Abstract
The objectives of this study are to investigate swallowing and its coordination with respiration in patients with obstructive sleep apnea (OSA). This is a prospective cohort study conducted in a tertiary referred Medical Center. A non-invasive method of assessing swallowing was used to detect the oropharyngeal swallowing parameters and the coordination with respiration during swallowing. The system used to assess swallowing detected: (1) movement of the larynx using a force-sensing resistor; (2) submental muscle activity using surface electromyography; and (3) coordination with respiration by measuring nasal airflow. Five sizes of water boluses (maximum 20 mL) were swallowed three times, and the data recorded and analyzed for each participant. Thirty-nine normal controls and 35 patients with OSA who fulfilled the inclusion criteria were recruited. The oropharyngeal swallowing parameters of the patients differed from the controls, including longer total excursion duration and shorter duration of submental muscles contraction. A longer swallowing respiratory pause (SRP), temporary coordination with respiration during swallowing, was demonstrated in the patients compared with the controls. The frequency of non-expiratory/expiratory pre- and postswallowing respiratory phase patterns of the patients was similar with the controls. There was significantly more piecemeal deglutition in OSA patients when clumping 10- and 20-mL water boluses swallowing together (p = 0.048). Oropharyngeal swallowing and coordination with respiration affected patients with OSA, and it could be detected using a non-invasive method. The results of this study may serve as a baseline for further research and help advance research methods in obstructive sleep apnea swallowing studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common disorder and a major public health problem due to potentially serious health consequences. OSA is reported to affect about 9–15 % of middle-aged adults and is more common in men [1]. It is characterized by repetitive episodes of partial or complete obstruction of the upper airway during sleep [2]. The clinical manifestations of OSA include snoring, nocturnal gasping, choking, nocturia, and other symptoms due to fragmented sleep [3, 4]. In addition, choking during sleep is a symptom of spontaneous swallowing impairment.
Sensation in the oropharynx and upper airway in patients with OSA has been proven to be impaired by nerve damage [5–7]. In addition, the absence of a gag reflex in severe OSA and the absence of palatal reflex in moderate OSA have been reported [8]. One study demonstrated that the patients with OSA had impaired swallowing reflex, longer latent time, and shorter inspiratory suppression time [9]. Another study reported that swallowing latency was shorter in patients with OSA [7]. Furthermore, Valbuza et al. used nasal fibroscopy to detect abnormalities in subclinical swallowing in patients with OSA which were shown to be due to premature oral leakage and pharyngeal stasis after swallowing (55 %), but not penetration or aspiration [10]. In contrast, none of these presentations were found in the control group [10]. Accordingly, the impact of OSA on swallowing function is still inconclusive, and the pathophysiology of subclinical swallowing disorders in OSA is still unclear. In recent decades, the role of the coordination of respiration with swallowing to ensure safe swallowing without aspiration has been emphasized [11–16]. However, this finely tuned mechanism is not completely understood in OSA.
To objectively analyze swallowing and coordination with respiration, invasive and non-invasive methods of studying swallowing including videofluoroscopic swallow study (VFSS), pharyngeal manometry [17–19], surface electromyography (sEMG) [20, 21], and swallowing sounds [22, 23] which simultaneously detect nasal airflow or respiration have been developed. The current trend of swallowing studies is toward the use of non-invasive and radiation free modalities, and non-invasive methods combining submental sEMG with piezoelectric sensors to indirectly characterize cricopharyngeal sphincter activity of normal and neuromuscular dysphagic swallowing movements have been demonstrated [24, 25]. Such novel non-invasive instruments have been used in combination with respiratory monitoring to analyze impairments in swallowing and coordination with respiration in studies on neuromuscular dysphagia [26] and aging [27]. Recently, new sensors such as bend sensors [28] and force-sensing resistors (FSRs) [29] have been used to detect thyroid cartilage excursion during swallowing. Therefore, the aim of the present study was to characterize physiological changes in swallowing–respiration coordination in patients with OSA using a non-invasive monitoring modality.
Materials and Methods
Patient Selection
This study was designed as a prospective cohort study in a tertiary referral medical center. Two questionnaires (Epworth Sleepiness Scale [30] and Snore Outcomes Survey [31]) were completed to exclude OSA or snoring symptoms in the control group. The exclusion criteria were a past history of dysphagia, cardiopulmonary disease, neurological disease, indigestion disorders, cancer, disease of the head and neck, and currently taking medications with known effects on swallowing or breathing. Ethics approval was granted by the Institutional Review Board of the Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan (no. 103-0910C). Each participant was able to understand verbal instructions and signed informed consent prior to participation.
