to seven times greater than in LAHR, and this is a main reason LAHR has a shorter length of stay compared to OAHR (2.4 vs. 4.3 days, respectively) [6, 34, 35]. In this study, even with limited open dissection, there were no superficial or deep wound infections, and the majority of patients underwent the “finned” mesh repair as a same day surgery (n = 71, 67 %) with an average hospital stay of 0.80 days (range 0–10 days). There was one instance of wound erythema-cellulitis (0.93 %) on a morbidly obese patient (BMI = 51), which was resolved with oral antibiotics, who was discharged on the same day as surgery.
Post-operative wound infections also increase the risk of mesh infection, which is a catastrophic complication associated with LAHR [31, 33]. Mesh infections cost over $75,000 per event and can result in multiple reoperations, potential mesh explantation, and long-term chronic wound complications [36–38]. OAHR has a significantly higher mesh infection rate compared to LAHR (10 vs. 0.78 %, respectively), and this is a major cause for concern when attempting open intraabdominal underlay of a composite mesh [29, 37]. The mesh infection rate in this study is comparable to LAHR with zero mesh infections observed in this study, which can be attributed to the limited dissection and low SSI rate. These results may also support a prophylactic effect of the IV antibiotics and gentamycin solution, and this correlation would need further investigation to be verified.
There were five patients that reported “needle-like pain” post-operatively. Physical examination revealed the cause to be polypropylene suture stumps extending beyond the knot used to secure the mesh “fin” within the defect. This was a new complication encountered in our series that we believe is preventable. After identifying the source of this pain, the operating surgeon began cutting the polypropylene suture at the base of the stump and burying the knot deeper within the tissue. No additional incidences of “suture stump” pain were observed after this adjustment, but there were not enough data to confirm whether or not this adjustment is effective. Additional monitoring of this particular outcome is necessary to provide further insight.
Recurrence represents the ultimate failure of any abdominal hernia repair, and long-term data show no significant difference between open and laparoscopic recurrence rates [4, 7, 14, 39]. Currently, the annual healthcare costs of abdominal wall hernia recurrence are estimated at $3.2BN in the USA, with $32MM in savings for each percentage point decrease in recurrence rates [40]. Several surgical aspects have proven effective in reducing recurrence, including mesh reinforcement with sufficient overlap, closure of the defect, and underlay mesh placement [18, 41, 42]. The “finned” mesh configuration combines all of these to create an ideal synergistic environment to prevent recurrence without increasing the risk of other complications.
Neither of the two patients who recurred in this study had reoperation at the time of this review, so the cause of each recurrence remains unknown. However, both patients were obese: a female with a BMI of 51 and a male with a BMI of 31. Obesity significantly increases the risk of recurrence, and the majority of the patients in this study were obese with a BMI over 30 (n = 64) [26, 27, 43, 44]. Fourteen (21.87 %) of these obese patients were having a recurrent hernia repaired, with five having multiple recurrences at the time of surgery. Post-operative recurrence rates were substantially reduced in obese patients from 21.87 to 3.12 %, and in morbidly obese patients (n = 4) from 25 to 5.25 %. Furthermore, out of the 19 patients with prior recurrences, 13 of them were obese (68.42 %), and none of these patients reported a post-operative recurrence (Table 4). Just as recurrence represents the ultimate failure, we feel that this lack of recurrence in high-risk patients represents the ultimate success.
One of the largest barriers to introducing a new technique is the additional training and knowledge required to perform a new skill. This particular hybrid method relies on a combination of already existing methods in open and laparoscopic repair while also mitigating the most difficult aspects of LAHR. The only new component of this hybrid procedure involves the incorporation in the mesh “fin” within the suture line of the closed defect, which demands a basic skillset that most surgeons already have or should be able to adopt easily. More surgeons in the same medical center as the authoring surgeon have adapted this particular technique, though a formal review of these additional patients has not yet been conducted. Additional surgeon feedback will be needed to analyze any learning curve associated with adapting this technique and also verify this new method’s effect on patient outcomes after ventral, incisional, and umbilical hernia repair.
Conclusion
The “finned” mesh method uses limited open dissection to enhance laparoscopic ventral hernia repair by suturing the mesh within the approximated defect. This new mesh configuration simplifies the most challenging aspects of LAHR and will encourage more surgeons to embrace the technique and its advantages. Our initial results indicate that it is a safe and efficient technique with a positive synergistic effect on recurrence (particularly in high-risk patients), operating time, mesh infection, wound infection, seroma rate, and length of stay. Future controlled studies are necessary to validate the outcomes observed in this study and any potential learning curve associated with this technique.
