Abstract
The Dynamic Swallow Study (DSS) is a methodology used to objectively and quantitatively assess swallowing kinematics during Videofluoroscopic Swallow Studies (VFSS). No DSS normative data exist delineating superior and anterior hyoid displacement (Hsup and Hant, respectively), nor the ratio between Hsup and Hant (SAratio). The aims of this study were to (1) establish normative data for Hsup, Hant, and SAratio and (2) assess the effects of age, sex, and bolus size on these measures in non-dysphagic patients, within the context of DSS. VFSSs were reviewed for consecutive elderly (≥ 65 years) and non-elderly (< 65 years) male and female non-dysphagic patients. Measurements of Hsup, Hant, and SAratio were made using a novel measurement methodology within the context of the Dynamic Swallow Study (DSS) protocol. Statistical analysis was performed to establish interaction effects and main effects of age, sex, and bolus size on Hsup, Hant, and SAratio. Descriptive statistics (mean ± standard deviations) are outlined for Hsup, Hant, and SAratio. Hsup was significantly effected by bolus size and age. Additionally, a significant three-way interaction of age, sex, and bolus size was observed. Hant was significantly effected by bolus size and sex, but no two- or three-way interactions were present. Neither bolus size, age, nor sex significantly effected SAratio. Age, sex, and bolus size normative data were established for Hsup, Hant, and SAratio for VFSS kinematic analysis. By outlining these measures, one can more thoroughly evaluate the areas of specific swallowing impairment, better determine the therapy targets, and track changes over time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Swallowing involves oral, pharyngeal, laryngeal, and esophageal structures working in tandem to efficiently propel food and liquid completely from the mouth into the stomach, whilst maintaining airway protection for the prevention of penetration and aspiration of boluses. Swallowing kinematics can be quantified spatially (how far structures move) and temporally (when structures move, and how fast they move, relative to each other and relative to bolus flow). Hyoid bone displacement can be measured by its overall displacement, or separated into its superior and anterior vectors [1, 2]. It is thought that superior hyoid displacement is an important contributing factor to epiglottic inversion and laryngeal vestibule closure for the prevention of penetration and aspiration of boluses before and during the swallow, while anterior hyoid displacement assists in mechanical opening of the pharyngoesophageal segment to allow for bolus clearance into the esophagus [3,4,5,6,7,8,9]. Research demonstrates that impairments in superior and anterior displacement of the hyoid bone place individuals at increased risk of penetration, aspiration, and post-swallow residue; however, it is important to note that some studies demonstrate impaired hyoid displacement that does not necessarily predict compromised laryngeal closure or bolus clearance [9,10,11,12,13,14,15].
Videofluoroscopic swallow studies (VFSS) are used to analyze spatial and temporal swallowing kinematics in addition to observing adverse airway protective events such as penetration and aspiration of foods and liquids. While many swallowing kinematic measurement methodologies exist, one standardized and frequently used approach to kinematic assessment is the Dynamic Swallow Study (DSS) protocol, originally developed by Rebecca Leonard and Katherine Kendall [11, 16,17,18,19,20,21]. The DSS protocol involves having an examinee establish a “pseudo-rest” position by way of holding a 1 cc liquid bolus in their mouth. After establishing the pseudo-rest position, the examinee performs a single cued swallow of 1, 3, and/or 20 cc nectar-thick liquids. Upon completion of the exam, the examiner can perform a frame-by-frame fluorographic analysis of a variety of spatial and temporal swallowing kinematics [11, 16, 22,23,24,25,26,27,28,29,30,31,32].
The “Hmax” is a DSS hyoid displacement measure describing the total distance traveled by the hyoid bone from the pseudo-rest position to the point of maximal anterior–superior hyoid displacement at or near the height of 1, 3, or 20 cc nectar-thick liquid swallows. While healthy volunteer DSS Hmax norms have been previously established, normative DSS data separately describing superior and anterior hyoid displacement, and their relationships to one another, have not been previously investigated [16]. Given the unique physiologic contributions of superior hyoid movement and anterior hyoid movement, establishing separate normative data within the context of a DSS protocol, and describing their kinematic relationship to one another, would provide meaningful information for clinical and research purposes.
The aims of this study were to (1) establish normative data for maximal superior hyoid displacement (Hsup), maximal anterior hyoid displacement (Hant), and the ratio of superior to anterior hyoid displacement (SAratio) in non-dysphagic patients within the context of the DSS maximal hyoid displacement (Hmax) measure and (2) assess the effects of age, sex, and bolus size on these measures.