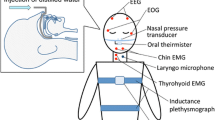
Non-invasive Assessment of Swallowing and Respiration Coordination (Fig. 1)
In this study, we used the same electrophysiological monitoring system (BIOPAC MP100 system, BIOPC System, Goleta, CA, USA) which is designed to record biological signals to assess swallowing and respiration as we used in our previous studies [27, 32]. Two surface electrodes were adhered at both sides of the midline under the chin for recording of submental muscles activities. For thyroid cartilage excursion, a FSR was attached to an air-filled plastic bulb onto an elastic belt which was placed around the subject’s neck (Fig. 1) [29]. The thyroid cartilage level at which to place the sensor was chosen according to our previous studies [27, 32]. Respiratory signals were detected by nasal airflow sensor transducing through nasal cannula which had been used to detect respiratory pause during sequential water swallowing previously [33].
The participants were first shown the equipment and given instructions about the study procedure. Each participant was then instructed to swallow five sizes (1, 3, 5, 10, and 20 mL) of water bolus, with each bolus being swallowed three consecutive times and recorded individually. The participants were blinded to the size of each bolus. The study procedure was similar to our previous studies [27, 32, 34]. Data were recorded using AcqKnowledge software (version 3.9.1a; BIOPAC Systems) for later off-line analysis. Software based on MatLab (version 2010; MathWorks, Natick, MA, USA) was used to analyze the parameters of oropharyngeal swallowing and respiration.
Definitions of the Parameters of Swallowing and Respiration (Fig. 2)
Latency and Duration of Oropharyngeal Swallowing
The submental sEMG and FSR signals were integrated for temporal analysis. The onset of the oral phase of swallowing was defined as the beginning of submentalis activity. The onset latency (OL) of laryngeal excursion was determined as the time from the onset of submentalis contraction to the onset of laryngeal excursion. For the laryngeal excursion signals, an asymmetric “W”-shaped waveform with a notch between the smaller first and larger second waveform was recorded [32]. The laryngeal excursion time (ET) was defined as the duration of the first wave representing the laryngeal upward excursion. The duration of the second wave represents the laryngeal downward excursion to return to the initial resting position. The total excursion time (TET) was calculated as the sum of the ET and 2nd deflection (Fig. 2) [25]. Such definitions of laryngeal excursions detected by sensors have previously been validated [24, 28, 35]. The motion of the hyoid bone is an important bony landmark for swallowing analysis in radiological VFSS studies [36, 37], and it has been observed to be positively correlated to [38] or synchronized with thyroid cartilage [39]. A recent study reported the use of a bend sensor to monitor thyroid cartilage motion [28, 40], and the waveforms detected by the bend sensor were correlated with hyoid bone movements by VFSS. However, a non-invasive method has the potential to be an auxiliary method to VFSS in the diagnosis of dysphagia in anatomically intact patients [28].
Definition of oropharyngeal swallowing and respiration parameters. Amp amplitude; D-SRPO-2nd DO duration from swallowing respiratory pause onset to 2nd deflection onset; OL onset latency; SRP swallowing respiratory pause; SRPO-L swallowing respiratory pause onset latency; sEMG surface electromyography; TET total excursion time
Respiratory Phase Patterns Pre- and Postswallowing
We defined four pre- and postswallowing respiratory phase patterns, including expiration–expiration (EX/EX), expiration–inspiration, inspiration–expiration, and inspiration–inspiration [17]. As the EX/EX pattern is the major pattern and the only physiologically protective respiratory phase pattern [21, 41, 42], we grouped the other three minor patterns into a non-EX/EX pattern [17, 27, 32, 43].
Swallowing Respiratory Pause (SRP)
SRP represents the duration of a stopped breath due to airway closure during swallowing and is a necessary protective respiratory phenomenon to allow for safe swallowing without aspiration [41]. This protective respiration pause is under central neural regulation, and it has been shown to be preserved in patients after laryngectomy during swallowing [41, 44].
Swallowing Respiratory Pause Onset Latency (SRPO-L)
SRPO-L is the duration from the onset of submentalis activity at the beginning of lingual motion for bolus propulsion to the swallowing respiratory pause onset (SRPO), and it is an important physiological event in swallowing–respiration coordination [45].
Duration from SRPO to 2nd Deflection Onset (D-SRPO-2nd DO)
D-SRPO-2nd DO is the duration between SRPO and onset of 2nd deflection. This time period indirectly represents the latent period between airway closure and the onset of cricopharyngeal sphincter relaxation from bolus arrival to passage [46].
