Abstract
Swallowing is an important physiological response that protects the airway. Although aspiration during sleep may cause aspiration pneumonia, the mechanisms responsible have not yet been elucidated. We evaluated the coordination between respiration and swallowing by infusing water into the pharynx of healthy young adults during each sleep stage. Seven normal subjects participated in the study. During polysomnography recordings, to elicit a swallow we injected distilled water into the pharynx during the awake state and each sleep stage through a nasal catheter. We assessed swallow latency, swallow apnea time, the respiratory phase during a swallow, the number of swallows, and coughing. A total number of 79 swallows were recorded. The median swallow latency was significantly higher in stage 2 (10.05 s) and stage 3 (44.17 s) when compared to awake state (4.99 s). The swallow latency in stage 3 showed a very wide interquartile range. In two subjects, the result was predominantly prolonged compared to the other subjects. There was no significant difference in the swallow apnea time between sleep stages. The presence of inspiration after swallowing, repetitive swallowing, and coughing after swallowing was more frequent during sleep than when awake. This study suggests that the coordination between respiration and swallowing as a defense mechanism against aspiration was impaired during sleep. Our results supported physiologically the fact that healthy adult individuals aspirate pharyngeal secretions during sleep.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Swallowing is an important physiological response that protects the airway by removing matter which could be aspirated, thereby preventing aspiration pneumonia. Thus, there must be some form of precise coordination between respiration and swallowing.
For some dysphagia patients, the coordination between respiration and swallowing leads to aspiration. It is recognized that aspirating food, liquid, or oropharyngeal secretions colonized by bacteria causes aspiration pneumonia [1–3]. Similarly, aspiration during sleep has also been shown to cause aspiration pneumonia [4]: Some elderly patients develop acute episodes of pneumonia due to increased aspiration during sleep [5] and even healthy adults aspirate small amounts of oropharyngeal secretions while asleep without major consequence [6]. The mechanisms responsible for this increase in aspiration during sleep have not yet been elucidated. Thus, understanding how the coordination between respiration and swallowing occurs during sleep when voluntary swallowing is absent is extraordinarily important [7].
No study, to our knowledge, has explored the coordination between respiration and swallowing during sleep. To better understand swallowing during sleep, we tested the hypothesis that the coordination between swallowing and respiration may be different in each sleep stage. We evaluated the coordination of respiration and swallowing elicited by infusing water into the pharynx of healthy young adults in each sleep stage. Then, we assessed swallow latency, swallow apnea time, the respiratory phase in swallow, the number of swallows, and coughing.
Materials and Methods
Subjects
Seven normal subjects (two males and five females) ranging in age from 25 to 30 years old participated in the study. All volunteers were in good health and free of neuromuscular and respiratory disorders. Current or ex-smokers were excluded from this study since pharyngeal reflexes may be affected by smoking. All participants gave their written informed consent. These experiments were performed under the approval of the Osaka University Ethics Committee (Osaka University Dental Hospital, No. H23-E19).
Study Procedures
Experimental Setup
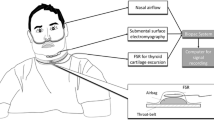
Figure 1 shows a diagram of the experiment. Polysomnography (PSG) was performed to assess sleep stages according to standard criteria [8, 9]. Standard measurements included electroencephalography (EEG), electrooculography (EOG), submental electromyography (EMG), and chest and abdominal respiratory impedance plethysmography. Nasal airflow was recorded by using nasal cannula connected to a pressure transducer and oral thermistor. In order to assess a swallow event, thyrohyoid EMG and a laryngeal microphone were added to the montage.
During full PSG recordings, we injected a small amount of distilled water into each subject’s pharynx during the awake state and each sleep stage through a nasal catheter (1.7 mm in diameter). The catheter was inserted through the nostrils and into the pharynx in order to elicit swallowing as reported previously [10–12].