Methods
Record Review
Records were reviewed for consecutive non-elderly (< 65) and elderly (≥ 65 years) male and female non-dysphagic patients who presented for VFSS at an outpatient, tertiary swallowing center. Non-dysphagic patients were included if VFSSs revealed a Functional Oral Intake Scale (FOIS) score of 1; a Penetration Aspiration Scale score ≤ 2; complete horizontal and vertical epiglottic inversion for all swallows during the VFSS; and normal (i.e., < 2 SD below mean) pharyngeal constriction ratio (PCR), hyolaryngeal approximation (HL), pharyngoesophageal segment opening (PESmax), and maximal hyoid displacement (Hmax) DSS displacement measures for 1, 3, and 20 cc nectar-thick liquid swallows [16, 33, 34]. Patients having the following criteria were excluded: a history of oral cavity, laryngeal, or pharyngeal surgical intervention (with the exception of routine dental work); a history of radiation therapy to the head and/or neck; diagnosis of a neurologic and/or neuromuscular disease; and/or a formal diagnosis of a structural- or motility-based esophageal abnormalities.
Videofluoroscopic Swallow Studies
Videofluoroscopic examinations were performed at the University of California San Francisco (UCSF) Medical Center in accordance with established and routine VFSS+DSS protocols. Two different fluoroscopic machines were used: the Axiom Luminos TF (Siemens Healthcare, USA) and the Luminos Agile Max (Siemens Healthcare, USA). Patients were presented with 1, 3, and 20 cc nectar-thick liquid barium boluses (40% w/v Varibar Nectar Barium Sulfate Suspension) and were instructed to (1) hold the liquid bolus in their mouth (i.e., the “pseudo-rest” position) and then (2) attempt to swallow the entire bolus in a single swallow when cued by the clinician. All video segments were recorded in a lateral viewing plane with an image-capturing rate of 25–30 images per second (depending on the capabilities of the fluoroscopy machine) and a magnification level of 1–2 ×. All VFSS videos were saved directly into a picture archiving and communication system (PACS) for later review and analysis.
Hyoid Displacement Measurement Methodology
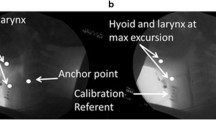
Both superior hyoid displacement (Hsup) and anterior hyoid displacement (Hant) were measured at the same points in time within the same video frames—during the pseudo-rest position (i.e., 1 cc bolus hold) and during maximal overall hyoid displacement (i.e., Hmax) at or near height of swallow for 1, 3, and 20 cc liquid swallows. The Hsup was defined as the change in vertical hyoid position from the pseudo-rest position (“Hsup-rest”) to the Hmax position (“Hsup-max”). The Hant was defined as the change in horizontal hyoid position from the pseudo-rest position (“Hant-rest”) to the Hmax position (“Hant-max”). The relationship between superior and anterior hyoid displacement was calculated by dividing Hsup by Hant, in order to get the superior-to-anterior hyoid displacement ratio (SAratio). See Figs. 1a–d for more detailed instructions on measurement methodology.
a Measurement methodology: Establish horizontal and vertical planes relative to the larynx during a pseudo-rest position (1 cc bolus hold) by connecting the anterior-inferior hyoid to anterior–superior tracheal air column. b Move the line established in Fig. 1a from the larynx to the spine with rotation or distortion. Ensure that the line crosses over the anterior-inferior point of cervical spine 2 (P1). Make note of a second identifiable point (P2) that is inferior to both P1 point and the hyoid bone but that also lays along the same line—extend the line inferiorly as necessary. In this example, P2 is along the superior border of C4. c Next, trace a line from the anterior-inferior hyoid bone to the P1–P2 line drawn in Fig. 1b until a 90º intersection is made. Extend the P1–P2 line superiorly as needed. Measure the distance from P2 to the 90º intersection (“Hsup-rest”) and the distance from the hyoid to the 90º intersection (“Hant-rest”). d Advance to the point of maximal overall hyoid displacement (Hmax). Repeat the instructions outlined in Fig. 1c by retracing a line using the original P1 and P2 points. Trace a line from the anterior-inferior hyoid until a 90º intersection is made. Measure from P2 to the 90º intersection (“Hsup-max”), and from the hyoid to the 90º intersection (“Hant-max”)
Data Abstraction and Reliability Testing
All measures were performed by one primary rater (JC). Ten percent of the video clips were selected at random and were repeated for analysis 1 week later by the primary rater to facilitate intra-rater reliability estimation. A second rater (JL) analyzed the same randomly selected video segments in order to assess inter-rater reliability. Both raters had ≥ 2 years of experience performing DSS kinematic analysis and were blinded to patient’s history and identity.