Dysphagia Limit and Piecemeal Deglutition
The maximum volume of a bolus that can be swallowed is defined as the “dysphagia limit.” When the size of the bolus placed in the mouth is larger than the dysphagia limit, it is necessary to divide the bolus into smaller pieces (piecemeal) which are then swallowed successively in multiple swallows. A dysphagia limit of less than 20 mL indicates inconspicuous dysphagia in neurogenic disorders [47].
Statistical Analysis
All statistical analyses were performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL). The data obtained from the three separately recorded swallowing trials of each bolus size those without piecemeal were averaged. Two-way repeated measures ANOVA (RMANOVA) tests were used. The independent variables were oropharyngeal parameters and SRP, and the groups and volume of boluses were two dependent variables to examine the interaction effect and main effect. For numbers and frequencies, the Chi-square test was used between groups. The level of α was selected at 0.05. A p value less than 0.05 was considered to be statistically significant.
Results
Characteristics of Subjects
Thirty-nine normal males (controls) and 35 male patients with OSA referred from our Department of Otorhinolaryngology-Head and Neck Surgery (age: 37.5 ± 6.0 vs. 39.1 ± 9.4 years, p = 0.41; body mass index: 24.1 ± 2.5 vs. 27.3 ± 3.9 kg/m2, p = 0.054) with no history of dysphagia were recruited (Table 1).
Parameters of Swallowing and Respiration
In a two-way RMANOVA model, OL showed no significant differences between groups of the patients with OSA and the normal controls (F = 1.802, p = 0.186). The OL differed among the five bolus type (F = 7.513, p ≤ 0.001) (Fig. 3a). There was no significant interaction between group and bolus type. However, the TET was significantly longer in the OSA patients than in the controls (F = 10.042, p = 0.002). The TET also showed significant difference for the five boluses (F = 4.083, p = 0.008) (Fig. 3b). There was no interaction between group and bolus type. Differences in ET between OSA patients and controls were not statistically significant (F = 0.277, p = 0.601) and also did not differ among five boluses within group (F = 2.188, p = 0.90) (Fig. 3c). There was no interaction between group and bolus type. In addition, a borderline statistic difference of groups was found (F = 3.882, p = 0.055), which indicates that the duration of submental sEMG contraction was shorter in the patients than in the controls. There was no significant effect for sizes of water bolus within group (F = 0.912, p = 0.458) and also no interaction between groups and sizes of water bolus. (Figure 3d). The amplitude of the submental sEMG in the patients and in the controls showed no significant difference (F = 0.462, p = 0.500) and also no effect by sizes of water bolus within group (F = 0.939, p = 0.408). There was no interaction between group and bolus size (Fig. 4a). In summary, for the temporal parameters of oropharyngeal swallowing, the patients with OSA had no delay in the onset of latency, but a longer duration of thyroid cartilage excursion than the normal controls. For submental sEMG, the duration of contraction was shorter in the patients but the amplitude showed no significant difference.
A main effect of groups was found (F = 4.902, p = 0.032), which indicates that the SRP was longer in the patients than in the controls. There was also a main effect for sizes of water bolus (F = 5.396, p = 0.002), but no interaction between groups and sizes of water bolus (Fig. 4b). The duration of D-SRPO-2nd DO in the patients and controls showed no statistical significance (F = 0.980, p = 0.327) and no effect by sizes of water bolus (F = 1.566, p = 0.205). There was no significant interaction between group and bolus size (Fig. 4c). There were no significant differences in the SRPO-L between the patients and controls (F = 0.010, p = 0.919) but had significant effect by sizes of water bolus (F = 5.463, p ≤ 0.001). There was no interaction between group and bolus size (Fig. 4d).
Respiratory Phase Patterns Pre- and Postswallowing
There were no significant differences in the number of swallows in pre- and postswallowing respiratory phase patterns between the patients and controls under each size of water bolus and all boluses clumped together (Table 2).
Piecemeal Deglutition
There was no significant difference in the piecemeal deglutition of neither 10 nor 20-mL water boluses between the patients and controls. However, significant difference was shown when clumped two boluses conditions together (p = 0.048) (Table 3).
Discussion
Dysphagia may cause dehydration, malnutrition, aspiration, pneumonia, and even fatal infection [48, 49]. Accordingly, understanding the pathophysiology of swallowing disorder and normal physiology of swallowing are both crucially important. There were less non-invasive tools used in the studying of OSA swallowing physiology. Accordingly, the purpose of this study was to detect the temporal parameters of oropharyngeal swallowing and its coordination with respiration in OSA using non-invasive method. This study demonstrated that subclinical dysphagia OSA patients had delayed pharyngeal phase presented by longer TET and coordinated with longer duration of SRP. The duration of submental sEMG contraction was shorter in the OSA patients. However, the frequency of pre- and postswallowing respiratory phase patterns was not significantly different between OSA patients and controls.
