Abstract
Hyoid position and swallowing-related displacement has been studied in healthy adults and adults with dysphagia, but research is limited in children. The aim of this study was to investigate feasibility of visualizing and measuring position and swallowing-related displacement of the hyoid bone in children. We explored relationships between hyoid displacement, age and aspiration risk scores. Pediatric swallowing data were extracted from a videofluoroscopy database containing recordings from 133 children aged 9 days to 21 years (mean 36 months, SD 3 years) referred for videofluoroscopy due to concerns regarding their feeding. Children presented with varying etiologies: neurological, structural, respiratory, and other diagnoses. Still shot images were extracted for the frame of hyoid peak position and a frame showing the hyoid at rest. Pixel-based image analysis software was used to measure hyoid position in three directions (X = anterior, Y = superior, XY = hypotenuse) relative to C4 vertebra. Difference between rest and peak position was used to measure hyoid displacement (X, Y and XY). The hyoid was not visible in children < 9 months, but could be reliably visualized and measured in 49 children. Descriptive statistics were collected for hyoid parameters. Age was significantly associated with rest (Y and XY) and peak (Y and XY) hyoid position parameters as well as anterior displacement. No significant associations were observed between hyoid parameters and aspiration risk scores. This study successfully explored hyoid visibility, position and swallowing-related displacement in a pediatric population. Hyoid can be reliably visualized and tracked through videofluoroscopy in children > 9 months of age.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Swallowing is a complex process through which children and adults receive essential nutrition and hydration in order to sustain life. Any anatomical, respiratory, or neurologic disturbance to this process can result in swallowing difficulties (dysphagia). Dysphagia often presents as penetration–aspiration (food/fluids/saliva entering the airway) or residue (food/fluids/saliva remaining in the mouth or pharynx after swallowing), posing a significant risk for respiratory sequelae in vulnerable populations [1,2,3].
Videofluoroscopy is a radiologic imaging technique that is commonly used in assessment of swallowing. The primary purpose of videofluoroscopy is to identify abnormalities in the physiology of the pharyngeal phase of swallowing, and to confirm the presence of penetration–aspiration and post-swallow residue [4]. Videofluoroscopy provides a clinician with dynamic visualization of the oropharynx and upper esophagus [5, 6] and is considered the most accurate tool for identifying penetration and aspiration in pediatric swallowing [7, 8].
During swallowing, the hyoid bone moves in an anterior–superior direction. Due to the muscle and ligament attachments between the hyoid, the epiglottis, the thyroid cartilage, and the arytenoid process, this motion contributes to closure of the laryngeal vestibule and deflection of the epiglottis [9]. Movement of the hyolaryngeal complex also facilitates opening of the upper esophageal sphincter (UES). Sub-atmospheric pressure generated by the opening of the UES works in combination with pressure applied behind the bolus by the base of tongue and pharynx to facilitate bolus movement into the esophagus [10,11,12]. Individuals presenting with a reduction in hyoid and laryngeal displacement during swallowing show reduced opening of the UES and reduced epiglottic deflection [13]. This is associated with risks of residue and aspiration before, during and after the swallow event [13].
Hyoid position and displacement have been investigated in both healthy and dysphagic adult populations through videofluoroscopy, both perceptually [14] and quantitatively [15, 16]. Quantitative measurement of hyoid movement requires the following components: (i) a stable origin from which to measure; (ii) a defined Cartesian coordinate system; (iii) a measurement scale; and (iv) normalization using a reference scalar, either in millimeters or an anatomical unit such as length of the C2–C4 cervical spine [16].
There is currently no research exploring the quantitative measurement of hyoid position and displacement in healthy or dysphagic pediatric populations. The physiological development of hyoid position and displacement in the early years of life is poorly understood and it remains unclear whether abnormalities in hyoid movement contribute to swallowing dysfunction in children. Parameters that characterize adult swallowing physiology cannot be generalized to pediatric populations due to anatomical and physiological differences in the developing child [1, 8, 17]. However, collating normative videofluoroscopic data in healthy children would require radiation exposure, which is unacceptable. Nevertheless, quantitative reference parameters of timing and displacement in pediatric swallowing are needed [1, 8, 18, 19], and current subjective methods of analysis have been criticized for poor inter-rater reliability [7, 20, 21]. Establishing quantitative reference values regarding hyoid movement in pediatric swallowing has the potential to inform our understanding of swallowing physiology and to guide treatment.