Statistical Analysis
Descriptive statistics were performed for demographic information, Hmax, Hsup, Hant, and SAratio. A mixed-design repeated-measures analysis of variance (ANOVA) with post hoc analysis and Bonferroni corrections, using bolus size (1, 3, 20 cc) as a within-subject factor, and age (elderly and non-elderly) and gender (male and female) as between subject-factors, was used to assess differences with Hsup, Hant, and SAratio as a function of age, sex, and bolus size. A p < 0.05 was set as the significance level for all statistical tests. Two-way random effects, absolute agreement, intraclass correlation coefficients (ICC) were used to calculate intra- and inter-rater reliabilities. Interpretation of ICC was judged to be ‘excellent’ if ≥ 0.90, ‘good’ if between 0.75 and 0.90, ‘moderate’ if between 0.50 and 0.75, and ‘poor’ if < 0.50 [35]. Statistical analyses were performed using SPSS statistical package version 24 (SPSS Inc., Chicago IL).
Results
Patient Demographics
One hundred sixty-five patients met the above inclusion–exclusion criteria and were included for initial study analysis. Four of the exams were extreme outliers (> 3 SD away from the Hmax mean) and were therefore excluded. A total of 161 exams were included in the final analysis. Non-elderly men (n = 39) had an average age of 50.3 years (± 12.1), with an age range from 18 to 64 years. Elderly men (n = 42) had an average age of 76.7 years (± 7.7), with an age range from 65 to 94 years. Non-elderly women (n = 46) had an average age of 46.5 years (± 13.8), with an age range from 18 to 64 years. Elderly women (n = 34) had an average age of 77.3 years (± 8.9), with an age range from 65 to 96 years.
Intra- and Inter-rater Reliabilities
Sixteen exams were selected at random and yielded a total of 102 repeated anterior and superior hyoid displacement measures. Intraclass correlation coefficient estimates demonstrated excellent inter-and intra-rater reliabilities for superior hyoid displacement and good intra-and inter-rater reliabilities for anterior hyoid displacement. For Hsup, inter-rater ICC was 0.929 (95% CI 0.866–0.962) and intra-rater ICC was 0.973 (95% CI 0.951–0.986). For Hant, inter-rater ICC was 0.821 (95% CI 0.639–0.908) and intra-rater ICC was 0.887 (95% CI 0.801–0.938).
Superior Hyoid Displacement (H sup)
Age, sex, and bolus size norms [mean ± standard deviation (range)] are outlined for Hsup (Table 1). Hsup was significantly effected by bolus size, F (2, 312) = 94.158, p < 0.005, and sex, F (1, 156) = 16.869, p < 0.0005, but not age (p > 0.05). Pairwise comparisons were performed for statistically significant differences between bolus size with Bonferroni corrections being made, revealing significant differences (p < 0.0005) between all three bolus sizes (i.e., 1 and 3 cc, 1 and 20 cc, and 3 and 20 cc). Three-way mixed ANOVA revealed a statistically significant three-way interaction between age, sex, and bolus size, F (2, 312) = 3.712, p = 0.026, partial η2 = 0.023 (Fig. 2). Statistical significance of a simple two-way interaction was accepted at a Bonferroni-adjusted alpha level of 0.025. There was a statistically significant simple two-way interaction of sex and age for 20 cc bolus size, F (1, 156) = 6.254, p = 0.013, but not for 1 cc or 3 cc bolus sizes (p > 0.05). Statistical significance of a simple simple main effect was accepted at a Bonferroni-adjusted alpha level of 0.025. There was a statistically significant simple simple main effect of age for males for 20 cc bolus size, F (1, 156) = 7.758, p = 0.006, but not for females (p > 0.05).