A non-invasive study tool using a piezoelectric sensor has been used with the sensor placed at the thyroid cartilage level [34] to examine the coordination of swallowing and respiration in three different age groups, and demonstrated worse coordination in older age groups [27]. In addition, differences in swallowing–respiration coordination have been reported in patients with a unilateral stroke compared with normal controls [32]. The results of the present study showed that a non-invasive method using a FSR to assess thyroid cartilage excursion can detect deviations in swallowing–respiration coordination in patients with OSA. This non-invasive method of assessing swallowing and respiration may be useful for follow-up after therapy for head and neck lesions in which dysphagia may be a related symptom. In addition, using this non-invasive method to compare the effectiveness of surgical treatment on swallowing or complicated dysphagia of head and neck surgery may be possible. Furthermore, this method may be used for long-term telemonitoring at home or institute for dysphagia in head and neck disorders [50].
To analyze the amplitude and contraction duration of submental sEMG in swallowing function is emphasized, currently. Peak amplitude of submental sEMG was identified to have weak-negative correlation with manometric pressure in normal healthy controls [51]. However, decreases in submental muscle activity were not evident in Huntington’s disease (HD) patients during saliva and water swallowing, tasks at 25 % of maximum expiratory pressure (EMT) recordings except during EMT at 75 %. The relative submental muscle weakness was observed only during a high-intensity task in early to mid-stage HD patients using the sEMG activities of submental muscles [52]. Kim et al. demonstrated that patients with unilateral middle cerebral artery (MCA) infarction within 2 months of stroke onset showed shorter duration of submental sEMG activity compared with those in healthy volunteers [53]. These findings in HD and unilateral MCA infarction are similar to our findings of shorter duration but no decrease amplitude of submental sEMG during water swallowing in subclinical dysphagia OSA patients.
Involuntary rhythmic breathing originates in the brain stem, and voluntary control of respiration is vital during swallowing [54, 55]. During swallowing, voluntary respiration suppresses involuntary respiration, and this fine-tuned interaction between voluntary and involuntary respiration is subconsciously coordinated in the brain stem in normal swallowing. However, preliminary data have demonstrated subclinical dysphagia with penetration using VFSS in OSA [10]. In the present study, we used a non-invasive method without radiation to assess parameters including OL, SRP, SRPO-L, D-SRPO-2nd DO, and pre- and postswallowing respiratory phase patterns, all of which are essential for safe swallowing. Our results suggested that the coordination of respiration with swallowing was affected in patients with OSA. However, there were no significant differences between subclinical dysphagic patients with OSA and normal controls in OL and SRPO-L, and future studies with a larger sample size or patients with clinically severe dysphagia may be needed to identify differences from normal controls using this non-invasive method. Also, D-SRPO-2nd DO is a hypothetical value used in our study that would be better to use the cricopharyngeal sphincter EMG directly [24, 35] in future studies.
A previous study has reported respiratory phase patterns of spontaneous swallowing in patients with OSA during sleep [56]. However, no previous studies have reported swallowing respiratory phase patterns in patients with OSA during voluntary swallowing. Our results revealed a similar frequency of respiratory phase pattern in the patients with OSA to the controls during voluntary swallowing, and the protective EX/EX respiratory phase pattern was the most common pre- and postswallowing respiratory phase pattern in both the controls and patients. One previous study reported no significant changes in respiration pattern and swallowing after total laryngectomy, suggesting that respiration is controlled by a generator center [44].
Swallowing is initiated orally and is coordinated with breathing mostly in the expiratory final phase. SRP is an important breathing pause that starts just before and persists through the pharyngeal phase [41]. To the best of our knowledge, there are limited preliminary reports of SRP in patients with OSA [57]. In the present study, we found a longer duration of SRP in the patients with OSA. A prolonged SRP has also been reported in elderly, amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and hemispheric stroke patients [27, 32, 33, 58, 59]. A longer excursion time of thyroid cartilage movement was compensated by a prolonged SRP to secure the airway for the delayed pharyngeal phase.