Several studies have successfully obtained and utilized timing and displacement measures of swallowing in pediatric populations [8, 18, 22, 23]. However, the hyoid is often reported to be inconsistently visualized and measures of hyoid position have frequently been left out of final analyses. This study is a preliminary exploration of the feasibility of obtaining hyoid position and displacement data in a pediatric population, applying a measurement technique well established in the adult literature [16] to videofluoroscopy recordings collected in children referred for swallowing assessment. Our research questions were as follows:
-
(i)
Can the hyoid be reliably visualized on videofluoroscopy in children?
-
(ii)
In cases where the hyoid can be visualized, how do measurements of hyoid position and displacement in children compare to those reported for adults? and
-
(iii)
Is hyoid displacement in children associated with age or aspiration status?
We hypothesized that the hyoid bone would become reliably visible in children around 2 years of age and older and that the position of the hyoid at rest would descend with advancing age. We predicted that older children in the study would present with hyoid displacement data similar to published adult data [16] and we predicted that there would be a reduction in superior hyoid displacement in children who aspirated compared to those who did not aspirate [15].
Methods
Participants
Pediatric swallowing data were extracted from The University of Auckland videofluoroscopy database, comprising recordings from 133 pediatric patients (65% male) referred for videofluoroscopy for suspected feeding difficulties between June 2014 and July 2015 at the Starship Children’s Hospital, Auckland, New Zealand. Participant chronological age ranged from 9 days to 21 years (mean age 36 months, SD 3 years) representing the full range of pediatric dysphagia management in a tertiary hospital with both a large neonatal unit as well as specialist outpatient follow-up into early adulthood for children with severe disabilities such as cerebral palsy. Children in the database presented with a number of different conditions, which were coalesced into the following groups: neurological diagnoses (acquired and congenital), structural diagnoses (congenital abnormalities of the structures involved in swallowing), respiratory diagnoses, and other (including cardiac, gastrointestinal tract, other or nil diagnoses). Ethical approval was obtained from The University of Auckland Human Participants Ethics committee.
Videofluoroscopy Procedure
All videofluoroscopy exams were completed according to a standardized protocol [18] using a Siemens Sireskop radiographic unit (Siemens, Munich, Germany) in lateral view. Videofluoroscopy was captured using continuous fluoroscopy recorded at 25 frames per second directly onto the hospital picture archiving and communication system (PACS). Videos were later exported to an external hard drive in AVI format for further analysis. Varibar® Thin Liquid barium sulfate powder for suspension (40% w/v) (E-Z-EM Canada Inc, Quebec, Canada) was used as per product instruction. Dependent on the child’s feeding stage and skill, the assessment captured events during (i) “midfeed sucking” for infants using a bottle, (ii) “midfeed drinking” for young children drinking sequentially from a sipper cup or, (iii) measured bolus sizes (5 ml, 10 ml, 20 ml, followed by a 100 ml sequential drinking task) in children who had adequate cup-drinking skills and were able to follow instructions.
Videofluoroscopy Rating
The first step in the videofluoroscopy rating procedure was inspection of the recorded images to determine whether or not the hyoid could be visualized at rest. This judgment was made by two independent raters, blinded to the child’s age and to each other’s ratings. There was 100% agreement between raters (Table 1). The hyoid was visible at rest in 60 of the 133 cases, which were carried forward for further rating.
Still shot images were extracted from each video clip as follows: (i) a frame showing the hyoid at rest; and (ii) a frame showing the hyoid at its peak anterior–superior position during the swallow. Rest position was captured from the start or end of each 20-s loop (younger child) or swallow (older child), dependent on where the child took a rest during that swallow sequence. The peak hyoid position still shot was captured from the swallow demonstrating the maximum (i.e., worst) penetration–aspiration score in the sequence or, in cases where no penetration–aspiration occurred, from the first swallow in the videofluoroscopic sequence. Frame selection was performed in duplicate by a second rater on a randomly selected 20% of the data for the purposes of calculating inter-rater reliability. There was 100% agreement on still shot frame selection for all frames (hyoid at rest, and hyoid peak position) (Table 1).