Experimental Protocol
All studies were conducted during regular nocturnal hours in an experimental bedroom. A nasal catheter was inserted through the subject’s nostrils to the uvula level of the pharynx. The subjects were asked to lay on their beds and sleep in a supine position. After confirming the wake state on the computer monitor from the PSG control room, we injected 0.4 mL of distilled water without the subject’s awareness. Three injections were performed on each subject while awake. Next, the lights were turned off and the subject was allowed to fall asleep. Once a stable sleep stage was achieved, we had planned to do three injections per each sleep stage [stage 1, stage 2, stage 3, and rapid eye movement (REM)] identified by a real-time PSG raw data, including EEG, EOG, and EMG, and confirmed the swallowing movement elicited from injections. We continued this experimental protocol of injections during sleep until the end of a first sleep cycle, or 120 min of sleep. At a later time, a trained sleep technologist manually scored the sleep stages for the full PSG study.
Data Analysis
Sleep stages were manually scored in 30-s epochs (American Academy of Sleep Medicine scoring manual) by an experienced and trained sleep technologist [8, 9]. As previously described [12], swallowing was identified by three simultaneous signals: an increase in thyrohyoid EMG activity, a signal from the laryngeal microphone, and a transient interruption of nasal airflow (swallowing apnea), as shown in Fig. 2.
a Raw data during the awake state. The swallow movement occurred 5.54 s after the injection of distilled water, which was preceded by inspiration flow and followed by expiration flow without coughing. b Raw data during stage 2. The swallow movement occurred 28.82 s after the injection of distilled water, which was preceded by inspiration flow and followed by inspiration flow. After a swallow movement, there were repetitive swallows and some coughing
The swallow latency time was defined as the time from the injection to the onset of swallowing [12]. Swallow apnea time was defined as the length of the time for nasal airflow signal to return to baseline (zero flow), thus indicating airway closure associated with swallowing [13]. Respiratory phases—inspiration or expiration—interrupted by a swallow and the respiratory phase following a swallow were determined using the nasal airflow signal. These respiratory phases were grouped into four patterns based on previous studies [14–16]: (1) swallows immediately preceded by and followed by inspiration flow (I–I); (2) swallows preceded by inspiration flow and followed by expiration flow (I–E); (3) swallows immediately preceded by and followed by expiration flow (E–E); and (4) swallows preceded by expiration flow and followed by inspiration flow (E–I). The number of swallows was assessed by counting the number of swallows per injection; repetitive swallowing was defined as two or more swallows per injection. Coughing was identified by disorderly airflow signals after swallowing [14].
Statistical Analysis
All data were analyzed by SPSS statistical software (SPSS Inc., Chicago, IL, USA). The swallow latency and swallow apnea time are presented as median (interquartile range). The Kruskal–Wallis analysis of variance assessed the differences in swallow latency and swallow apnea time between sleep stages. When the analysis of variance showed a p value <0.05, comparisons between sleep stages were performed using the Mann–Whitney test with Bonferroni correction. Respiratory phase, repetitive swallowing, and coughing were expressed as absolute and relative frequencies. A p value of <0.05 was considered to indicate statistical significance.
Results
All seven normal subjects completed the trial, and data were obtained only in the first sleep cycle, or 120 min of sleep. In each subject, it was possible to record swallowing after injection during the awake state and stage 3. In six subjects, we identified swallowing in stages 1 and 2. In four subjects, we were able to successfully perform the procedure during REM sleep. A total number of 79 swallows (20 swallows in awake state, 18 in stage 1, 25 in stage 2, 11 in stage 3, and 5 in REM) were recorded.
Table 1 shows the swallow latency and swallow apnea time during different sleep stages. A REM swallow was recorded in only four out of seven subjects because three subjects became completely awake prior to achieving REM. Thus, the data of swallow latency and swallow apnea time during REM were not included in these statistics.