Piecemeal deglutition is a protective phenomenon that allows for safe swallowing when the bolus volume exceeds a person’s swallowing limit. Previous studies have demonstrated that assessment of the swallowing limit can objectively and sensitively detect dysphagia in neurological diseases [25, 47, 60]. However, piecemeal deglutition has not been reported in patients with OSA, and to the best of our knowledge, this is the first study to demonstrate an increase in the probability of piecemeal deglutition in patients with OSA who have subclinical dysphagia, although the increase was only modestly significant.
Piecemeal deglutition and a longer SRP are both considered to be protective phenomena to allow for safety swallowing without aspiration. Our results showed a prolonged SRP in the patients with OSA in all bolus sizes, but not in piecemeal deglutition. Accordingly, we hypothesize that patients with OSA and subtle dysphagia used as a prolonged SRP as compensation before piecemeal deglutition. We also found similar results in our previous unilateral stroke study [32]. Clinically, we suggest that feeding with a small bolus is safer, and that the non-invasive method used in this study can be used to detect swallowing limits.
The deviations in respiratory–swallowing coordination patterns shown in this study have also been demonstrated in neurological diseases [23, 32, 61, 62]. We suggest that the non-invasive method we used to monitor swallowing and respiration parameters in patients with OSA may be useful for future research studies. The non-invasiveness of this modality has advantages for longitudinal follow-up and treatment outcome studies which need multiple assessments. Moreover, this non-invasive method can be used for various foods with different tastes, volumes, and consistency of the bolus in swallowing studies that can be performed without exposure to radiation for otolaryngologic patients including those with OSA. Further studies are required on swallowing–respiration coordination to enable greater understanding of the effect of OSA and the effectiveness of treatment for swallowing–respiration coordination in patients with OSA.
Our results suggest that this non-invasive tool is useful to uncover subclinical dysphagia of OSA patients. Of future application, it may be used clinically to assess the dysphagia limit and timed tests for swallowing capacity and speed in patients with OSA, and also in otolaryngologic disorders such as head and neck lesions to assess dysphagia even in subclinical or mild cases. The development of wearable, portable, or telemetering applications is currently underway [29, 50].
However, this non-invasive method is not without limitation. It is not well suited to determine the safety of swallowing, due to this instrumental test cannot detect pharyngeal residue, penetration, and aspiration [63, 64]. Besides, it can observe neither the tongue movements of oral phase nor the esophageal phase of swallowing. Moreover, the defects of anatomical structures over head and neck regions, at the locations for sensors placements, will make this examination difficulty in data recording.
Conclusion
Subclinical dysphagia in patients with OSA was detected using a non-invasive swallowing and respiration assessment tool, which also showed that oropharyngeal swallowing and its coordination with respiration was affected. In future, more voluntary swallowing studies in patients with OSA and otolaryngologic disorders can be performed using this method.
References
Ferini-Strambi L, Fantini ML, Castronovo C. Epidemiology of obstructive sleep apnea syndrome. Minerva Med. 2004;95(3):187–202.
Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22 (5):667–9.
Chuang LP, Hsu SC, Lin SW, Ko WS, Chen NH, Tsai YH. Prevalence of snoring and witnessed apnea in Taiwanese adults. Chang Gung Med J. 2008;31(2):175–81.
Fleisher KE, Krieger AC. Current trends in the treatment of obstructive sleep apnea. J Oral Maxillofac Surg. 2007;65(10):2056–68. doi:10.1016/j.joms.2006.11.058.
Friberg D, Gazelius B, Hokfelt T, Nordlander B. Abnormal afferent nerve endings in the soft palatal mucosa of sleep apnoics and habitual snorers. Regul Pept. 1997;71(1):29–36.
Friberg D, Gazelius B, Lindblad LE, Nordlander B. Habitual snorers and sleep apnoics have abnormal vascular reactions of the soft palatal mucosa on afferent nerve stimulation. Laryngoscope. 1998;108(3):431–6.
Jobin V, Champagne V, Beauregard J, Charbonneau I, McFarland DH, Kimoff RJ. Swallowing function and upper airway sensation in obstructive sleep apnea. J Appl Physiol. 2007;102(4):1587–94. doi:10.1152/japplphysiol.00439.2006.
Valbuza JS, de Oliveira MM, Conti CF, Prado LB, Carvalho LB, do Prado GF. Oropharyngeal examination as a predictor of obstructive sleep apnea: pilot study of gag reflex and palatal reflex. Arq Neuropsiquiatr. 2011;69(5):805–8.
Teramoto S, Ishii T, Matsuse T. Relationship between swallowing function and gas exchange during day and night in patients with obstructive sleep apnea syndrome. Dysphagia. 2001;16(4):249–53.