In 11 cases, the raters were unable to reliably visualize the hyoid at peak position due to obstruction by the shadow of the mandible or to motion artifact; these cases were excluded from further analysis (Fig. 1). The final dataset comprised 49 cases (31 male; 18 female; mean age 75 months, range 10–253, SD 61). Diagnoses for these children were as follows: 18 had a neurological etiology (e.g., Trisomy 21, seizures); 8 a structural diagnosis (e.g., cleft palate); 5 a respiratory diagnosis; and 18 a cardiac, other or nil diagnosis. The distribution of swallows within the dataset included 17 × midfeed sucking swallows, 13 × midfeed unspecified quantity swallows, 3 × 5 ml swallows, 14 × 10 ml swallows a,nd 2 × 20 ml swallows.
Penetration–Aspiration
Penetration–Aspiration Scale (PAS) scores were recorded for each child using the 8-point PAS scoring system [24]. Children with maximum PAS scores of 3 (material enters the laryngeal vestibule and there is visible post-swallow laryngeal residue) and higher (including all deep laryngeal penetration and aspiration events) were considered to have an airway concern [25]. All recorded images were blind rated by two experienced speech-language pathologists for inter-rater reliability. Agreement for PAS ratings between the two raters was excellent [26] (Table 1).
Image Analysis
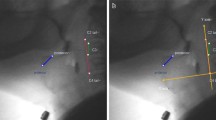
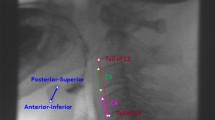
Hyoid position was measured using digital imaging software (ImageJ, Research Services Branch, National Institute of Mental Health, Maryland, USA). The following coordinates were tracked on all still shot frames: (i) the anterior inferior corner of the C4 vertebra (origin); (ii) the anterior inferior corner of the C2 vertebra (Y vector); and (iii) the anterior inferior corner of the hyoid (Fig. 2). Detailed information regarding the image tracking procedure has been previously described [16]. All hyoid measures were derived in normalized cervical units (% C2–C4 distance) in order to control for size differences across children.
All tracking data were exported to a Microsoft excel file with an embedded macro, which calculated the following parameters (all expressed in % C2–C4 units): (i) Peak hyoid position (X), (ii) Peak hyoid position (Y), (iii) Peak hyoid position (XY), (iv) Rest hyoid position (X), (v) Rest hyoid position (Y), (vi) Rest hyoid position (XY), (vii) Anterior hyoid displacement (X), (viii) Superior hyoid displacement (Y), and (ix) Hypotenuse hyoid displacement (XY).
Reliability of Hyoid Measures
After initial hyoid measures were taken, the distribution of data points was inspected visually and apparent outliers were re-measured by the original researcher. If original measures were not consistent with re-measured data, values were re-checked by the second researcher. Inter-rater reliability for all hyoid measures was completed by an independent rater blinded to child age and the other rater’s score. Agreement was good to excellent for all hyoid measures [26] (Table 1).
Data Analysis
Statistical analyses were performed using IBM SPSS statistics v 24 (SPSS inc. Chicago, Illinois, USA). Visualization was plotted across four age categories: 0–9, 10–24, 25–48, 49 + months. Point bi-serial correlation was calculated to investigate the relationship between the continuous variable of age and the dichotomous variable of visibility of the hyoid. Descriptive statistics explored means, range, standard deviation (SD), standard errors (SE), and 95% confidence intervals (CI). ANOVAs were performed exploring differences in each hyoid parameter with factors of penetration–aspiration status (PAS score < vs ≥ 3) and a continuous covariate of age in months. Scatter plots were created to display hyoid measures with increasing age. A binary variable of < 72 months and > 72 months was chosen based on (i) an observation of a stabilizing in hyoid measures > 72 months and a reasonable sample size in each group (< 72 months n = 34; > 72 months n = 15). Modified forest plots were constructed to compare the confidence intervals for these data with hyoid measurement values previously collected in a sex-balanced sample of healthy young adults on 5 and 10 ml 20% w/v thin liquid barium swallows ([16]; Molfenter and Steele, personal communication). Adult data bolus volumes of 5 ml and 10 ml were used for comparison, as these were likely to most closely represent the volumes swallowed by children in our study. Inter-rater reliability for PAS and hyoid measures were analyzed using intra-class correlation (ICC). The Cicchetti interpretation of ICC values for strength of agreement was used: poor (0.00–0.39), fair (0.40–0.59), good (0.60–0.74), and excellent (0.75–1.00) [26].