References
DeFrances CJ, Cullen KA, Kozak LJ (2007) National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13:1–209
Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, IJzermans JN, Boelhouwer RU, de Vries BC, Salu MK, Weredlsma JC, Brujninckx CM, Jeekel J (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343:392–398
Nguyen MT, Berger RL, Hicks SC, Davila JA, Li LT, Kao LS, Liang MK (2014) Comparison of outcomes of synthetic mesh vs suture repair of elective primary ventral herniorrhaphy: a systematic review and meta-analysis. JAMA Surg 149:415–421
Sajid MS, Bokhari SA, Mallick AS, Cheek E, Baig MK (2009) Laparoscopic versus open repair of incisional/ventral hernia: a meta-analysis. Am J Surg 197:64–72
Olmi S, Scaini A, Cesana GC, Erba L, Croce E (2007) Laparoscopic versus open incisional hernia repair: an open randomized controlled study. Surg Endosc 21:555–559
Arita NA, Nguyen MT, Nguyen DH, Berger RL, Lew DF, Suliburk JT, Askenasy EP, Kao LS, Liang MK (2014) Laparoscopic repair reduces incidence of surgical site infections for all ventral hernias. Surg Endosc. doi:10.1007/s00464-014-3859-1
Liang MK, Berger RL, Li LT, Davila JA, Hicks SC, Kao LS (2013) Outcomes of laparoscopic vs open repair of primary ventral hernias. JAMA Surg 148:1043–1048
Itani K, Hur K, Kim LT, Anthony T, Berger DH, Reda D, Neumayer L, Investigators Veterans Affairs Ventral Incisional Hernia (2010) Comparison of laparoscopic and open repair with mesh for the treatment of ventral incisional hernia: a randomized trial. Arch Surg 145:322–328
Funk LM, Perry KA, Narula VK, Mikami DJ, Melvin WS (2013) Current national practice patterns for inpatient management of ventral abdominal wall hernia in the United States. Surg Endosc 27:4104–4112
Aher CV, Kubasiak JC, Daly SC, Janssen I, Deziel DJ, Millikan KW, Myers JA, Luu MB (2014) The utilization of laparoscopy in ventral hernia repair: an update of outcomes analysis using ACS-NSQIP data. Surg Endosc. doi:10.1007/s00464-014-3798-x
Carter SA, Hicks SC, Brahmbhatt R, Liang MK (2014) Recurrence and pseudorecurrence after laparoscopic ventral hernia repair: predictors and patient-focused outcomes. Am Surg 80:138–148
den Hartog D, Eker HH, Tuinebreijer WE, Kleinrensink GJ, Stam HJ, Lange JF (2010) Isokinetic strength of the trunk flexor muscles after surgical repair for incisional hernia. Hernia 14:243–247
Shestak KC, Edington HJ, Johnson RR (2000) The separation of anatomic components technique for the reconstruction of massive midline abdominal wall defects: anatomy, surgical technique, applications, and limitations revisited. Plast Reconstr Surg 105:731–738
Sauerland S, Walgenbach M, Habermalz B, Seiler CM, Miserez M (2011) Laparoscopic versus open surgical techniques for ventral or incisional hernia repair. Cochrane Database Syst Rev. doi:10.1002/14651858.CD007781.pub2
Al-Harazi A, Goel R, Tan CT, Cheah WK, Lomanto D (2014) Laparoscopic ventral hernia repair: defining the learning curve. Surg Laparosc Endosc Percutan Tech 24:475–477
Bencini L, Sanchez LJ (2004) Learning curve for laparoscopic ventral hernia repair. Am J Surg 187:378–382
Booth JH, Garvey PB, Baumann DP, Selber JC, Nguyen AT, Clemens MW, Liu J, Butler CE (2013) Primary fascial closure with mesh reinforcement is superior to bridged mesh repair for abdominal wall reconstruction. J Am Coll Surg 217:999–1009
Nguyen DH, Nguyen MT, Askenasy EP, Kao LS, Liang MK (2014) Primary fascial closure with laparoscopic ventral hernia repair: systematic review. World J Surg 38:3097–3104
Lambrecht J (2011) Overlap-coefficient for the relationship between mesh size and defect size in laparoscopic ventral hernia surgery. Hernia 15:473–474
Guerin G, Turquier F (2013) Impact of the defect size, the mesh overlap, and the fixation depth on ventral hernia repairs: a combined experimental and numerical approach. Hernia 17:647–655
Diana M, Callari C, D’Agostino J, Wu HS, Mutter D, Marescaux J (2014) Laparoscopic tension-free abdominal wall repair: impact of mesh size and of different fixation devices in a consecutive series of 120 patients. Surg Laparosc Endosc Percutan Tech 24:461–464
Morales-Conde S, Suarez-Artacho G, Socas-Mocias M, Barranco-Moreno A (2015) Retroprosthetic seroma after laparoscopic ventral hernia repair: incidence, risk factors and clinical significance. Hernia. doi:10.1007/s10029-015-1352-y
Susmallian S, Gewurts G, Ezri T, Charuzi I (2001) Seroma after laparoscopic repair of hernia with PTFE patch: is it really a complication? Hernia 5:139–141