Valbuza JS, de Oliveira MM, Zancanella E, Conti CF, Prado LB, Carvalho LB, GE Prado do. Swallowing dysfunction related to obstructive sleep apnea: a nasal fibroscopy pilot study. Sleep Breath. 2011;15(2):209–13. doi:10.1007/s11325-010-0474-9.
Hiss SG, Strauss M, Treole K, Stuart A, Boutilier S. Swallowing apnea as a function of airway closure. Dysphagia. 2003;18(4):293–300. doi:10.1007/s00455-003-0021-y.
Davenport PW, Bolser DC, Morris KF. Swallow remodeling of respiratory neural networks. Head Neck. 2011;33(Suppl 1):S8–13. doi:10.1002/hed.21845.
Broussard DL, Altschuler SM. Central integration of swallow and airway-protective reflexes. Am J Med. 2000;108(Suppl 4a):62s–7s.
Barlow SM. Oral and respiratory control for preterm feeding. Curr Opin Otolaryngol Head Neck Surg. 2009;17(3):179–86. doi:10.1097/MOO.0b013e32832b36fe.
Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003;550(Pt 1):287–304. doi:10.1113/jphysiol.2003.039966.
Bolser DC, Pitts TE, Morris KF. The use of multiscale systems biology approaches to facilitate understanding of complex control systems for airway protection. Curr Opin Pharmacol. 2011;11(3):272–7. doi:10.1016/j.coph.2011.06.002.
Martin-Harris B, Brodsky MB, Michel Y, Ford CL, Walters B, Heffner J. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg. 2005;131(9):762–70. doi:10.1001/archotol.131.9.762.
Martin-Harris B, Brodsky MB, Price CC, Michel Y, Walters B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. J Appl Physiol. 2003;94(5):1735–43. doi:10.1152/japplphysiol.00806.2002.
Paydarfar D, Gilbert RJ, Poppel CS, Nassab PF. Respiratory phase resetting and airflow changes induced by swallowing in humans. J Physiol. 1995;483(Pt 1):273–88.
Wilson SL, Thach BT, Brouillette RT, Abu-Osba YK. Coordination of breathing and swallowing in human infants. J Appl Physiol Respir Environ Exerc Physiol. 1981;50(4):851–8.
Hiss SG, Treole K, Stuart A. Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults. Dysphagia. 2001;16(2):128–35.
Selley WG, Flack FC, Ellis RE, Brooks WA. The exeter dysphagia assessment technique. Dysphagia. 1990;4(4):227–35.
Selley WG, Ellis RE, Flack FC, Bayliss CR, Pearce VR. The synchronization of respiration and swallow sounds with videofluoroscopy during swallowing. Dysphagia. 1994;9(3):162–7.
Ertekin C, Pehlivan M, Aydogdu I, Ertas M, Uludag B, Celebi G, Colakoglu Z, Sagduyu A, Yuceyar N. An electrophysiological investigation of deglutition in man. Muscle Nerve. 1995;18(10):1177–86. doi:10.1002/mus.880181014.
Ertekin C, Aydogdu I, Yuceyar N, Tarlaci S, Kiylioglu N, Pehlivan M, Celebi G. Electrodiagnostic methods for neurogenic dysphagia. Electroencephalogr Clin Neurophysiol. 1998;109(4):331–40.
Terzi N OD, Prigent H, Denise P, Normand H, Lofaso F. Noninvasive monitoring of breathing and swallowing interaction. In: EMG Methods for evaluating muscle and nerve function. 2012. http://www.intechopencom/download/pdf/25870chap21. doi:10.5772/25779.
Wang CM, Chen JY, Chuang CC, Tseng WC, Wong AM, Pei YC. Aging-related changes in swallowing, and in the coordination of swallowing and respiration determined by novel non-invasive measurement techniques. Geriatr Gerontol Int. 2015;15(6):736–44. doi:10.1111/ggi.12343.
Li Q, Hori K, Minagi Y, Ono T, Chen YJ, Kondo J, Fujiwara S, Tamine K, Hayashi H, Inoue M, Maeda Y. Development of a system to monitor laryngeal movement during swallowing using a bend sensor. PLoS One. 2013;8(8):e70850. doi:10.1371/journal.pone.0070850.
Shieh WY, Wang CM, Chang CS. Development of a portable non-invasive swallowing and respiration assessment device. Sensors (Basel). 2015;15(6):12428–53. doi:10.3390/s150612428.
Chen NH, Johns MW, Li HY, Chu CC, Liang SC, Shu YH, Chuang ML, Wang PC. Validation of a Chinese version of the epworth sleepiness scale. Qual Life Res. 2002;11(8):817–21.