Results
Figure 3 illustrates the frequency distributions of reliable hyoid visibility in the larger dataset, by participant age, broken down into age ranges of 0–9, 10–24, 25–48, 49 + months. A noticeable increase in hyoid visibility was seen with increasing age, such that the hyoid could not be visualized in any of the children aged 9 months or younger but was visible at rest in 75% of children aged 10 months and older (rb = 0.41, p < 0.001).
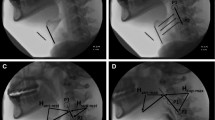
Among the 49 children in whom the hyoid was visible, 35 (i.e., 71%) showed no evidence of airway concern with PAS scores of 1 or 2. The remaining 14 cases showed airway invasion as follows: 1 X PAS of 4; 2 X PAS of 5; 3 X PAS of 7; and 8 X PAS of 8. Descriptive statistics for the hyoid parameters measured are displayed in Table 2. The relationship between age and the nine hyoid parameters of interest is presented in Fig. 4a–c. These modified forest plots display a comparison of hyoid position at rest (Fig. 4a), peak position (Fig. 4b) and hyoid displacement (Fig. 4c) measures for children < 72 months and > 72 months with adult norms [16].
Forest plots comparing hyoid position measures for children with adult norms [15]
Discussion
Explorations of objective measures of swallowing physiology and particularly hyoid position and movement in pediatric populations are sparse. Previous studies have reported inconsistent visualization of the hyoid in pediatric videofluoroscopy and for this reason have not been able to report on hyoid movement [18, 23]. Our study successfully employed the use of a measurement technique that had previously only been used in adults, on a population of children who presented with feeding difficulties [16]. The hyoid could be visualized through videofluoroscopy in the majority of children over 9 months of age. This study demonstrates that hyoid position and displacement can be reliably measured in children. The study’s results provide new opportunities to further our understanding of the kinematics of hyoid movement during swallowing in children.
Before 9 months of the age, the hyoid appeared faint through X-ray imaging due to reduced ossification of the hyoid bone and consequently coordinates required for image analysis could not be seen. This corroborates previous postpartum radiograph studies reporting ossified hyoids in infants older than 6 months [27]. The hyoid could not be visualized in any child under 9 months of age, and in a small number of children from the older age groups suggesting some variability in infant bone development in the cohort. This aligns with previous findings of inconsistent visualization of the hyoid in eight dysphagic children aged 2–30 months [23].
A number of studies have quantified hyoid movement in adults, providing hyoid position and displacement data for both normative and dysphagic adult populations. Whilst it may not be expected that a child’s hyoid position and displacement would be the same as an adult’s, comparison between normative adult data gives us insight into how differently this physiologic phenomenon performs from birth through to adulthood. Hyoid position values in children infrequently fell within the 95% confidence intervals of adults. As expected, rest (minimal) hyoid position displayed the greatest deviation from adult norms, with the exception of X position, both in the younger (< 72 months) and older (≥ 72 months) groups. This verifies established developmental understanding of a heightened position of the hyoid and larynx in infants, which descends in the first year of life [28, 29]. This relatively elevated hyoid position at rest is thought to provide increased airway protection during the vulnerable early stage of life. Our data are validated by previous ontogenetic data which show that in the neonate, the hyoid sits at rest opposite C2 and C3 and by 2 years old it has descended to sit in its adult position opposite C3 and C4 [28].
Comparisons between the older cohort of children (≥ 72 months) and normal adults exhibit a trend towards closer values with overlapping 95% confidence intervals for peak hyoid position (XY), minimal hyoid position (X) and all three displacement measures. This validates the hyoid measure values as the data show approximating adult norms as children’s age increases. Caution must be taken, however, in comparing 5 ml/10 ml fixed volume boluses in the adults with the varied bolus sizes swallowed by the children with even matched bolus volumes inevitably proportionally larger in a small child compared to a large adult. Comparisons of adult norms with the current pediatric data found wider variation (wider confidence intervals) in the pediatric data both in the younger (< 72 months) and older (≥ 72 months) groups. This may indicate wider variation in a cohort referred with feeding difficulties or more likely represents a wider variability in the developing child. This deserves further investigation.