Anterior Hyoid Displacement (H ant)
Age, sex, and bolus size norms [mean ± standard deviation (range)] are outlined for Hant (Table 2). Hant was statistically effected by bolus size, F (2, 312) = 6.845, p = 0.001, and age, F (1, 156) = 10.449, p = 0.001, but not sex (p > 0.05). Pairwise comparisons were performed for statistically significant differences between bolus size with Bonferroni corrections being made, revealing significant differences (p < 0.01) between 1 and 3 cc and 1 and 20 cc, but not between 3 and 20 cc (p = 0.497). Three-way mixed ANOVA was run to understand the effects of age, sex, and bolus size on Hant. There were no statistically significant three- or two-way interactions between age, sex, and bolus size (p > 0.05). Statistical significance of a simple simple main effect was accepted at a Bonferroni-adjusted alpha level of 0.025.
Superior-to-Anterior Ratio of Hyoid Displacement (SAratio)
Age, sex, and bolus size norms [mean ± standard deviation (range)] are outlined for SAratio (Table 3). SAratio was not significantly effected by bolus size, age, or sex (p > 0.05). A three-way mixed ANOVA was run to understand the effects of age, sex, and bolus size on SAratio. There were no statistically significant three- or two-way interactions between bolus size, age, and sex for SAratio (p > 0.05).
Discussion
This study establishes normative data for superior and anterior hyoid displacement, and the ratio between these two measures, for elderly and non-elderly males and females for 1, 3, and 20 cc liquid boluses within the context of the Hmax DSS measurement. Superior and anterior hyoid displacement varied significantly by bolus size. Sex was noted to significantly impact superior hyoid displacement but not anterior hyoid displacement. Age was seen to significantly impact anterior hyoid displacement, but not superior hyoid displacement. Neither bolus size, age, nor sex significantly impacted the ratio of superior to anterior hyoid displacement. Generally speaking, superior and anterior displacements became larger with increasing bolus sizes, and was larger in males compared to females, and non-elderly when compared to elderly. These findings are similar with previous studies, which demonstrate that hyoid displacement increases with increasing bolus sizes, and may be impacted by age and sex [11, 16,17,18, 29].
The hyoid bone was found to frequently move an average of two to three times more superiorly than as it does anteriorly. In some instances, anterior displacement was noted to be negative, in other words, moving slightly posterior at time of maximal overall hyoid displacement. It is unclear if these patterns for increasingly larger superior-to-anterior hyoid displacement ratios and large variability of anterior hyoid displacement reflect outcomes inherent to this measurement methodology (both superior and anterior measures taken at the same point in time), or if they truly demonstrate a highly variable nature in anterior hyoid movement in normal swallow function. This result is significant, as findings of marginal-to-nil (or even negative) anterior hyoid displacements relative to large superior hyoid displacements may not reflect a disrupted swallow pattern, but rather be a variant of normal healthy swallow, as was observed with multiple exams in the present study. This finding would also call into question the extent to which anterior hyoid displacement plays in functional airway protection and pharyngoesophageal segment opening in non-dysphagic and dysphagic patients.
Two key points should be noted when extrapolating the data for swallow function interpretation. Firstly, Hsup and Hant displacement measures are made by establishing vertical and horizontal planes relative to the orientation of the larynx. This methodology varies from many studies evaluating superior and anterior hyoid displacement norms, which use vertical and horizontal planes relative to the lateral fluoroscopic viewing plane or anterior aspects of the spine, and as a result, superior movement appears generally longer and anterior movement appears generally shorter than previously reported [6, 18, 21, 36,37,38]. Secondly, it should be recognized that while maximal anterior and superior hyoid displacements tend to occur within close temporal proximity to each other, these displacement measures often occur at different time points during the swallow. Hsup and Hant may therefore not reflect true maximal superior and anterior hyoid displacements outside of the context of DSS’s Hmax. However, Hmax was chosen as the single point of analysis for Hsup and Hant measurements in hopes of capitalizing on the excellent reliability that was previously reported for Hmax. This method proved successful, as the reliability of the present two measures also appear to have good-to-excellent intra- and inter-rater reliabilities. Further, the Hmax was chosen as the single point of analysis for the two measures in this study in an effort to minimize clinical burden by collapsing three separate hyoid displacement time frames into one, therefore reducing the time required to collect and calculate the measures. All that is needed to obtain Hmax is a Pythagoreans theorem calculation, rather than an additional measurement, where Hmax = (H 2sup + H 2ant )0.5.