Chen NH, Li HY, Gliklich RE, Chu CC, Liang SC, Wang PC. Validation assessment of the Chinese version of the snore outcomes survey. Qual Life Res. 2002;11(6):601–7.
Wang CM, Shieh WY, Chen JY, Wu YR. Integrated non-invasive measurements reveal swallowing and respiration coordination recovery after unilateral stroke. Neurogastroenterol Motil. 2015. doi:10.1111/nmo.12634.
Aydogdu I, Tanriverdi Z, Ertekin C. Dysfunction of bulbar central pattern generator in ALS patients with dysphagia during sequential deglutition. Clin Neurophysiol. 2011;122(6):1219–28. doi:10.1016/j.clinph.2010.11.002.
Wang CMCC, Chen CPC, Tseng WC, Chen JY. Noninvasive swallowing test for young healthy adults: finding the best location to monitor thyroid cartilage movements. J Med Biol Eng. 2014;34:393–8.
Ertekin C, Celik M, Secil Y, Tarlaci S, Kiyloglu N, Aydogdu I. The electromyographic behavior of the thyroarytenoid muscle during swallowing. J Clin Gastroenterol. 2000;30(3):274–80.
Ueda N, Nohara K, Kotani Y, Tanaka N, Okuno K, Sakai T. Effects of the bolus volume on hyoid movements in normal individuals. J Oral Rehabil. 2013;40(7):491–9. doi:10.1111/joor.12060.
Nagy A, Molfenter SM, Peladeau-Pigeon M, Stokely S, Steele CM. The effect of bolus volume on hyoid kinematics in healthy swallowing. Biomed Res Int. 2014;2014:738971. doi:10.1155/2014/738971.
Steele CM, Bailey GL, Chau T, Molfenter SM, Oshalla M, Waito AA, Zoratto DC. The relationship between hyoid and laryngeal displacement and swallowing impairment. Clin Otolaryngol. 2011;36(1):30–6. doi:10.1111/j.1749-4486.2010.02219.x.
Yokoyama M, Mitomi N, Tetsuka K, Tayama N, Niimi S. Role of laryngeal movement and effect of aging on swallowing pressure in the pharynx and upper esophageal sphincter. Laryngoscope. 2000;110(3 Pt 1):434–9. doi:10.1097/00005537-200003000-00021.
Li Q, Minagi Y, Hori K, Kondoh J, Fujiwara S, Tamine K, Inoue M, Maeda Y, Chen Y, Ono T. Coordination in oro-pharyngeal biomechanics during human swallowing. Physiol Behav. 2015;147:300–5. doi:10.1016/j.physbeh.2015.05.004.
Costa MM, Lemme EM. Coordination of respiration and swallowing: functional pattern and relevance of vocal folds closure. Arq Gastroenterol. 2010;47(1):42–8.
Boden K, Cedborg AI, Eriksson LI, Hedstrom HW, Kuylenstierna R, Sundman E, Ekberg O. Swallowing and respiratory pattern in young healthy individuals recorded with high temporal resolution. Neurogastroenterol Motil. 2009;21(11):1163. doi:10.1111/j.1365-2982.2009.01352.x.
Brodsky MB, McFarland DH, Dozier TS, Blair J, Ayers C, Michel Y, Gillespie MB, Day TA, Martin-Harris B. Respiratory-swallow phase patterns and their relationship to swallowing impairment in patients treated for oropharyngeal cancer. Head Neck. 2010;32(4):481–9. doi:10.1002/hed.21209.
Charbonneau I, Lund JP, McFarland DH. Persistence of respiratory-swallowing coordination after laryngectomy. J Speech Lang Hear Res. 2005;48(1):34–44.
Hiss SG, Strauss M, Treole K, Stuart A, Boutilier S. Effects of age, gender, bolus volume, bolus viscosity, and gustation on swallowing apnea onset relative to lingual bolus propulsion onset in normal adults. J Speech Lang Hear Res. 2004;47(3):572–83. doi:10.1044/1092-4388(2004/044).
Kendall KA, Leonard RJ, McKenzie S. Airway protection: evaluation with videofluoroscopy. Dysphagia. 2004;19(2):65–70.
Ertekin C, Aydogdu I, Yuceyar N. Piecemeal deglutition and dysphagia limit in normal subjects and in patients with swallowing disorders. J Neurol Neurosurg Psychiatry. 1996;61(5):491–6.
Feinberg MJ, Knebl J, Tully J, Segall L. Aspiration and the elderly. Dysphagia. 1990;5(2):61–71.
Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756–63. doi:10.1161/01.STR.0000190056.76543.eb.