Hyoid rest position does not necessarily differ in adults with dysphagia compared with those without dysphagia. However, hyoid peak position and displacement are significantly reduced in some adults with dysphagia [30, 31]. In adults, such reductions in hyoid movement have been associated with penetration–aspiration [15]. Differences in peak hyoid position and displacement measures were not significantly associated with airway compromise in this study. Penetration–aspiration rates were, however, low and larger cohorts may show different findings.
Limitations
Although this study had a moderate total sample size, when children were separated by etiology, statistical power was not adequate for further analysis. Penetration–aspiration rates were low, perhaps preventing statistically significant findings. Patient compliance presents a problem when working with a pediatric population leading to the removal of a number of cases due to movement. The exclusion of children due to their videofluoroscopy not containing an appropriate rest position could be eliminated from future studies by measuring only hyoid peak position (and not displacement), as a rest position is not needed in order to determine peak X, Y, or XY hyoid position. Previous studies have suggested that selection of a rest frame can introduce measurement error associated with the selection of the true rest position within a swallow [16]. Using only positional data would eliminate rest frame selection as a potential measurement error during the image analysis stage, with accurate rest frames also being difficult to attain in children.
The image quality on videofluoroscopy became a barrier to accurate tracking and measurement of the hyoid in some cases. While image contrast and brightness can be modified during analysis, fluoroscopy penetration is controlled by the fluoroscope operator at the time of videofluoroscopy. While sufficient fluoroscopic penetration is needed in order to ensure adequate visualization of anatomical features, low dose settings are often used to limit radiation exposure in children [32]. Once the VFSS is completed, altering the quality of the image is no longer possible. This was a limitation beyond the control of researchers in our study. Future research in this area should ensure that at the time of videofluoroscopy, all essential landmarks required for measurement are visualized clearly.
The children in our final analysis consumed a variety of differing bolus sizes and consistencies. The range in developmental stages of the population in our study meant that bolus size and consistency type could not be the same for each child. However, previous studies of hyoid kinematics in adults have reported significant differences in hyoid movement through a range of bolus volumes (5–20 ml) [16], suggesting that bolus size may impact results in our study. Bolus volume scaled to participant size, while perhaps rife with administrative complications, may allow clearer patterns in hyoid kinematics in relation to size (i.e., age).
Conclusions and Future Directions
The results from our preliminary study indicate that hyoid position and displacement can be accurately measured in the pediatric population from videofluoroscopy using digital imaging techniques. Whilst results from this study are preliminary and require additional independent replication to ascertain validity, this is the first step in establishing possible criteria for hyoid position and displacement in children. In an ideal world, further analysis in this area would look into defining hyoid position and displacement in a population of healthy children for comparison. However, this is limited by radiation exposure. In reality, larger cohorts comparing values across etiologies and in children of varying severity would add value.
Change history
10 September 2020
This letter notifies the readers of the Dysphagia journal of an error in the original published version of this manuscript, describing hyoid position during swallowing in children using an available pediatric dataset. A previously available open source spreadsheet tool had been used to calculate the position of the hyoid bone on lateral view videofluoroscopic images. An error in the mathematical formula built into the spreadsheet resulted in a reversal of reported results for measures of peak hyoid position in the X and Y planes of measurement. This erratum provides corrections to the results and interpretations of the original manuscript.
References
Newman LA. Infant swallowing and dysphagia. Curr Opin Otolaryngol Head Neck Surg. 1996;4(3):182–6.
Goldfield EC, Smith V. Preterm infant swallowing and respiration coordination during oral feeding: relationship to dysphagia and aspiration. Curr Pediatr Rev. 2010;6(2):143–50.
Arslan SS, Demir N, Dolgun BA, Karaduman AA. Development of a new instrument for determining the level of chewing function in children. J Oral Rehabil. 2016;43(7):488–95.
Arvedson JC, Lefton-Greif MA. Pediatric videofluoroscopic swallow studies: a professional manual with caregiver guidelines. San Antonio: Communication Skill Builders/Psychological Corporation; 1998.
Kidder TM, Langmore S, Martin B. Indications and techniques of endoscopy in evaluation of cervical dysphagia: comparison with radiographic techniques. Dysphagia. 1994;9(4):256–61.
Fuller S, Leonard R, Aminpour S, Belafsky P. Validation of the pharyngeal squeeze maneuver. Otolaryngology. 2009;140(3):391–4.