The swallow latency median was significantly higher in stage 3 (44.17 s) when compared to awake (4.99 s) or stage 1 (5.07 s). The swallow latency median in stage 2 (10.05 s) was also significantly higher compared to the awake state (4.99 s) (Fig. 3). The swallow latency in stage 3 showed a very wide interquartile range; therefore, we evaluated intersubject variation. Figure 4 shows the swallow latency of stage 3 in each subject. In two subjects (#6, #7), the results were predominantly prolonged compared to the other subjects. There was no significant difference in the swallow apnea time between sleep stages (Fig. 5).
Swallow latency during sleep stages. The median of swallow latency was significantly higher in stage 3 when compared to awake (p < 0.01) and stage 1 (p < 0.05). The median of swallow latency in stage 2 was also significantly higher compared to awake (p < 0.05). Box plot Median values are represented by horizontal lines within the box; the interquartile range by box edges. T-bars represent minimum and maximum value within the range. An open circle represents outliner cases (values between one-and-a-half to three box lengths above box edges) and an asterisk represents an extreme case (values more than three box lengths above box edges)
Swallow apnea time during sleep stages. There was no significant difference in the swallow apnea time between sleep stages. Box plot Median values are represented by horizontal lines within the box; the interquartile range by box edges. T-bars represent minimum and maximum value within the range. Open circles represent outliner cases (values between one-and-a-half to three box lengths above box edges) and an asterisk represents an extreme case (values more than three box lengths above box edges)
Table 2 and Fig. 6 show the distribution of the timing of swallows in reference to the phase of the respiratory cycle for each sleep stage. During the awake state, 45 % of the swallow patterns were I–E, whereas during sleep, the swallow patterns more frequently were E–I independent of sleep stage.
Percentage of respiratory phase with swallowing during sleep stages and the awake state. During the awake state, 45 % of swallowing patterns wereI–E, whereas during sleep, the most frequent swallowing pattern was E–I, independent of sleep stage. I–I inspiration–swallow–inspiration, I–E inspiration–swallow–expiration, E–E expiration–swallow–expiration, E–I expiration–swallow–inspiration, REM rapid eye movement
The respiratory phase after swallowing requires a separate analysis because an increase in the frequency of the swallows followed by inspiration has been observed in individuals with a higher risk of aspiration [13, 17]. Table 3 and Fig. 7 show the percentage of the respiratory phase after swallowing, number of swallows, and coughs after swallowing in all sleep stages. During the awake state, 70 % of swallows were followed by expiration. During sleep, the prevalence of inspiration following a swallow was 88.9 % for swallowing in stage 1, 88.0 % in stage 2, 63.6 % in stage 3, and 100 % in REM. Repetitive swallowing was seen in 20.0 % of the swallows in the awake state, 55.6 % in stage 1, 40.0 % in stage 2, 72.6 % in stage 3, and 80.0 % in REM. Coughing after swallowing was not seen while awake or during stage 1, but swallows were followed by coughing in 16.0 % of stage 2 swallows, 45.5 % of those in stage 3, and 20.0 % in REM.
Percentage of respiratory phase after swallowing, number of swallows, and coughing after swallowing. The presence of inspiration after swallowing, repetitive swallowing, and coughing after swallowing was more frequent during sleep than awake. S1 non-REM stage 1, S2 non-REM stage 2, S3 non-REM stage 3, REM rapid eye movement
Discussion
In this study, we evaluated the coordination of respiration and swallowing as a reflexive swallow movement during sleep. We found that the swallow latency was significantly higher in stages 2 and 3 when compared to the awake state. Also, the presence of inspiration after swallowing, repetitive swallowing, and coughing after swallowing was more frequent during sleep than when awake.
On a clinical basis, a swallow was reported to be classified as one of the two types: a voluntary swallow or a reflexive swallow [7]. Voluntary swallows occur with a desire to eat or drink (e.g., during meal time) while awake. Reflexive swallows are the unconscious movement to swallow saliva (e.g., during sleep). It is a protective reflex action to ensure food particles or saliva is not aspirated.