Esteves GP, Silva Junior EP, Nunes LG, Greco CS, Melo PL. Configurable portable/ambulatory instrument for the analysis of the coordination between respiration and swallowing. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:90–3. doi:10.1109/iembs.2010.5626157.
Huckabee ML, Butler SG, Barclay M, Jit S. Submental surface electromyographic measurement and pharyngeal pressures during normal and effortful swallowing. Arch Phys Med Rehabil. 2005;86(11):2144–9. doi:10.1016/j.apmr.2005.05.005.
Reyes A, Cruickshank T, Thompson J, Ziman M, Nosaka K. Surface electromyograph activity of submental muscles during swallowing and expiratory muscle training tasks in Huntington’s disease patients. J Electromyogr Kinesiol. 2014;24(1):153–8. doi:10.1016/j.jelekin.2013.09.009.
Kim HR, Lee SA, Kim K, Leigh JH, Han TR, Oh BM. Submental muscle activity is delayed and shortened during swallowing following stroke. PM R. 2015;7(9):938–45. doi:10.1016/j.pmrj.2015.05.018.
Miller AJ. Neurophysiological basis of swallowing. Dysphagia. 1986;1:91–100.
Jean A. Brainstem control of swallowing: localization and organization of the central pattern generator for swallowing. In: Taylor A, editor. Neurophysiology of the jaws and teeth. London: McMillan Press; 1990. pp. 294–321.
Sato K, Umeno H, Chitose S, Nakashima T. Sleep-related deglutition in patients with OSAHS under CPAP therapy. Acta Otolaryngol. 2011;131(2):181–9. doi:10.3109/00016489.2010.520166.
Arici S, Gurgor N, Secil Y, Peker S, Incesu TK, Ozdemirkiran T, Tokucoglu F, Ce P, Celebisoy M, Ertekin C. P10.15 Oropharyngeal swallowing and obstructive sleep apnea syndrome. Clin Neurophysiol. 2011;122(supplement 1):S108. doi:10.1016/S1388-2457(11)60382-9.
Gurgor N, Arici S, Kurt Incesu T, Secil Y, Tokucoglu F, Ertekin C. An electrophysiological study of the sequential water swallowing. J Electromyogr Kinesiol. 2013;23(3):619–26. doi:10.1016/j.jelekin.2012.12.003.
Beckmann Y, Gurgor N, Cakir A, Arici S, Incesu TK, Secil Y, Ertekin C. Electrophysiological evaluation of dysphagia in the mild or moderate patients with multiple sclerosis: a concept of subclinical dysphagia. Dysphagia. 2015;30(3):296–303. doi:10.1007/s00455-015-9598-1.
Aydogdu I, Kiylioglu N, Tarlaci S, Tanriverdi Z, Alpaydin S, Acarer A, Baysal L, Arpaci E, Yuceyar N, Secil Y, Ozdemirkiran T, Ertekin C. Diagnostic value of “dysphagia limit” for neurogenic dysphagia: 17 years of experience in 1278 adults. Clin Neurophysiol. 2014;. doi:10.1016/j.clinph.2014.06.035.
Troche MS, Huebner I, Rosenbek JC, Okun MS, Sapienza CM. Respiratory-swallowing coordination and swallowing safety in patients with Parkinson’s disease. Dysphagia. 2011;26(3):218–24. doi:10.1007/s00455-010-9289-x.
Martin-Harris B. Clinical implications of respiratory-swallowing interactions. Curr Opin Otolaryngol Head Neck Surg. 2008;16(3):194–9. doi:10.1097/MOO.0b013e3282febd4b.
Rugiu MG. Role of videofluoroscopy in evaluation of neurologic dysphagia. Acta Otorhinolaryngol Ital. 2007;27(6):306–16.
Bastian RW. The videoendoscopic swallowing study: an alternative and partner to the videofluoroscopic swallowing study. Dysphagia. 1993;8(4):359–67.
Acknowledgments
This study was supported by Grants from the National Science Council, Taiwan (NSC-101-2314-B-182A-007-MY2) and Chang Gung Memorial Hospital, Taiwan (CMRPG5C0023).
Funding
The study was financially supported by Grants from the National Science Council, Taiwan (NSC-101-2314-B-182A-007-MY2) and Chang Gung Memorial Hospital, Taiwan (CMRPG5C0023).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Wang, CM., Li, HY., Lee, L.A. et al. Non-invasive Assessment of Swallowing and Respiration Coordination for the OSA Patient. Dysphagia 31, 771–780 (2016). https://doi.org/10.1007/s00455-016-9740-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-016-9740-8