Rea R, Falco P, Izzo D, Leongito M, Amato B (2012) Laparoscopic ventral hernia repair with primary transparietal closure of the hernia defect. BMC Surg. doi:10.1186/1471-2482-12-S1-S22
Birch DW (2007) Characterizing laparoscopic incisional hernia repair. Can J Surg 50:195–201
Vidovic D, Jurisic D, Franjic BD, Glavan E, Ledinsky M, Bekavac-Beslin M (2006) Factors affecting recurrence after incisional hernia repair. Hernia 10:322–325
Sailes FC, Wallis J, Guelig D, Mirzabeigi M, Long WD, Crawford A, Moore JH Jr, Copit SE, Tuma GA, Fox J (2010) Synthetic and biological mesh in component separation: a 10-year single institution review. Ann Plast Surg 64:696–698
Earle D, Seymour N, Fellinger E, Perez A (2006) Laparoscopic versus open incisional hernia repair: a single-institution analysis of hospital resource utilization for 884 consecutive cases. Surg Endosc 20:71–75
Carlson MA, Frantzides CT, Shostrom VK, Laguna LE (2008) Minimally-invasive ventral herniorrhaphy: an analysis of 6,266 published cases. Hernia 12:9–22
Nguyen MT, Li LT, Hicks SC, Davila JA, Suliburk JW, Leong M, Kao LS, Berger DH, Liang MK (2013) Readmission following open ventral hernia repair: incidence, indications, and predictors. Am J Surg 206:942–949
Sanchez VM, Abi-Haider YE, Itani KM (2011) Mesh infection in ventral incisional hernia repair: incidence, contributing factors, and treatment. Surg Infect (Larchmt) 12:205–210
Liang MK, Li LT, Nguyen MT, Berger RL, Hicks SC, Kao LS (2014) Abdominal reoperation and mesh explantation following open ventral hernia repair with mesh. Am J Surg 208:670–676
Hawn MT, Gray SH, Snyder CW, Graham LA, Finan KR, Vick CC (2011) Predictors of mesh explantation after incisional hernia repair. Am J Surg 202:28–33
Kaoutzanis C, Leichtle SW, Mouawad NJ, Welch KB, Lampman RM, Cleary RK (2013) Postoperative surgical site infections after ventral/incisional hernia repair: a comparison of open and laparoscopic outcomes. Surg Endosc 27:2221–2230
Pierce RA, Spitler JA, Frisella MM, Matthews BD, Brunt LM (2007) Pooled data analysis of laparoscopic vs. open ventral hernia repair: 14 years of patient data accrual. Surg Endosc 21:378–386
Le D, Deveney CW, Reaven NL, Funk SE, McGaughey KJ, Martindale RG (2013) Mesh choice in ventral hernia repair: so many choices so little time. Am J Surg 205:602–607
Cobb WS, Carbonell AM, Kalbaugh CL, Jones Y, Lokey JS (2009) Infection risk of open placement of intraperitoneal composite mesh. Am Surg 75:762–768
Hanna M, Dissanaike S (2015) Mesh ingrowth with concomitant bacterial infection resulting in inability to explant: a failure of mesh salvage. Hernia 19(2):339–344
Ballem N, Parikh R, Berber E, Siperstein A (2008) Laparoscopic versus open ventral hernia repairs: 5 year recurrence rates. Surg Endosc 22:1935–1940
Poulose BK, Shelton J, Phillips S, Moore D, Nealon W, Penson D, Beck W, Holzman MD (2012) Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 16:179–183
LeBlanc KA, Whitaker JM, Bellanger DE, Rhynes VK (2003) Laparoscopic incisional and ventral hernioplasty: lessons learned from 200 patients. Hernia 7:118–124
Hawn MT, Snyder CW, Graham LA, Gray SH, Finan KR, Vick CC (2010) Long-term follow-up of technical outcomes for incisional hernia repair. J Am Coll Surg 210:648–657
Stey AM, Russel MM, Sugar CA, Hall BL, Zingmond DS, Lawson EH, Ko CY (2015) Extending the value of the National Surgical Quality Improvement Program claims dataset to study long-term outcomes: rate of repeat ventral hernia repair. Surgery. doi:10.1016/j.surg.2014.12.027
Raftopoulos I, Courcoulas AP (2007) Outcomes of laparoscopic ventral hernia repair in morbidly obese patients with a body mass index exceeding 35 kg/m2. Surg Endosc 21:2293–2297
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Christoudias owns US Patent #8,343,231 and US Patent #8,663,256 pertaining to the “finned” mesh hernia repair method and mesh, and reports an equity interest in Apollo Surgical Industries, Inc. (Teaneck, NJ), outside the submitted work. Maritsa Nunziata reports equity interest in Apollo Surgical Industries, Inc. (Teaneck, NJ), outside the submitted work.
Rights and permissions
About this article
Cite this article
Christoudias, G., Nunziata, M. A simplified laparoscopic approach to ventral hernia repair: a new “finned” mesh configuration with defect closure. Surg Endosc 30, 2632–2640 (2016). https://doi.org/10.1007/s00464-015-4480-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4480-7