The main limitation of this study is that analyses were performed on “non-dysphagic” individuals previously seen in an outpatient otolaryngology clinic rather than on healthy volunteers. Strict inclusion–exclusion criteria were established to rule out abnormal physiology and people with compromised bolus clearance and airway protection; however abnormal physiology not representative of healthy volunteers cannot be ruled out. However, one could argue that even if the findings of the present study were not representative of “normal healthy” physiology, the present information is still valuable in understanding the deviant non-dysphagic physiology that still results in complete bolus clearance and airway protection.
Conclusion
This study presents age, sex, and bolus size norms for Hsup, Hant, and SAratio, taken at time at maximal hyoid displacement (Hmax), within the context of a Dynamic Swallow Study protocol for videofluoroscopic swallow studies. Establishing normative data for anterior and superior hyoid displacement should allow clinicians using DSS to more thoroughly identify areas of swallowing impairment, determine targets for therapeutic intervention, and track individual physiologic changes over time.
References
Pearson WG, Langmore SE, Zumwalt AC. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2011;26:345–51. https://doi.org/10.1007/s00455-010-9315-z.
Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–9.
Thompson TZ, Obeidin F, Davidoff AA, et al. Coordinate mapping of hyolaryngeal mechanics in swallowing. J Vis Exp. 2014;87(10):51476. https://doi.org/10.3791/51476.
Molfenter SM, Steele CM. Kinematic and temporal factors associated with penetration-aspiration in swallowing liquids. Dysphagia. 2014;29:269–76. https://doi.org/10.1007/s00455-013-9506-5.
Sivarao DV, Goyal RK. Functional anatomy and physiology of the upper esophageal sphincter. Am J Med. 2000;108(4A):27S–37S.
Nishikubo K, Mise K, Ameya M, Hirose K, Kobayashi T, Hyodo M. Quantitative evaluation of age-related alteration of swallowing function: videofluoroscopic and manometric studies. Auris Nasus Larynx. 2015. https://doi.org/10.1016/j.anl.2014.07.002.
Ekberg O. Closure of the laryngeal vestibule during deglutition. Acta Otolaryngol. 1982;93(1–2):123–9.
Ekberg O, Sigurjónsson SV. Movement of the epiglottis during deglutition - A cineradiographic study. Gastrointest Radiol. 1982;7:101–7. https://doi.org/10.1007/BF01887619.
Steele CM, Bailey GL, Chau T, et al. The relationship between hyoid and laryngeal displacement and swallowing impairment. Clin Otolaryngol. 2011;36:30–6. https://doi.org/10.1111/j.1749-4486.2010.02219.x.
Kraaijenga SAC, van der Molen L, Heemsbergen WD, Remmerswaal GB, Hilgers GB, van der Brekel MW. Hyoid bone displacement as parameter for swallowing impairment in patients treated for advanced head and neck cancer. Eur Arch Oto-Rhino-Laryngol. 2017. https://doi.org/10.1007/s00405-016-4029-y.
Kendall KA, Leonard RJ. Hyoid movement during swallowing in older patients with dysphagia. Arch Otolaryngol Head Neck Surg. 2001;127:1224–9. https://doi.org/10.1001/archotol.127.10.1224.
Paik NJ, Kim SJ, Lee HJ, Jeon JY, Lim JY, Han TR. Movement of the hyoid bone and the epiglottis during swallowing in patients with dysphagia from different etiologies. J Electromyogr Kinesiol. 2008;18:329–35. https://doi.org/10.1016/j.jelekin.2006.09.011.
Wang TG, Chang YC, Chen WS, Lin PH, Hsiao TY. Reduction in hyoid bone forward movement in irradiated nasopharyngeal carcinoma patients with dysphagia. Arch Phys Med Rehabil. 2010;91:926–31. https://doi.org/10.1016/j.apmr.2010.02.011.
Ekberg O. Defective closure of the laryngeal vestibule during deglutition. Acta Otolaryngol. 1982;93(1–6):309–17.
Ekberg O. Epiglottic dysfunction during deglutition in patients with dysphagia. Arch Otolaryngol. 1983;109:376–80.
Leonard R, Kendall K. Dysphagia assessment and treatment planning: a team approach. 3rd ed. San Diego: Plural Publishing, Inc.; 2014.
Leonard RJ, Kendall KA, McKenzie S, Gonçalves MI, Walker A. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15(3):146–52. https://doi.org/10.1007/s004550010017.