In this study, the swallow latency median was significantly higher in stage 2 (10.05 s) and stage 3 (44.17 s) when compared to the awake state (4.99 s). According to previous research, swallow latency while awake was 1.2 ± 0.1 s in healthy control subjects, 5.2 ± 0.6 s in older people without a history of pneumonia, and 12.5 ± 3.0 s in older people with aspiration pneumonia [18]. Subjects whose swallow latency was longer than 5 s were reported to be at high risk of developing pneumonia [19]. Another study classified swallowing into three groups depending on swallow latency: low-risk group (<3.0 s), intermediate-risk group (3.0–6.0 s), and high-risk group (>6.0 s) [10]. Based on these studies, the swallow latencies in stage 2 (10.5 s) and in stage 3 (44.17 s) were classified as a high-aspiration risk group. This result may suggest that even in healthy subjects, the swallowing reflex while asleep may be depressed to the same level as dysphagia patients who are awake. Furthermore, this result physiologically supports the fact that half of all healthy adults aspirate small amounts of oropharyngeal secretions while asleep [6].
There was a wide range in swallow latency in stage 3. Also, two subjects showed a defined prolonged swallow latency compared to the other subjects. Thus, this type of analysis may reflect a marker of arousability. In our data, all swallows were associated with an arousal, which suggests that the swallowing reflex may be related with arousability. It was reported that a subset of subjects with or without OSA seem to have greater vulnerability in arousability [20, 21]. In OSA patients, it has been shown that frequent arousal may contribute to the development of OSA.
Swallowing apnea has been considered beneficial for the coordination between respiration and swallowing, where breathing is resumed when the bolus has passed the pharynx and the laryngeal inlet [22]. In the current study, the swallow apnea time did not differ between the sleep stages. In awake and upright positions, swallow apnea time is most often cited to range from approximately 0.5–1.5 s [23–26]. In our study, the swallow apnea time ranged from 2.04 to 3.57 s, which is longer than the results of previous studies on awake subjects. In terms of a relationship between the number of swallows and the swallow apnea time, the swallow apnea time was significantly prolonged in the repetitive swallow group (3.61 s) when compared to the single swallow group (1.87 s). This result suggests that a long swallow apnea time may be a sign of an accidental swallow, which would require a repetitive swallow reflex.
During deglutition, a strongly preferred exhalation after swallow has been identified in healthy adults [24, 27, 28]. A disruption of the normal breathing–swallowing pattern, such as inhaling after swallowing, could put patients at an increased risk of aspiration since the negative pressure of inhalation has the potential to draw food and liquid residue toward the lungs [24, 28]. Several studies [17, 29, 30] have reported on the respiratory pattern after swallowing in awake dysphagia patients. Terzi and colleagues reported that the percentage of swallows followed by inspiration was about 50 % in neuromuscular patients compared with nearly 0 % in the control subjects [29]. Other studies reported that swallowing in patients with brain, spinal cord, and peripheral neurological diseases was followed by inspiration more frequently than expected (91 % of patients compared to 9 % of normal subjects, p < 0.001) [17]. There was one study where 70.6 ± 17.8 % of swallows were followed by inspiration and 28.8 ± 18.3 % of swallows were followed by expiration during sleep in patients with obstructive sleep apnea [30]. In our study, inspiration following a swallow was shown to occur with 66.7 % of swallows, and this confirms that in normal subjects, swallowing followed by inspiration is common during sleep; hence, further studies are necessary to assess this process in neuromuscular disease and sleep disturbances. The frequency of repetitive swallowing and coughing, which are described as the index for aspiration risk, was seen more often in the sleep state when compared to the awake state in our study.
The current study had several limitations. Because of the complexity of the study, the sample size was small, especially for the swallows during REM. Some subjects became completely awake after swallows were elicited by infusion of water and did not go back to sleep. Specifically, in stage 3, we observed that the frequency of coughing after swallowing was high (50 %). Furthermore, the stimulation of coughing led to the awake state, which made it difficult to achieve REM stage sleep.