Duncan DR, Larson K, Hester L, McSweeney ME, Rosen R. The clinical feeding evaluation has poor reliability in the assessment of pediatric swallow function and causes delays in the diagnosis of aspiration. Gastroenterology. 2017;152(5):S708–9.
Newman LA, Cleveland RH, Blickman JG, Hillman RE, Jaramillo D. Videofluoroscopic analysis of the infant swallow. Invest Radiol. 1991;26(10):870–3.
Logemann JA, Kahrilas PJ, Cheng J, et al. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol. 1992;262(2):G338–41.
Mendelsohn MS, McConnel FM. Function in the pharyngoesophageal segment. Laryngoscope. 1987;97:483–9.
Leonard R, Kendall K. Dysphagia assessment and treatment planning—a team approach. London: Singular Publishing Ltd; 2018.
Cook IJ, Dodds WJ, Dantas RO, et al. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol. 1989;257:G748–59.
Choi KH, Ryu JS, Kim MY, Kang JY, Yoo SD. Kinematic analysis of dysphagia: significant parameters of aspiration related to bolus viscosity. Dysphagia. 2011;26(4):392–8.
Perlman A, Booth B, Grayhack J. Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia. 1994;9(2):90–5.
Molfenter SM, Steele CM. Kinematic and temporal factors associated with penetration-aspiration in swallowing liquids. Dysphagia. 2014;29(2):269–76.
Molfenter SM, Steele CM. Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. J Speech Lang Hear Res. 2014;57:768–78.
Newman LA, Keckley C, Petersen MC, Hamner A. Swallowing function and medical diagnoses in infants suspected of dysphagia. Pediatrics. 2001;108(6):e106–9.
Henderson M, Miles A, Holgate V, Peryman S, Allen J. Development and validation of quantitative objective videofluoroscopic swallowing measures in children. J Pediatr. 2016;178:202–5. https://doi.org/10.1016/j.jpeds.2016.07.050.
Tutor JD, Gosa MM. Dysphagia and aspiration in children. Pediatr Pulmonol. 2012;47(4):321–37.
Stoeckli SJ, Huisman TAGM, Seifert BAGM, Gosa MM, Martin-Harris BJW. Interrater reliability of videofluoroscopic swallow evaluation. Dysphagia. 2003;18(1):53–7.
Lee JW, Randall DR, Evangelista LM, Kuhn MA, Belafsky PC. Subjective assessment of videofluoroscopic swallow studies. Otolaryngology. 2017;156(5):901–5.
Gosa MM, Suiter DM, Kahane JC. Reliability for identification of a select set of temporal and physiologic features of infant swallows. Dysphagia. 2015;30(3):356–72.
Rommel N, Dejaeger E, Bellon E, Smet M, Veereman-Wauters G. Videomanometry reveals clinically relevant parameters of swallowing in children. Int J Pediatr Otolaryngol. 2006;70(8):1397–405.
Rosenbek J, Robbins J, Roecker E, Coyle J, Woods J. A penetration-aspiration scale. Dysphagia. 1996;11:93–8.
Steele CM, Grace-Martin K. Reflections on clinical and statistical use of the penetration-aspiration scale. Dysphagia. 2017;32(5):601–16.
Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6(4):284–91.
Reed MH. Ossification of the hyoid bone during childhood. Can Assoc Radiol J. 1993;44(4):273–6.
Lieberman DE, McCarthy RC, Hiiemae KM, Palmer JB. Ontogeny of postnatal hyoid and larynx descent in humans. Arch Oral Biol. 2001;46:117–28.
Miller AJ. Deglutition. Physiol Rev. 1982;62:129–84.
Curtis DJ. Radiologic evaluation of oropharyngeal swallowing. In: Gelfand DW, Richter JE, editors. Diagnosis and treatment of dysphagia. New York: Igaku-Shoin; 1989. p. 161–82.
Dodds WJ. The physiology of swallowing. Dysphagia. 1989;3:171–8.
Steele C, Molfenter S, Peladeau-Pigeon M, Stokely S. Challenges in preparig contrast media for videofluoroscopy: letter to the editor. Dysphagia. 2013;28:464–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Riley, A., Miles, A. & Steele, C.M. An Exploratory Study of Hyoid Visibility, Position, and Swallowing-Related Displacement in a Pediatric Population. Dysphagia 34, 248–256 (2019). https://doi.org/10.1007/s00455-018-9942-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-018-9942-3