Molfenter SM, Steele CM. Physiological variability in the deglutition literature: hyoid and laryngeal kinematics. Dysphagia. 2011;26(1):67–74. https://doi.org/10.1007/s00455-010-9309-x.
Dodds WJ, Man KM, Cook IJ, Kahrilas PJ, Stewart ET, Kern MK. Influence of bolus volume on swallow-induced hyoid movement in normal subjects. Am J Radiol. 1988;150:1307–9.
Leonard R, McKenzie S. Hyoid-bolus transit latencies in normal swallow. Dysphagia. 2006;21:183–90. https://doi.org/10.1007/s00455-006-9025-8.
Kim Y, McCullough GH. Maximum hyoid displacement in normal swallowing. Dysphagia. 2008;23(3):274–9. https://doi.org/10.1007/s00455-007-9135-y.
Kendall KA, Leonard RJ. Pharyngeal constriction in elderly dysphagic patients compared with young and elderly nondysphagic controls. Dysphagia. 2001;16:272–8. https://doi.org/10.1007/s00455-001-0086-4.
Leonard RJ, Kendall KA, Johnson R, McKenzie S. Swallowing in myotonic muscular dystrophy: a videofluoroscopic study. Arch Phys Med Rehabil. 2001;82:979–85. https://doi.org/10.1053/apmr.2001.23962.
Kendall KA, Leonard RJ. Videofluoroscopic upper esophageal sphincter function in elderly dysphagic patients. Laryngoscope. 2002;112:332–7.
Kendall KA, Leonard RJ, McKenzie SW. Sequence variability during hypopharyngeal bolus transit. Dysphagia. 2003;18:85–91. https://doi.org/10.1007/s00455-002-0086-z.
Kendall KA, Ellerston J, Heller A, Houtz DR, Zhang C, Presson AP. Objective measures of swallowing function applied to the dysphagia population: a one year experience. Dysphagia. 2016;31:538–46. https://doi.org/10.1007/s00455-016-9711-0.
Kendall KA. Oropharyngeal swallowing variability. Laryngoscope. 2002;112(3):547–51. https://doi.org/10.1097/00005537-200203000-00025.
Kendall KA, Leonard RJ, Mckenzie S. Common medical conditions in the elderly: impact on pharyngeal bolus transit. Dysphagia. 2004;19:71–7. https://doi.org/10.1007/s00455-003-0502-z.
Leonard R, Kendall KA, Mckenzie S. Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia. 2004;19:133–41. https://doi.org/10.1007/s00455-003-0508-6.
Kendall KA, Leonard RJ, Mckenzie S. Airway protection: evaluation with videofluoroscopy. Dysphagia. 2004;19:65–70. https://doi.org/10.1007/s00455-003-0500-1.
Kendall KA, Leonard RJ. Bolus transit and airway protection coordination in older dysphagic patients. Laryngoscope. 2001;111:2017–21.
Kendall KA, Mckenzie S, Leonard RJ, Gonçalves MI, Walker A. Timing of events in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15:74–83. https://doi.org/10.1007/s004550010004.
Crary MA, Carnaby Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86:1516–20. https://doi.org/10.1016/j.apmr.2004.11.049.
Rosenbek JC, Robbins J, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63. https://doi.org/10.1016/j.jcm.2016.02.012.
Ishida R, Palmer JB, Hiiemae KM. Hyoid motion during swallowing: factors affecting forward and upward displacement. Dysphagia. 2002;17(4):262–72. https://doi.org/10.1007/s00455-002-0064-5.
Sia I, Carvajal P, Carnaby-Mann GD, Crary MA. Measurement of hyoid and laryngeal displacement in video fluoroscopic swallowing studies: variability, reliability, and measurement error. Dysphagia. 2011;27(2):1–6. https://doi.org/10.1007/s00455-011-9352-2.
Ekberg O. The normal movements of the hyoid bone during swallow. Invest Radiol. 1986;21(5):408–10.
Acknowledgements
The authors thank Michelle Troche, PhD, CCC-SLP for contributions to manuscript review and statistical guidance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors James Curtis, Jonelyn Langenstein, and Sarah Schenider declare that they each have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Curtis, J., Langenstein, J. & Schneider, S. Superior and Anterior Hyoid Displacement During Swallowing in Non-Dysphagic Individuals. Dysphagia 33, 602–609 (2018). https://doi.org/10.1007/s00455-018-9878-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-018-9878-7