Another limitation is the possibility that some stimulation other than the injection of water may elicit the arousal and consequently the swallow. Noise has been associated with sleep disruption and arousals [31, 32]. The level of these noises ranged from 40 to 65 dB, which approximately corresponds to bird calls and restaurant conversations, respectively. In our study, all subjects slept in a experimental bedroom, which had sound proof equipment. Therefore, we consider that the influence of noise was minimal.
Conclusion
This current study found that the swallow latency was significantly higher in stages 2 and 3 when compared to the awake state, and the pattern of an inspiration phase after swallowing and repetitive swallowing and coughing after swallowing occurred more often during sleep than while awake. Our results suggest that the coordination between respiration and swallowing as a defense mechanism for aspiration is impaired during sleep. For healthy people, this change has little influence on their health: Even if aspiration occurs during sleep, it will likely not lead to aspiration pneumonia. However, our results supported physiologically the fact that most of the healthy adult individuals experience nightly aspiration of pharyngeal secretion during sleep.
Abbreviations
- EEG:
-
Electroencephalography
- EMG:
-
Electromyography
- EOG:
-
Electrooculography
- PSG:
-
Polysomnography
- REM:
-
Rapid eye movement
References
Logemann JA. Update on clinical trials in Dysphagia. Dysphagia. 2006;21:116–20.
Ashford J, McCabe D, Wheeler-Hegland K, Frymark T, Mullen R, Musson N, Schooling T, Hammond CS. Evidence-based systematic review: oropharyngeal dysphagia behavioral treatments. Part III—Impact of dysphagia treatments on populations with neurological disorders. J Rehabil Res Dev. 2009;46:195–204.
Baijens LW, Speyer R. Effects of therapy for dysphagia in Parkinson’s disease: systematic review. Dysphagia. 2009;24:91–102.
Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665–71.
Kikuchi R, Watabe N, Konno T, Mishina N, Sekizawa K, Sasaki H. High incidence of silent aspiration in elderly patients with community-acquired pneumonia. Am J Respir Crit Care Med. 1994;150:251–3.
Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111:1266–72.
Ertekin C. Voluntary versus spontaneous swallowing in man. Dysphagia. 2011;26(2):183–92.
Iber C, Ancoli-Israel S, Chesson AL, et al. The AASM manual for the scoring of sleep associated events: rules, terminology and technical specifications. 1st ed. Westchester: American Academy of Sleep Medicine; 2007.
Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Brain Information Service/Brain Research Institute, University of California; 1968.
Ebihara T, Takahashi H, Ebihara S, Okazaki T, Sasaki T, Watando A, Nemoto M, Sasaki H. Capsaicin troche for swallowing dysfunction in older people. J Am Geriatr Soc. 2005;53(5):824–8.
Nishino T, Hiraga K. Coordination of swallowing and respiration in unconscious subjects. J Appl Physiol (1985). 1991;70(3):988–93.
Nishino T, Takizawa K, Yokokawa N, Hiraga K. Depression of the swallowing reflex during sedation and/or relative analgesia produced by inhalation of 50% nitrous oxide in oxygen. Anesthesiology. 1987;67:995–8.
Gross RD, Atwood CW Jr, Ross SB, Eichhorn KA, Olszewski JW, Doyle PJ. The coordination of breathing and swallowing in Parkinson’s disease. Dysphagia. 2008;23(2):136–45.
Kijima M, Isono S, Nishino T. Coordination of swallowing and phases of respiration during added respiratory loads in awake subjects. Am J Respir Crit Care Med. 1999;159(6):1898–902.
Kijima M, Isono S, Nishino T. Modulation of swallowing reflex by lung volume changes. Am J Respir Crit Care Med. 2000;162(5):1855–8.
Yamamoto F, Nishino T. Phasic vagal influence on the rate and timing of reflex swallowing. Am J Respir Crit Care Med. 2002;165(10):1400–3.
Hadjikoutis S, Pickersgill TP, Dawson K, Wiles CM. Abnormal patterns of breathing during swallowing in neurological disorders. Brain. 2000;123(Pt 9):1863–73.
Nakazawa H, Sekizawa K, Ujiie Y, Sasaki H, Takishima T. Risk of aspiration pneumonia in the elderly. Chest. 1993;103(5):1636–7.
Nakajoh K, Nakagawa T, Sekizawa K, Matsui T, Arai H, Sasaki H. Relation between incidence of pneumonia and protective reflexes in post-stroke patients with oral or tube feeding. J Intern Med. 2000;247(1):39–42.
Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004.
Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, Schwab RJ, Loring SH, Malhotra A, White DP, Wellman A. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014;190(8):930–7.
Martin-Harris B. Clinical implications of respiratory–swallowing interactions. Curr Opin Otolaryngol Head Neck Surg. 2008;16(3):194–9.
Hiss SG, Treole K, Stuart A. Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults. Dysphagia. 2001;16(2):128–35.
Martin-Harris B, Brodsky MB, Michel Y, Ford CL, Walters B, Heffner J. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg. 2005;131(9):762–70.
Martin BJ, Logemann JA, Shaker R, Dodds WJ. Coordination between respiration and swallowing: respiratory phase relationships and temporal integration. J Appl Physiol (1985). 1994;76(2):714–23.
Butler SG, Postma GN, Fischer E. Effects of viscosity, taste, and bolus volume on swallowing apnea duration of normal adults. Otolaryngol Head Neck Surg. 2004;131(6):860–3.
Preiksaitis HG, Mayrand S, Robins K, Diamant NE. Coordination of respiration and swallowing: effect of bolus volume in normal adults. Am J Physiol. 1992;263(3 Pt 2):R624–30.
Klahn MS, Perlman AL. Temporal and durational patterns associating respiration and swallowing. Dysphagia. 1999;14(3):131–8.
Terzi N, Orlikowski D, Aegerter P, Lejaille M, Ruquet M, Zalcman G, Fermanian C, Raphael JC, Lofaso F. Breathing-swallowing interaction in neuromuscular patients: a physiological evaluation. Am J Respir Crit Care Med. 2007;175(3):269–76.
Sato K, Nakashima T. Sleep-related deglutition in patients with sleep apnea–hypopnea syndrome. Ann Otol Rhinol Laryngol. 2009;118(1):30–6.
Stanchina ML, Abu-Hijleh M, Chaudhry BK, Carlisle CC, Millman RP. The influence of white noise on sleep in subjects exposed to ICU noise. Sleep Med. 2005;6(5):423–8.
Saremi M, Grenèche J, Bonnefond A, Rohmer O, Eschenlauer A, Tassi P. Effects of nocturnal railway noise on sleep fragmentation in young and middle-aged subjects as a function of type of train and sound level. Int J Psychophysiol. 2008;70(3):184–91.
Acknowledgments
K.O. is the guarantor of the paper and takes responsibility for the integrity for the work as a whole, from inception to published articles. K.N., E.T., and S.T. contributed to conception and design. J.A.F., N.T.A., and A.A.L. contributed to drafting the manuscript for important intellectual content. F.R.A. contributed to analysis, interpretation, and drafting the manuscript. This work was supported by a Scientific Research Grant (C) (25463239) from the Japan Society for the Promotion of Science (JSPS). We thank Mrs. Mary Wong for her assistance in the statistical analysis and Ms. Samantha Song for her editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Okuno, K., Nohara, K., Takai, E. et al. Sleep Stage Coordination of Respiration and Swallowing: A Preliminary Study. Dysphagia 31, 579–586 (2016). https://doi.org/10.1007/s00455-016-9719-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-016-9719-5